Abstract
In-frame fusion KIF5B (the-kinesin-family-5B-gene)-RET transcripts have been characterized in 1–2% of non-small cell lung cancers and are known oncogenic drivers. The RET tyrosine kinase inhibitor, vandetanib, suppresses fusion-induced, anchorage-independent growth activity. In vitro studies have shown that vandetanib is a high-affinity substrate of breast cancer resistance protein (Bcrp1/Abcg2) but is not transported by P-glycoprotein (P-gp), limiting its blood-brain barrier penetration. A co-administration strategy to enhance the brain accumulation of vandetanib by modulating P-gp/Abcb1- and Bcrp1/Abcg2-mediated efflux with mTOR inhibitors, specifically everolimus, was shown to increase the blood-brain barrier penetration. We report the first bench to bed-side evidence that RET inhibitor combined with an mTOR inhibitor is active against brain-metastatic RET-rearranged lung cancer and the first evidence of blood-brain barrier penetration. A 74 year old female with progressive adenocarcinoma of the lung (wild-type EGFR and no ALK rearrangement) presented for therapy options. A deletion of 5’RET was revealed by FISH assay, indicating RET-gene rearrangement. Because of progressive disease in the brain, she was enrolled in a clinical trial with vandetanib and everolimus (NCT01582191). Comprehensive genomic profiling revealed fusion of KIF5B (the-kinesin-family-5B-gene) and RET, in addition to AKT2 gene amplification. After 2 cycles of therapy a repeat MRI brain showed a decrease in the intracranial disease burden and PET /CT showed systemic response as well. Interestingly, AKT2 amplification seen is a critical component of the PI3K/mTOR pathway, alterations of which has been associated with both de novo and acquired resistance to targeted therapy. The addition of everolimus may have both overcome the AKT2 amplification to produce a response in addition to its direct effects on the RET gene. Our case report forms the first evidence of blood-brain-barrier penetration by vandetanib in combination with everolimus.Further research is required in this setting.
Keywords: RET, mTOR, Lung cancer, Brain metastases, Blood brain barrier, RET fusion, AKT, Next generation sequencing, Vandetanib, Everolimus, exceptional responder, outlier responder
Introduction
Discovery of ‘driver oncogenes’ and molecularly targeted therapies with inhibitory drugs targeting the oncogenes have revolutionized the therapeutic landscape of non-small cell lung cancer (NSCLC). Targeted therapeutics are replacing chemotherapy even in the front-line setting. In addition to tumors harboring the EGFR mutations that respond to EGFR tyrosine kinase inhibitors (erlotinib, geftinib)[1] and ALK rearranged tumors that remarkably respond well to ALK inhibitors (crizotinib, ceretinib), recently RET (rearranged during transfection) fusions have been discovered in NSCLC[1, 2]. RET is a receptor tyrosine kinase that is primarily expressed in cells of the nervous system[2, 3]. It has been identified as a proto-oncogene. Recombination of the RET locus with a partner gene has been shown to result in cellular transformation[3]. In the TCGA dataset RET rearrangement was observed in 2 of 513 lung adenocarcinoma cases[4]. Other studies have identified RET rearrangement in 1–2% of cases[2, 5–7] and at an incidence of 6% in non-small cell lung cancer patients lacking other known driver mutations[7]. Multiple activating RET fusions with distinct fusion partners have been described in cancer[4, 8], with the KIF5B-RET and CCDC6-RET fusions being most the common variants in NSCLC. Several multi-kinsae inhibitors with activity against RET have been approved by the US FDA in various indications, including vandetanib, cabozantinib, ponatinib, sunitinib, regorafenib, and sorafenib. Preclinical studies demonstrated that cells transformed by KIF5B-RET are sensitive to treatment with vandetanib, sorafenib, and sunitinib[2, 9, 10]. These agents and other drugs targeting RET are in clinical trials in NSCLC and other solid tumors. Preliminary data from an ongoing Phase 2 study reported a partial response in an NSCLC patient harboring the KIF5B-RET fusion treated with cabozantinib[7]. Another case reported an ongoing 5 month response to vandetanib in a patient with NSCLC harboring a CCDC6-RET fusion[11]. However, a retrospective evaluation of RET biomarker status and outcome to vandetanib monotherapy in four phase III randomized NSCLC trials failed to show any differential advantage in RET fusion patients[12]. In addition the blood brain barrier penetration of vandetanib in RET rearranged lung cancer is not reported. Our case report forms the first bench-to-bedside evidence of blood-brain-barrier penetration by RET inhibitor vandetanib combined with the mTOR inhibitor everolimus.
Case history
A 74-year-old Caucasian female who was a former light smoker presented with worsening dyspnea and weight loss. A chest x-ray revealed a right pleural effusion. Thoracentesis drained 1.8 liters of bloody fluid. An FNA cytologic examination revealed adenocarcinoma of the lung (wild-type EGFR and no ALK rearrangement). An MRI of the brain demonstrated brain metastases, for which she underwent gamma knife radiosurgery. PET/CT revealed the presence of metastatic osseous lesions at T2, L1, and hemipelvis. She underwent 6 cycles of carboplatin and pemetrexed with zoledronic acid, followed by docetaxel, for 8 cycles. A follow-up MRI of the brain revealed progression of disease, with multiple enhancing lesions throughout the brain parenchyma that were 1 to 6 mm in size. She underwent whole-brain radiation therapy. A re-staging CT chest showed a right soft tissue nodule in the lower lobe that measured 1.7 cm, and loculated right pleural effusion. She presented to our institution for further treatment options. A 50-gene panel based on exome sequencing was negative. A deletion of 5’RET was revealed by FISH assay, indicating RET-gene rearrangement (Figure-1). Because of progressive disease in the brain, she was enrolled in a clinical trial with vandetanib (300 mg Q daily) and everolimus (10 mg Q daily) (NCT01582191). The time interval between the whole brain radiation therapy and re-staging MRI brain was 4.5 months. Fortuitously, comprehensive genomic profiling was also performed in parallel and revealed fusion of KIF5B (the-kinesin-family-5B-gene) and RET (Figure-2), in addition to AKT2 and several gene amplifications (Table 1). The patient tolerated therapy reasonably well, with grade-2 diarrhea that required loperamide. Her performance status and quality of life improved. After 2 cycles of therapy a repeat MRI of the brain showed a decrease in the intracranial disease burden (Figure-3-A–D). A PET showed response in the right hemithorax as well (Figure-3-E,F).
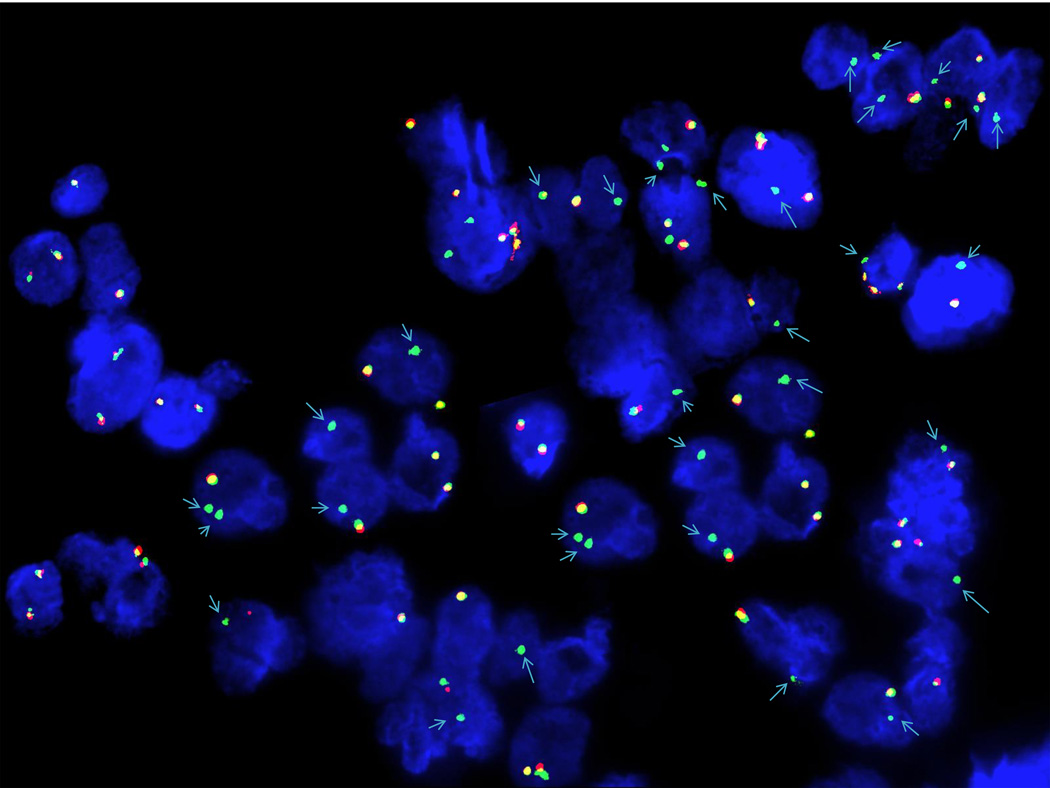
Figure 1.
Fluorescence in situ hybridization (ISH) of paraffin-embedded tissue sections from pleura, right biopsy. The ISH technique was performed using a Clear-View FISH RET dual-color breakapart probe from Cymogen DX. The probe hybridizes to band 10q11.21 (cmyo-orange on the centromeric side and cymo-green on the telomeric side of the RET gene breakpoint). Deletion of 5’-RET (red signal) was observed in 47.0% of the interphases scanned, indicating a RET gene rearrangement.
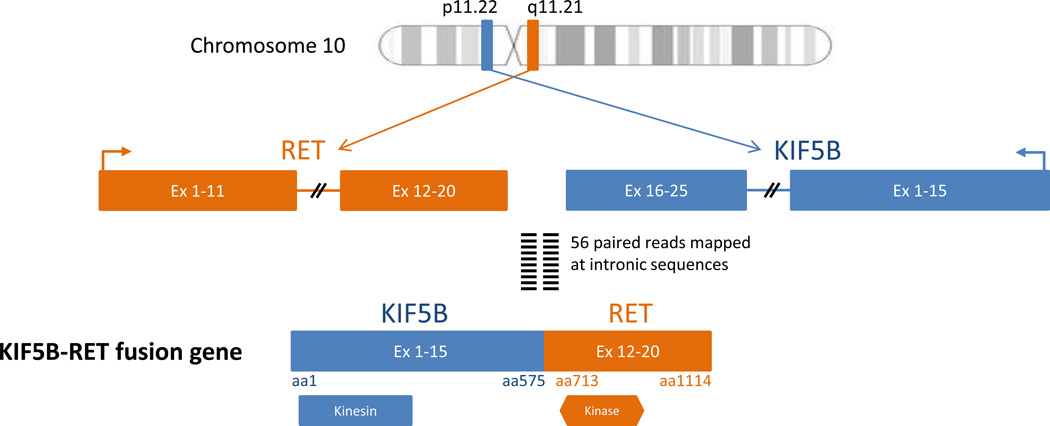
Figure 2.
Schematic representation of KIF5B-RET fusion gene. An inversion rearrangement event within chromosome 10 involves exons 1 to 15 of Kinesin-1 heavy chain protein (KIF5B), containing the kinesin motor domain and exons 12 to 20 of RET proto-oncogene, containing the protein tyrosine kinase domain.
Table 1.
Copy number alterations in addition to the KIF5B-RET fusion. Interestingly AKT2 amplification seen is a critical component of the PI3K/mTOR pathway, alterations of which has been associated with both de novo and acquired resistance to targeted therapy. The addition of the rapamycin analog everolimus may have both overcome the AKT amplification to produce a response in addition to its direct effects on the RET gene. In addition there was full homozygous loss of CDKN2A/B.
| Gene | Copy Number | Position |
|---|---|---|
| MDM2 | 9 | chr12:69153996-69277205 |
| NFKBIA | 10 | chr14:35871177-35873850 |
| NKX2-1 | 10 | chr14:36947748-37023501 |
| CCNE1 | 8 | chr19:30253918-30360638 |
| AKT2 | 8 | chr19:40701261-40791492 |
| AXL | 8 | chr19:41725275-41765809 |
| CDKN2A | 0 | chr9:21853212-21998002 |
| CDKN2B | 0 | chr9:21998748-22101832 |
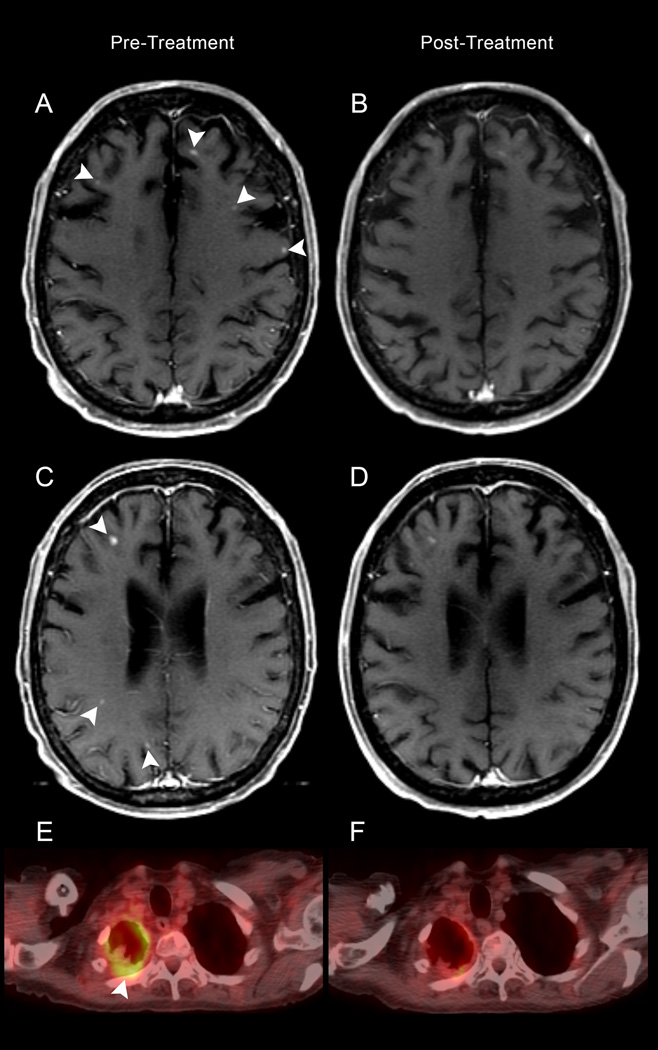
Figure 3.
A–E: Pretreatment MR (A, and C) demonstrates enhancing lesions (arrowheads) on the axial T1-weighted images, decreased in number and size following treatment (B and D). FDG- PET CT reveals pleural hypermetabolism in the right hemithorax (E) with decreased avidity post-therapy (F).
Discussion
We report the first evidence that RET inhibitor-based therapy is active against brain-metastatic RET-rearranged lung cancer and the first evidence of blood-brain barrier penetration. In-frame fusion KIF5B-RET transcripts have been characterized in 1–2% of non-small cell lung cancers [2, 6]. The KIF5B-RET fusion reported here is predicted to activate the RET kinase, resulting in oncogenic transformation[2, 9, 10, 13] The RET tyrosine kinase inhibitor, vandetanib, suppresses fusion-induced, anchorage-independent growth activity; it has been translated to the clinic and has been described in published case reports[14]. Moreover, vandetanib targets RET as well as tumor endothelium[15]. However, single agent vandetanib response in lung cancer has been low [16] and monotherapy with vandetanib failed to show any differential advantage in RET fusion patients in a large retrospective study[12].
In vitro studies have shown that vandetanib is a high-affinity substrate of breast cancer resistance protein (Bcrp1/Abcg2) but is not transported by P-glycoprotein (P-gp), limiting its blood-brain barrier penetration[17]. A co-administration strategy to enhance the brain accumulation of vandetanib by modulating P-gp/Abcb1- and Bcrp1/Abcg2-mediated efflux with mTOR inhibitors, specifically everolimus, was shown to increase the blood-brain barrier penetration[17]. Our case report forms the first bench-to-bedside evidence of blood-brain-barrier penetration by vandetanib in combination with everolimus. Interestingly, AKT2 amplification seen is a critical component of the PI3K/mTOR pathway, alterations of which has been associated with both de novo and acquired resistance to targeted therapy. AKT2 (also known as PKB-beta) encodes an intracellular serine/threonine kinase that is one of three members of the AKT gene family[18]. Activation of AKT2 has been implicated in multiple malignancies[18]. Alterations in the PI3K/AKT signaling pathway are frequent in lung cancer[19]. In the TCGA datasets, AKT2 amplification was found in 1.3% of lung adenocarcinoma cases and 4.5% of lung squamous cell carcinoma cases[20]. There are no US FDA approved therapies to address the amplification of AKT2. The mTOR inhibitors everolimus and temsirolimus are US FDA approved for the treatment of other tumor types, and these agents can impact the AKT pathway. Clinical studies have shown some activity of everolimus, either alone or in combination with other therapies, in NSCLC[21, 22].
The addition of everolimus may have both overcome the AKT amplification to produce a response in addition to its direct effects on the RET gene[23, 24]. This leads to the hypothesis that combined RET and mTOR pathway inhibition may overcome the innate and/or acquired resistance to RET-targeted monotherapy, as has been demonstrated in other systems[24–27]. In conclusion, RET inhibitor-based therapy may be active against brain metastatic RET-rearranged lung cancer due to potential blood-brain barrier penetration. Further research is required in this setting.
Supplementary Material
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported by Cancer Center Support Grant no. CA 016672
Footnotes
Conflicts of interest disclosure: SMA, CM and VAM are employees of and have equity interest in Foundation Medicine.
References
- 1.Kohno T, Tsuta K, Tsuchihara K, et al. RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci. 2013;104:1396–1400. doi: 10.1111/cas.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 4.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84:121–126. doi: 10.1016/j.lungcan.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 7.Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013;3:630–635. doi: 10.1158/2159-8290.CD-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshihara K, Wang Q, Torres-Garcia W, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2014 doi: 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 11.Falchook GS, Ordonez NG, Bastida CC, et al. Effect of the RET Inhibitor Vandetanib in a Patient With RET Fusion-Positive Metastatic Non-Small-Cell Lung Cancer. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.50.5016. [DOI] [PubMed] [Google Scholar]
- 12.Adam Platt PE, Morten John, Ji Qunsheng, Donald Emma, Womack Chris, Su Xinying, Zheng Li, Gladwin Amanda, Vasselli James Robert, Lee Jin Soo, De Boer Richard H, Herbst Roy S The RET Analysis Team. Retrospective evaluation of RET biomarker status and outcome to vandetanib in four phase III randomized NSCLC trials. Journal of Clinical Oncology, 2013 ASCO Annual Meeting Abstracts. 2013;31(15_suppl):8045. (May 20 Supplement), 2013. [Google Scholar]
- 13.Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautschi O, Zander T, Keller FA, et al. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J Thorac Oncol. 2013;8:e43–e44. doi: 10.1097/JTO.0b013e31828a4d07. [DOI] [PubMed] [Google Scholar]
- 15.Beaudry P, Nilsson M, Rioth M, et al. Potent antitumor effects of ZD6474 on neuroblastoma via dual targeting of tumor cells and tumor endothelium. Mol Cancer Ther. 2008;7:418–424. doi: 10.1158/1535-7163.MCT-07-0568. [DOI] [PubMed] [Google Scholar]
- 16.Heymach JV, Lockwood SJ, Herbst RS, et al. EGFR biomarkers predict benefit from vandetanib in combination with docetaxel in a randomized phase III study of second-line treatment of patients with advanced non-small cell lung cancer. Ann Oncol. 2014;25:1941–1948. doi: 10.1093/annonc/mdu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minocha M, Khurana V, Qin B, et al. Co-administration strategy to enhance brain accumulation of vandetanib by modulating P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors. Int J Pharm. 2012;434:306–314. doi: 10.1016/j.ijpharm.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu AX, Testa JR, Hamilton TC, et al. AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res. 1998;58:2973–2977. [PubMed] [Google Scholar]
- 19.Scrima M, De Marco C, Fabiani F, et al. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): new insights on the role of phosphatydil-inositol-3 kinase. PLoS One. 2012;7:e30427. doi: 10.1371/journal.pone.0030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadimitrakopoulou VA, Soria JC, Jappe A, et al. Everolimus and erlotinib as second- or third-line therapy in patients with advanced non-small-cell lung cancer. J Thorac Oncol. 2012;7:1594–1601. doi: 10.1097/JTO.0b013e3182614835. [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009;20:1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 23.Gild ML, Landa I, Ryder M, et al. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr Relat Cancer. 2013;20:659–667. doi: 10.1530/ERC-13-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapa I, Saggiorato E, Giachino D, et al. Mammalian target of rapamycin pathway activation is associated to RET mutation status in medullary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2146–2153. doi: 10.1210/jc.2010-2655. [DOI] [PubMed] [Google Scholar]
- 25.Subbiah V, Naing A, Brown RE, et al. Targeted morphoproteomic profiling of Ewing's sarcoma treated with insulin-like growth factor 1 receptor (IGF1R) inhibitors: response/resistance signatures. PLoS One. 2011;6:e18424. doi: 10.1371/journal.pone.0018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbiah V, Westin SN, Wang K, et al. Targeted therapy by combined inhibition of the RAF and mTOR kinases in malignant spindle cell neoplasm harboring the KIAA1549-BRAF fusion protein. J Hematol Oncol. 2014;7:8. doi: 10.1186/1756-8722-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal R, Koenig K, Rohren E, Subbiah V. Combined Antiangiogenic and Mammalian Target of Rapamycin Inhibitor Targeted Therapy in Metaplastic Breast Cancer Harboring a PIK3CA Mutation. J Breast Cancer. 2014;17:287–290. doi: 10.4048/jbc.2014.17.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.