Abstract
Background
Dermatologic adverse events (AEs) are some of the most frequently observed toxicities of immune-checkpoint inhibitor therapy, but have received little attention. The drugs, pembrolizumab and nivolumab are recently approved inhibitors of the PD-1 receptor that have overlapping AE profiles however, the incidence, relative risk (RR), and clinico-morphological pattern of the associated dermatologic AEs is not known.
Methods
We conducted a systematic review of the literature, and performed a meta-analysis of dermatologic AEs with the use of pembrolizumab and nivolumab in cancer patients. An electronic search was conducted using the PubMed, and Web of Science, and on the ASCO and ESMO meeting abstracts’ library for potentially relevant oncology trials employing the drugs at FDA-approved doses and reporting dermatologic AEs. The incidence, relative risk and 95% CIs were calculated using either random- or fixed-effects models based on the heterogeneity of included studies. The clinical presentation, histology of affected areas, and management strategies based on institutional experience, are also presented.
Results
Rash, pruritus and vitiligo were the most frequently reported AEs. The calculated incidence of all-grade rash with pembrolizumab and nivolumab was 16.7% (RR=2.6) and 14.3% (RR=2.5), respectively. Other significant all-grade AEs included pruritus [pembrolizumab: incidence, 20.2% (RR=49.9); nivolumab: incidence, 13.2% (RR=34.5)] and vitiligo [pembrolizumab: incidence, 8.3% (RR=17.5); nivolumab: 7.5% (RR=14.6)]. Interestingly, all the vitiligo events were reported in trials investigating melanoma. The RR for developing dermatologic toxicities in general, was 2.95 with pembrolizumab, and 2.3 with nivolumab.
Conclusion
We found that pembrolizumab and nivolumab are both associated with dermatologic AEs, primarily low-grade rash, pruritus, and vitiligo, which are reminiscent of those seen with ipilimumab. Knowledge of these findings is critical for optimal care, maintaining dose intensity, and health-related quality of life, in cancer patients receiving PD-1 inhibitors.
Keywords: adverse events, immune-checkpoint, immunotherapy, incidence, nivolumab, PD-1, pembrolizumab, programmed death-1, pruritus, rash, risk, targeted therapies, vitiligo
INTRODUCTION
Recent developments in cancer immunology at the cellular and molecular level have generated a renewed interest in the immunotherapy of cancer. The cytotoxic T lymphocyte antigen-4 (CTLA-4) signalling pathway, and more recently, the programmed death receptor-1 (PD-1)/PD ligand-1 (PD-L1/PD-L2) signalling pathway are being increasingly recognized as critical mediators (“checkpoints”) of tumor-induced immune suppression. While the former (CTLA-4, with its ligands CD80 and CD86) is crucial in attenuating the activation of naive and memory T cells (in the early phase) after binding with the T cell receptor, the latter is active against T cell activity in peripheral tissues (PD-1, with its ligands PD-L1/-L2) in chronic inflammatory states, and infection or cancer, thus suppressing autoimmunity.1 More specifically, interaction between the ligand and PD-1 receptor delimits the body’s normal anti-tumor immune response by inhibiting T-cell proliferation, down-modulating cytokines and anti-apoptotic molecules, and promoting induced T-reg cell proliferation—ultimately conferring immune resistance in tumor cells.2–5
Preclinical and clinical data have shown that pharmacological inhibition of the PD-1 signaling pathway is more beneficial (viv-a-vis CTLA-4 blockade), in terms of antitumor effects and adverse event (AE) rates.6–9 This led to the eventual approval of two novel anti-PD-1 receptor IgG4-κ monoclonal antibodies (mAbs)—pembrolizumab (humanized; Keytruda®, Merck & Co., Inc., Whitehouse Station, NJ), and nivolumab (fully human; Opdivo®, Bristol-Myers Squibb Company, Princeton, NJ). At the time of this writing, both drugs were indicated for the treatment of unresectable or metastatic melanoma and metastatic non-small cell lung cancer (NSCLC), with nivolumab being additionally approved for renal cell carcinoma; the precise treatment regimen with either agent however varies, and is subject to certain underlying criteria (e.g. BRAFV600 mutational status, prior treatment with ipilimumab/platinum-based chemotherapy/antiangiogenic therapy, or PD-L1 expression).10,11 In general, these drugs inhibit the key immune-compromising interaction between the tumor cell PD-L1 (B7-H1)/PD-L2 (B7-DC) and T cell PD-1 receptors.12
In terms of AEs, their overall safety profiles appear impressive. However, the potential for developing dermatologic AEs remains significant—moreover, the pattern is understood to be different in frequency and character from most other chemotherapy and targeted therapy-induced AEs.13 Some of these AEs manifest with signs of autoimmunity (“immune-related adverse events”, irAEs), and are thought to be due to treatment-related nonspecific hyperfunctioning of the immune system. Our current understanding mostly stems from the ipilimumab experience, wherein the development of rash, pruritus, alopecia, and vitiligo (in 20–30% of patients)14 may lead to anticancer therapy dose modifications and/or termination, besides impairing patients’ health-related quality of life (HRQoL). Since very little is known about PD-1 inhibitor-induced dermatologic AEs, we sought to determine the incidence and risk, and describe the clinical characteristics in patients evaluated at our oncodermatology program.
PATIENTS AND METHODS
Data sources and search strategy
We conducted a systematic search of the literature to identify clinical trials of pembrolizumab and nivolumab, that reported dermatologic AEs. The Medline (via PubMed) and Thomson-Reuters’ Web of Science databases were searched for studies published between January, 1960, and July, 2015. The following generic drug names and synonyms were used as search terms: pembrolizumab (MK-3475/lambrolizumab/Keytruda) and nivolumab (BMS-963558/ONO-4538/MDX-1106/Opdivo). In addition, pertinent abstracts from the American Society of Clinical Oncology’s (ASCO) and the European Society for Medical Oncology’s (ESMO) annual meetings/congresses were reviewed. The latest manufacturer’s package inserts were also retrieved.
Study selection and screening process
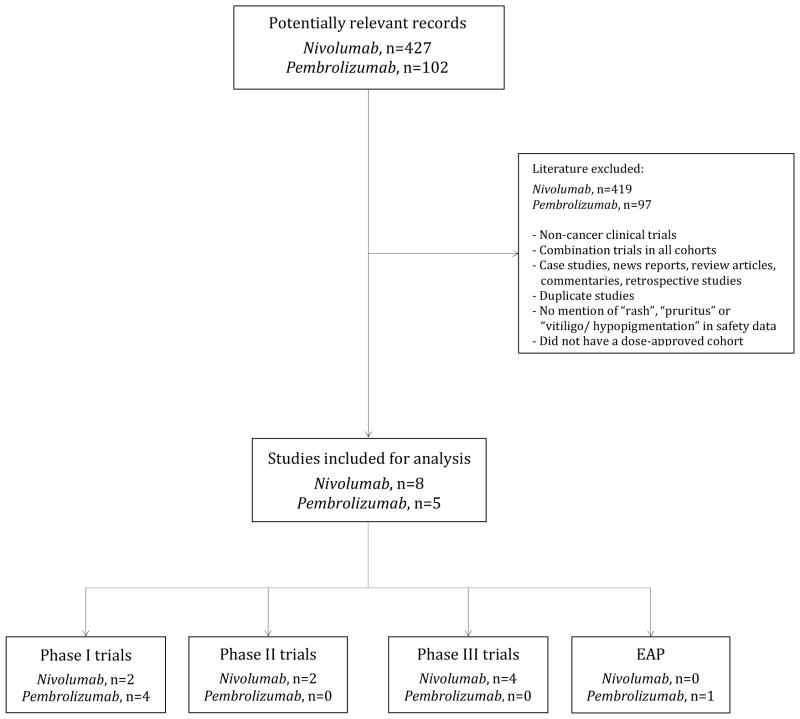
Our selection criteria included all prospective clinical trials (and/or cohorts) that: 1) investigated the utility of pembrolizumab or nivolumab at the FDA-approved dose in the treatment of cancer; 2) clearly reported a dermatologic AE in their safety data, with or without the clinical severity grading; and 3) were published in the English language (Fig. 1). We excluded any trials that: 1) involved combination regimens with other therapies/modalities (e.g. targeted therapy, chemotherapy, radiation therapy, other immunotherapies); and 2) did not list a dermatologic AE exclusively in any arm/cohort (Fig 1). Within the trials included for analysis, for skin eruptions, data was recorded only for instances of “rash”; others e.g. “rash maculopapular”, “macular/erythematous rash”, “pruritic rash”, “eczema”, “dermatitis”, “drug eruption” were excluded because of possible duplication in reporting within the same trial (the excluded data was not significant). In the event of duplicates, ambiguity or publications reporting on the same study population, only the most recent, relevant and/or comprehensive publication (abstract/manuscript) was retained. Any discrepancy in selection was resolved by consensus.
Fig 1.
Flow diagram showing the selection process for clinical trials included in the final analysis.
Data extraction and Clinical Endpoints
The data abstraction was conducted by two authors (V.R.B. and B.B.) independently and then reviewed. The variables extracted included the name of the first author, year of publication, clinical trial identifier (e.g. NCT#), study design, overall enrollment numbers, number of treatment arms/cohorts (experimental/control) and dosing details, all-grade and high-grade AEs among those evaluated for safety, the underlying cancer indication, AE reporting threshold, and the clinical severity grading system used. When AEs were reported as percentages, we manually calculated the numbers and rounded down to the nearest whole number, or excluded the publication if this was not possible.
The safety data in each clinical trial was examined for the clinical endpoints. The Common Terminology Criteria for Adverse Events (CTCAE) version 4.0,15 issued by the National Cancer Institute (NCI), was predominantly used by all trials included in the analysis. We included the data from all trials reporting grade ≥ 1 rash, pruritus, or hypopigmentation/vitiligo. For dermatologic AEs where the available data did not lend itself to a statistical analysis, we employed descriptive methods.
Meta-analytic Strategy
All statistical analyses were performed using version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, N.J., USA).16 The total number of patients with all-grade and high-grade rash, pruritus, and vitiligo were extracted from the clinical trials as delineated above (Fig 1). For each clinical trial, the incidence of rash, pruritus, and vitiligo were calculated, and the 95% confidence intervals (CI) were derived. The relative risk (RR) of AEs among patients assigned to the respective PD-1 receptor inhibitor was calculated and compared with those assigned to chemotherapy. To graphically represent the meta-analysis, forest plots were constructed.
For the meta-analysis, both the fixed-effects model (weighted with inverse variance) and the random-effects model were considered.17 For each meta-analysis, Cochran’s Q statistic was first calculated to assess the heterogeneity of the included trials. For P value <0.1, the assumption of homogeneity was deemed invalid, and the random-effects model was used.18 Otherwise, results from both the fixed-effects model and the random-effects model were evaluated, and if they were similar, only fixed-effects model results were reported. A two-tailed P value <0.05 was considered as statistically significant.
Case series
We obtained a waiver of research authorization from the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board (IRB) for this portion of the study. The electronic medical records of patients who were evaluated by a dermatologist (M.E.L.) for an AE that developed during treatment with pembrolizumab or nivolumab were identified using an institutional data mining service—the Information Systems’ data delivery group, DataLine. Subsequently, the corresponding clinical notes, pathology slides/reports of available skin biopsy specimens, and clinical photographs were examined and relevant data was recorded.
RESULTS
Our search strategy yielded a total of 427 potentially relevant records related to nivolumab and 102 records for pembrolizumab. Of these, 8 studies [phase I (n=2),19,20 phase II (n=2),21,22 phase III randomized controlled (n=4)8,23–25] investigating nivolumab, and 5 studies [phase I (n=4),26–29 expanded access program (n=1)30] of pembrolizumab were included in the final statistical analysis (Table 1).
Table 1.
Characteristics of all oncology clinical trials included in the final analysis.†
| Trial (first author, year) | Trial Design | Investigational arm | Control Arm(s)/Cohorts | Total Enrollment* | Treated patients** (assessed for AEs) | Underlying Malignancy | CTCAE version |
|---|---|---|---|---|---|---|---|
| Brahmer et al., 201523 | Phase III, randomized, open-label | Nivolumab 3mg/kg q2wks. | Docetaxel 75mg/m2 of BSA q3wks. | 352 | 131 | Advanced squamous-cell NSCLC | 4 |
| Larkin et al., 20158 | Phase III, randomized, double-blind | Nivolumab 3mg/kg q2wks. + Ipilimumab-matched placebo | Ipilimumab 3mg/kg q3wks.+ Nivolumab-matched placebo OR Nivolumab 1mg/kg q3wks. + Ipilimumab 3mg/kg q3wks. |
1296 | 313 | Unresectable stage III/IV malignant melanoma | 4 |
| Weber et al., 201524 | Phase III, randomized controlled, open-label | Nivolumab 3mg/kg q2wks. | Investigator choice of chemotherapy | 631 | 268 | Unresectable stage III/IV malignant melanoma | 4 |
| Robert et al., 201525 | Phase III, randomized, double-blind | Nivolumab 3mg/kg q2wks. + Dacarbazine-matched placebo | Dacarbazine 1000mg/m2 q3wks. + Nivolumab matched-placebo | 518 | 206 | Untreated stage III/IV melanoma (without a BRAF mutation) | 4 |
| Rizvi et al., 201521 | Phase II, single-arm | Nivolumab 3mg/kg q2wks. | X | 140 | 117 | Advanced refractory squamous NSCLC | 4 |
| Hamanishi et al., 201522 | Phase II, non-randomized, single-arm, open-label | Nivolumab 3mg/kg q2wks. | X | 20 | 10 | Advanced or relapsed, platinum-resistant ovarian cancer | X |
| Weber et al., 201320 | Phase I, open-label, multi-dose, dose-escalation study | Nivolumab 3mg/kg q2wks. (Cohort 6)† | Nivolumab 1mg/kg q2wks. + Peptide vaccine (Cohort 1) Nivolumab 3mg/kg q2wks. + Peptide vaccine (Cohort 2) Nivolumab 10mg/kg q2wks. + Peptide vaccine (Cohort 3) Nivolumab 3mg/kg q2wks. + Peptide vaccine (Cohort 4)† Nivolumab 3mg/kg q2wks. + Peptide vaccine (Cohort 5)†† |
90 | 41 | Ipilimumab-refractory or Ipilimumab-naïve melanoma | 4 |
| Topalian et al., 201219 | Phase I | Nivolumab 3mg/kg q2wks. | X | 296 | 50 | Advanced melanoma, NSCLC, RCC, castration-resistant prostate cancer, or CRC | 3 |
| Robert et al., 201427 | Phase I, open-label | Pembrolizumab 2mg/kg q3wks. | X | 178 | 89 | Ipilimumab-refractory advanced melanoma | 4 |
| Garon et al., 201528 | Phase I | Pembrolizumab 2mg/kg q3wks. | X | 495 | 6 | Advanced NSCLC | 4 |
| Hamid et al., 201326 | Phase I | Pembrolizumab 2mg/kg q3wks. | X | 135 | 22 | Advanced Melanoma | 4 |
| Perdon et al., 201530 | EAP | Pembrolizumab 2mg/kg q3wks. | X | 62 | 60 | Advanced melanoma | NA |
| Patnaik et al., 201529 | Phase I | Pembrolizumab 2mg/kg q3wks. | X | 31 | 7 | Advanced solid tumors | 4 |
Abbreviations: Adverse events, AEs; Colorectal cancer, CRC; Renal cell cancer, RCC; Non-small-cell lung cancer, NSCLC; NA, not available
The relative risk was calculated and compared only in trials with chemotherapy (as controls);
Represents the overall number of patients treated with the drug at all doses in the trial and/or cohort;
Represents the number of patients who were treated with the drug at the FDA-approved dose in the trial and/or cohort, and were included in the analysis;
For cohorts 4 and 6, only grade 1 and 2 dose-limiting toxicities for ipilimumab were allowed;
Cohort 5 patients were required to have had grade 3 dose-limiting toxicity for ipilimumab.
Incidence of all-grade and high-grade (grades 3/4) rash and relative risk
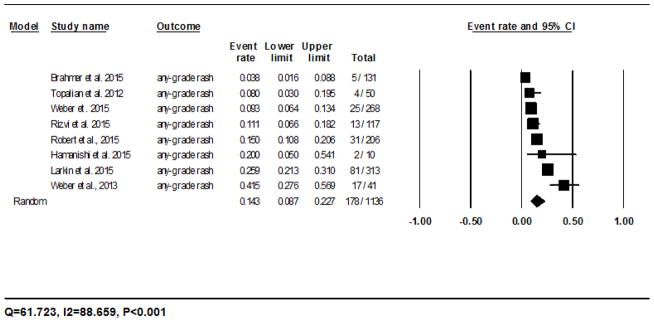
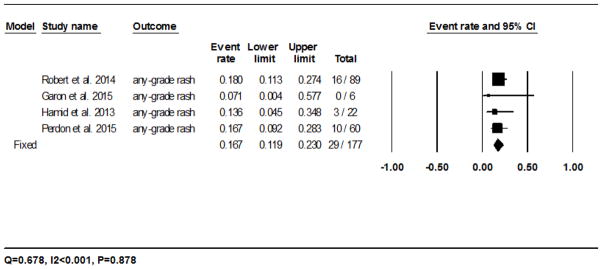
The calculated incidence of all-grade rash with nivolumab was 14.3% (95% CI: 8.7–22.7%) in 178/1136 patients analyzed using the random-effects model, and ranged from 3.8% (5/131)23 to 41.5% (17/41)20 (Fig. 2A). The RR of developing all-grade rash to nivolumab as compared to the chemotherapy controls was 2.5 (95% CI: 1.3–5.0; P=0.008). For pembrolizumab, the incidence was 16.7% (95% CI: 11.9–23.0%) in 29/177 patients as per the fixed-effects model, and ranged from 0% (0/6)28 to 18% (16/89)27 (Fig. 2B). The RR of developing all-grade rash as compared to the chemotherapy controls was 2.6 (95% CI: 1.2–5.6; P=0.011).
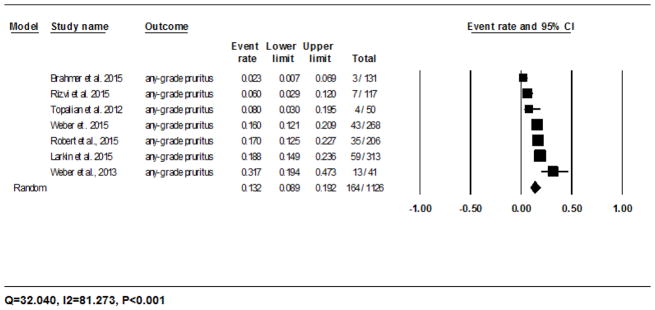
Fig 2.
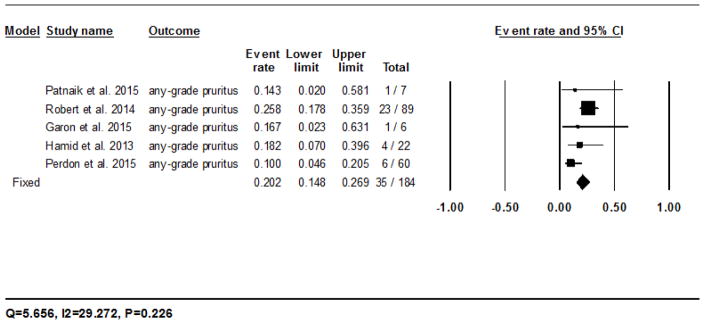
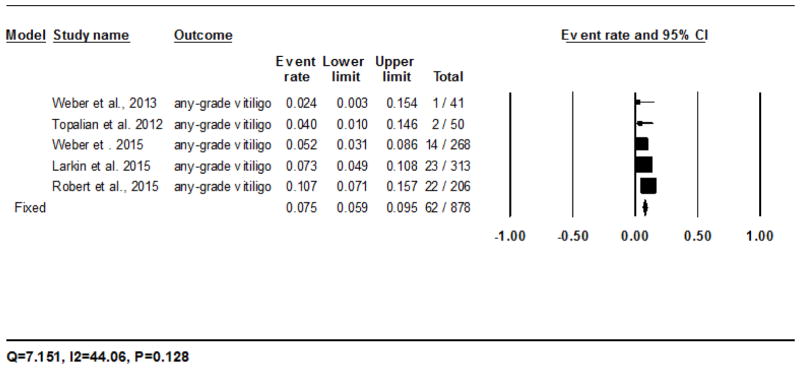
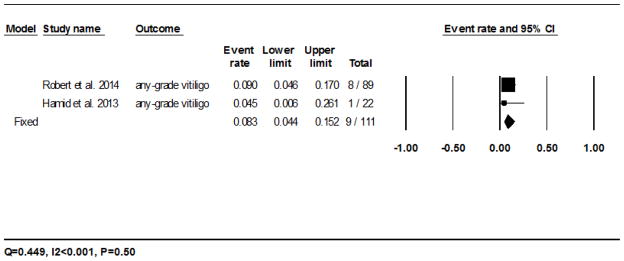
Forest plot corresponding to the main random-effects meta-analysis, including risk estimates quantifying the relationship between treatment with the PD-1 inhibitors, nivolumab and pembrolizumab, and the development of: all-grade rash to nivolumab (Fig 2A) and pembrolizumab (Fig 2B), all-grade pruritus to nivolumab (Fig 2C) and pembrolizumab (Fig 2D), all-grade vitiligo to nivolumab (Fig 2E) and pembrolizumab (Fig 2F). The size of the square box represents each risk estimate, and is proportional to the weight that the risk estimate contributed to the summary risk estimate (diamond symbol). ■ = risk estimate in each trial; horizontal lines “––“ = 95% CI; ◆ = summary risk estimate
The incidence of high-grade rash was low with both drugs [nivolumab – 1.2% (95% CI: 0.5–2.9%) in 8/1136 patients analyzed using the random-effects model, and ranged from 0% (0/131)23 to 10% (1/10)22; pembrolizumab – 1.7% (95% CI: 0.4–6.7%) in 1/171 patients analyzed using the fixed-effects model, and ranged from 0% (0/89)27 to 4.5% (1/22)26]. The RR for developing high-grade rash during treatment with nivolumab and pembrolizumab (as compared to the chemotherapy controls) was 0.7 (95% CI: 0.15–3.0; P=0.613) and 0.4 (95% CI: 0.04–4.1; P=0.424), respectively.
Incidence of all-grade and high-grade (grade 3) pruritus and relative risk
The calculated incidence of all-grade pruritus with nivolumab was 13.2% (95% CI: 8.9–19.2%) in 164/1126 patients analyzed using the random-effects model, and ranged from 2.3% (3/131)23 to 31.7% (13/41)20 (Fig. 2C). The RR of developing all-grade pruritus during treatment with nivolumab as compared to the chemotherapy controls was 34.5 (95% CI: 2.1–550.6; P=0.012). For pembrolizumab, it was 20.2% (95% CI: 14.8–26.9%) in 35/184 patients as per the fixed-effects model, and ranged from 10% (6/60)30 to 25.8% (23/89)27 (Fig. 2D). The RR of developing all-grade pruritus during treatment with pembrolizumab as compared to the chemotherapy controls was 49.9 (95% CI: 3.0–806.0; P=0.006).
The incidence of high-grade pruritus was low with both drugs [nivolumab – 0.5% (95% CI: 0.2–1.3%) in 2/1126 patients analyzed using the fixed-effects model; pembrolizumab – 2.3% (95% CI: 0.7–7.6%) in 1/177 patients analyzed using the fixed-effects model]. The RR of developing high-grade pruritus during treatment with nivolumab and pembrolizumab (as compared to the chemotherapy controls) was 1.5 (95% CI: 0.08–26.4; P=0.782) and 2.2 (95% CI: 0.09–53.3; P=0.63), respectively.
Incidence of all-grade and high-grade (grade 2) hypopigmentation/vitiligo and relative risk
All the vitiligo events were noted in trials of melanoma.8,19,20,24–27 The calculated incidence of all-grade vitiligo with nivolumab was 7.5% (95% CI: 5.9–9.5%) in 62/878 patients analyzed using the fixed-effects model, and ranged from 2.4% (1/41)20 to 10.7% (22/206)25 (Fig. 2E). The RR of developing all-grade vitiligo during treatment with nivolumab was 14.6 (95% CI: 0.9–235.0; P=0.058) as compared to the chemotherapy controls. For pembrolizumab, the incidence was 8.3% (95% CI: 4.4–15.2%) in 9/111 patients as per the fixed-effects model, and ranged from 4.5% (1/22)26 to 9% (8/89)27 (Fig. 2F). The RR of developing all-grade vitiligo during treatment with pembrolizumab was 17.5 (95% CI: 1.03–296.44; P=0.048) as compared to the chemotherapy controls.
The incidence of high-grade vitiligo was low with both drugs [nivolumab – 0.4% (95% CI: 0.1–1.3%); pembrolizumab – 1.1% (95% CI: 0.2–7.4%)] as per the fixed-effects model. The RR of developing high-grade vitiligo during treatment with nivolumab was 0.35 (95% CI: 0.01–8.6; P=0.521) as compared to the chemotherapy controls; the RR for pembrolizumab could not be calculated due to lack of data.
In general, as compared to the chemotherapy controls, the overall RR for developing dermatologic AEs (rash, pruritus, vitiligo) with pembrolizumab was 2.95 (95% CI: 1.5–5.7%; P=0.001), and with nivolumab it was 2.3 (95% CI: 1.3–4.1%; P=0.004).
Other Dermatologic Adverse Events
The findings for other AEs were pooled, since the available data from the included studies was insufficient for a meta-analysis. In patients treated with nivolumab (at any dose), these included xerosis (5.3%, 20/373),25,31 alopecia (2%, 10/502),19,25 stomatitis (1.5%, 2/131),23 urticaria (1.4%, 6/427),19,23 photosensitivity reaction (1.4%, 3/206),25 hyperhidrosis (0.9%, 2/206),25 and skin exfoliation (0.7%, 1/131).23 In patients treated with pembrolizumab (at any dose), these included xerosis (2.4%, 31/1264),7,27,28,32 hair color changes (1.1%, 8/690),7,26 alopecia (0.9%, 5/555),7 and impaired healing (14%, 1/7).29
Case Series: Clinical characteristics and histopathology
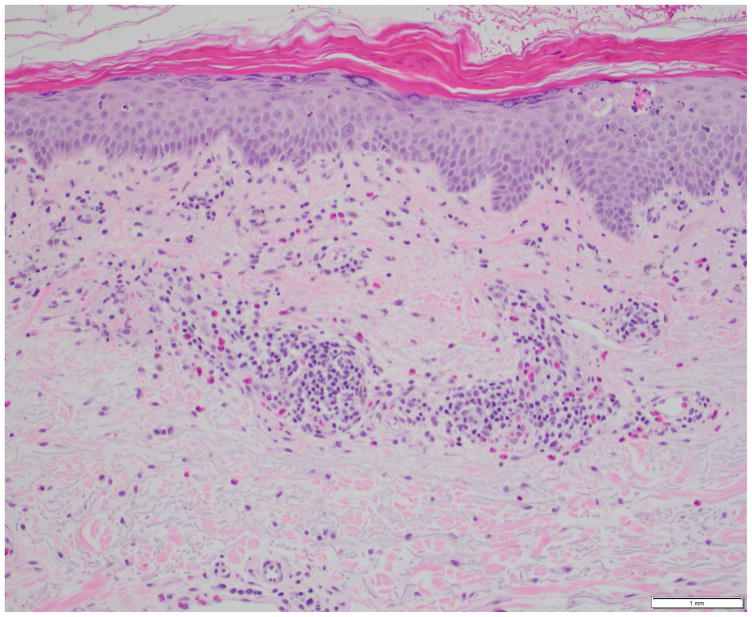
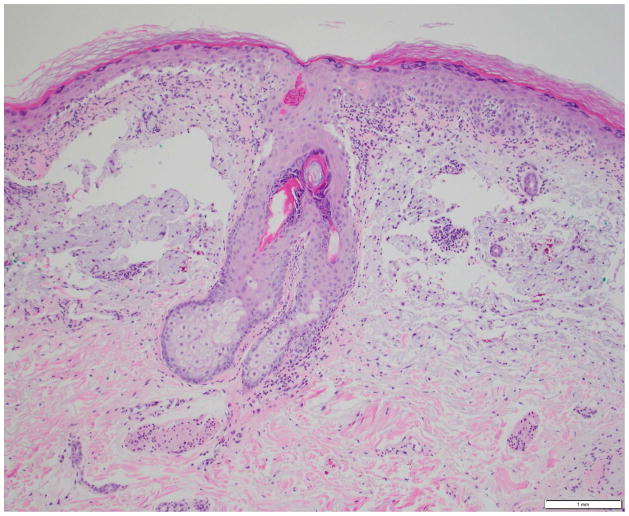
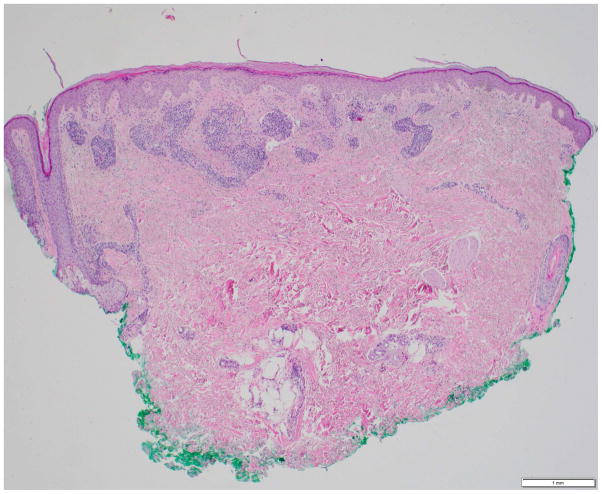
Our institutional data yielded 9 patients with nivolumab-induced rash, and 5 patients with pembrolizumab-induced rash—the clinical and histopathological characteristics are summarized in Table 2, and depicted in Fig. 4. Majority of the patients (12/14) had grade 1/2 rash, and of those, treatment had been interrupted in 3 patients (nivolumab, n=2; pembrolizumab, n=1). The onset of rash after initiation of treatment, ranged from 3 weeks to 2 years in the case of nivolumab-treated patients, and 6 weeks to 20 weeks in pembrolizumab-treated patients. Clinically, it consisted of erythematous macules/papules/plaques (sometimes associated with a scale), with or without pruritus (Fig. 3A–C). The lesions were predominantly localized to the trunk and extremities (upper>lower). At the time of eruption, the peripheral blood eosinophil counts in all patients were within normal limits (WNL) however, autoantibody tests were not clinically indicated. On histopathology, a lichenoid reaction pattern/interface dermatitis, with mild superficial and perivascular lymphohistiocytic infiltrates and scattered eosinophils, was frequently noted (Fig. 4). In the management, medium-to-high potency topical corticosteroids generally sufficed, although oral antihistamines and corticosteroids were occasionally needed (Table 2).
Table 2.
Clinical characteristics of drug eruptions in adult cancer patients receiving nivolumab and pembrolizumab.
| Age, sex |
Underlying malignancy |
Medication, Dose |
Allergies | Onset of rash (post-1st dose) |
CTCAE Grade v4.03 |
Clinical description* | Treatment (Outcome) |
Histopathologic description |
Dose reduction/ interruption due to rash |
|---|---|---|---|---|---|---|---|---|---|
| 72, F | Urothelial cancer (Stage IV) | Nivolumab, 3mg/kg q2w | Benzonatate, Erythromycin, Loratadine, Penicillin, TMP/SMX | 3 weeks | 1 | Multiple (>100), slightly raised, erythematous, mildly scaly papules, scattered diffusely on both upper extremities and trunk | Triamcinolone cream 0.1%, bid (Improved) | Lichenoid dermatitis | No |
| 18, M | High-Grade astrocytoma | Nivolumab, 3mg/kg q2w | NKDA | 4 weeks | 3 | Multiple (>100), discrete, slightly raised, pink to erythematous papules (coalescing at places) on all the extremities, trunk, buttocks; cheilitis | Prednisone 20mg tid, Clobetasol spray 0.05%, bid (Improved) | Mild vacuolar interface dermatitis with superficial perivascular lymphoeosinophilic infiltrate | No |
| 73, M | NSCLC | Nivolumab, 3mg/kg q2w | NKDA | 12 weeks | 2 | Few, light pink, monomorphic macules, follicular at places; some coalescing into patches on the chest, abdomen, back and arms | Medrol Dosepak 4mg (as directed)†, Triamcinolone cream 0.1%, bid (Improved) | Perivascular lymphocytic dermatitis with a few eosinophils | Yes (Interruption) |
| 56, F | Breast cancer | Nivolumab, 3mg/kg q2w | Acetaminophen, Amoxicillin, Oxycodone | 96 weeks | 2 | Few, well-demarcated, erythematous, mildly scaly papules and plaques, scattered haphazardly on the back, buttocks, posterior aspect of left arm, and bilateral lower extremities | Medrol Dosepak 4mg, (as directed)† Triamcinolone cream 0.1%, bid (Improved) | Pityriasiform, lichenoid and spongiotic dermatitis | No |
| 85, M | SCLC (Stage IV) | Nivolumab, 3mg/kg q2w | NKDA | 12 weeks | 2 | Few, non-scaly, erythematous papules and plaques on the chest/upper abdomen and lower back | Clobetasol lotion 0.05%, bid (Improved) | Spongiotic, vesicular and superficial perivascular dermatitis with eosinophils (Fig. 4A) | Yes (Interruption upon progressing to Grade 3, months later) |
| 64, F | Melanoma, metastatic (BRAF V600E) | Nivolumab, 3mg/kg q2w | Penicillin, Statins | 8 weeks | 1 | Few, discrete, erythematous papules on the upper trunk | Triamcinolone spray, bid (Improved) | Spongiotic, lichenoid and focal intra-epidermal acantholytic dermatitis with lymphocytes and eosinophils (Fig. 4B) | No |
| 54, M | Melanoma, metastatic | Nivolumab, 3mg/kg q2w | NKDA | 36 weeks | 1 | Multiple (>100), erythematous papules with ill-defined borders (few, coalescing at places) on the upper chest and extending down to the abdomen with excoriations), and back | Clobetasol cream 0.05%, bid, Fexofenadine 180mg, od, Diphenhydramine 25mg, qHS (Improved) | Interface, perivascular and periadnexal lymphocytic dermatitis, with a few plasma cells and minor vascular damage | No |
| 70, F | MALT lymphoma | Nivolumab, 1mg/kg q2w | NKDA | 14 weeks | 1 | Few, mildly scaly, erythematous macules, papules (some mildly scaly) and a plaque on bilateral lower extremities | Fluocinonide cream 0.05%, bid (Improved) | Lichenoid interface dermatitis | No |
| 53, M | Multiple Myeloma | Nivolumab, 1mg/kg q2w | Penicillin | 5 weeks | 1 | Erythematous macules and papules on the extremities, penis with a few excoriations | Hydrocortisone cream 2.5%, Diphenhydramine 50mg, qHS (Improved) | NA | No |
| 79, M | Melanoma, metastatic | Pembrolizumab, 10mg/kg q2w | Penicillin | 6 weeks | 2 | Erythematous, discrete, eczematous patches on the abdomen, back, and bilateral lower extremities (some with impetiginized crust); yellow-crusted plaque with underlying erythema on the right shoulder (Fig. 3A) | Triamcinolone cream 0.1%, bid, Clobetasol cream 0.05%, bid, Hydroxyzine 10mg, q8h PRN (Improved) | Psoriasiform, spongiotic and superficial dermal lymphoeosinophilic dermatitis on the left back; sparse perivascular lymphocytic dermatitis on the left medial back | No |
| 80, M | Melanoma, metastatic; CLL | Pembrolizumab, 2mg/kg q3w | NKDA | 8 weeks | 3 | Oval red macules (some with eroded scale) on the chest, back, arms; erosions on the upper and lower lips (Fig. 3B) | Medrol Dosepak 4mg (as directed)†, Clobex spray 0.05%, bid, Triamcinolone cream 0.1%, bid (Improved) | Lichenoid and perivascular lymphocytic dermatitis with dysmaturation and apoptosis of keratinocytes (Fig. 4C) | Yes (Interruption) |
| 62, M | Melanoma, metastatic | Pembrolizumab, 2mg/kg q3w | Penicillin | 20 weeks | 2 | Few, pink to erythematous papules and psoriasiform plaques on the dorsal aspect of extremities; papules on the abdomen | Clobetasol foam 0.05%, bid, Diphenhydramine 25mg, qHS (Improved) | Lichenoid dermatitis | No |
| 44, F | Primary mediastinal B-cell lymphoma | Pembrolizumab, 10mg/kg q3w | Oxycodone | 12 weeks | 1 | Flat-topped, slightly scaly shiny (lichenoid) papules on the dorsal aspects of the hands, forearms, lateral neck and upper chest (sun-exposed areas) | Triamcinolone cream 0.1%, bid (Improved) | Superficial and deep perivascular, periadnexal and interface dermatitis with vasculopathic changes and keratinocyte dysmaturation (Fig. 4D) | No |
| 80, M | NSCLC, metastatic | Pembrolizumab, 2mg/kg q3w | Cephalexin | ~8.5 weeks | 2 | Multiple erythematous macules and papules on the upper extremities and trunk | Clobetasol foam/spray, bid, Hydroxyzine 25mg, bid (Lost to follow-up) | Lichenoid interface dermatitis with intraepidermal acantholysis and keratinocyte apoptosis | Unknown (Lost to follow-up) |
At the time of presentation.
Day 1: 8 mg PO before breakfast, 4 mg after lunch and after dinner, and 8 mg at bedtime; Day 2: 4 mg PO before breakfast, after lunch, and after dinner and 8 mg at bedtime; Day 3: 4 mg PO before breakfast, after lunch, after dinner, and at bedtime; Day 4: 4 mg PO before breakfast, after lunch, and at bedtime; Day 5: 4 mg PO before breakfast and at bedtime; Day 6: 4 mg PO before breakfast; May be tapered over 12 days (to decrease chance of dermatitis flare-up).
Fig 4.
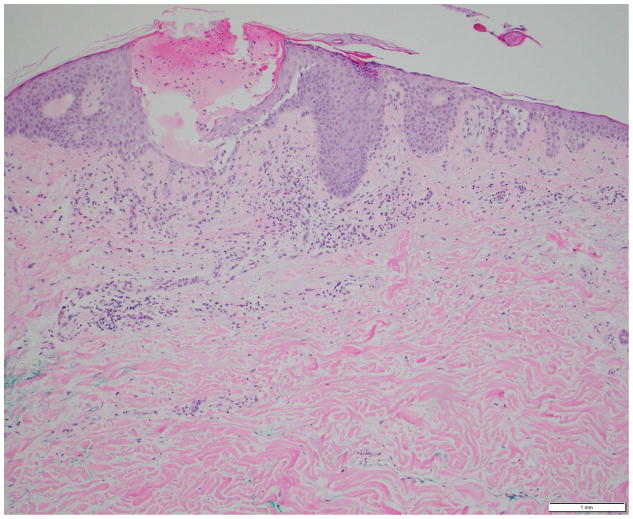
Photomicrographs showing the histopathological features of skin rash with the PD-1 inhibitors, nivolumab (Figs. 4A, 4B) and pembrolizumab (Figs. 4C, 4D): Fig 4A. Spongiotic and focally intraepidermal acantholytic dermatitis with lymphocytes and eosinophils, Fig 4B. Psoriasiform and spongiotic dermatitis with lymphocytes and eosinophils, Fig 4C. Lichenoid interface dermatitis, Fig 4D. Interface and perivascular lymphocytic dermatitis. (Refer to Table 2 for the clinical descriptions).
Fig 3.
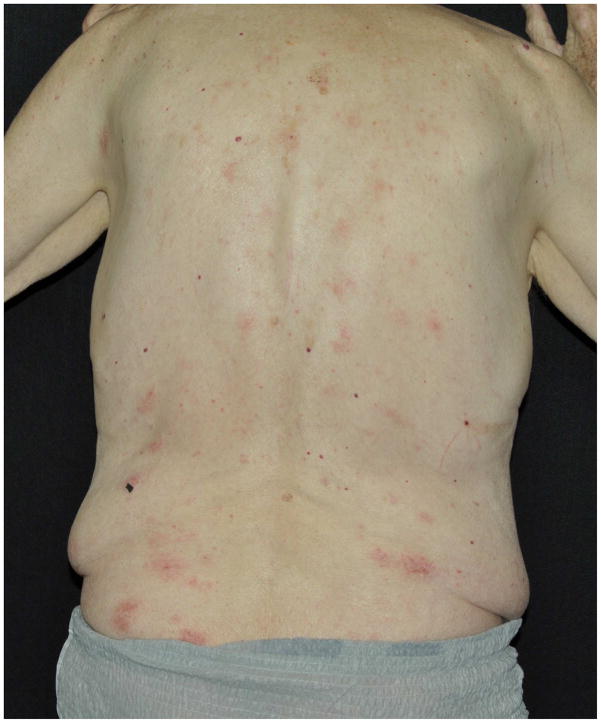
(A, B) Clinical photographs of skin rash in patients being treated with PD-1 inhibitors (graded as per the NCI-CTCAE):
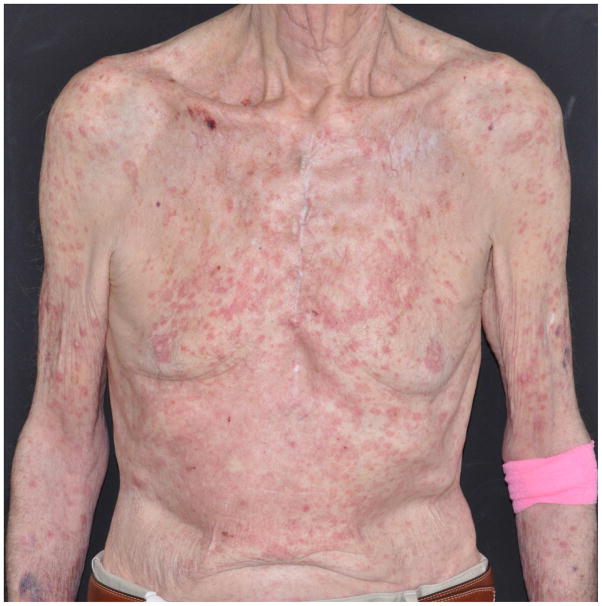
A. Grade 2 rash in a 79-year-old man, appearing after 6 weeks of therapy with pembrolizumab (10mg/kg q2wks.) for metastatic melanoma.
B. Grade 3 rash on the trunk and upper extremities of a 80-year-old man, appearing after 2 months of therapy with pembrolizumab (2mg/kg q2wks.) for stage IV melanoma.
DISCUSSION
Our study is the first to estimate the burden of dermatologic AEs with the use of PD-1 receptor inhibitors in cancer immunotherapy. The most commonly reported AEs were rash, pruritus, and vitiligo—the incidence and risk of developing all-grade and high grade events however, appears to be low with both the drugs, pembrolizumab (P) and nivolimumab (N). Pembrolizumab is a humanized mAb, which may have a slightly greater immunogenic potential than nivolumab (fully human mAb). Nevertheless, the AEs were mostly mild in severity (grades 1/2). The other AEs reported include xerosis (P, N), alopecia (P, N), stomatitis (N), urticaria (N), photosensitivity reactions (N), hyperhidrosis (N), skin exfoliation (N), and hair color changes (P), occurring at various doses.
Our findings suggest that the dermatologic AEs with these drugs appear to mirror the onset and pattern of irAEs seen with CTLA-4 blockade (ipilimumab),14,33 and occur irrespective of the underlying cancer type being treated.33 Ipilimumab acts by inhibiting an immune-checkpoint (CTLA-4) that allows tumor cells to evade immune responses, and it also has the potential to trigger autoimmune damage in previously protected normal cells. The skin is a major organ affected by this ‘enhanced’ autoimmunity.34 Given that pembrolizumab and nivolumab target another immune-checkpoint (PD-1 receptor), it could be possible that a similar mechanism may be responsible for the resulting AEs. While the underlying mechanisms are not clear, the spectrum of dermatologic AEs from both classes of immune-checkpoint inhibitors appear to be strikingly similar (rash, pruritus, vitiligo). In the studies included in our meta-analysis, a majority of patients had been treated for melanoma, but it is not clear whether this could have influenced the development and/or pattern of AEs.
As can be inferred from our case series (Table 2), the rashes generally manifest with erythematous macules, papules, and plaques, predominantly localized to the trunk and extremities, and may be associated with pruritus. Maculopapular eruptions, responsive to low-potency topical corticosteroids, were recently reported in 29% (24/83) of patients in a retrospective analysis of 2 pembrolizumab trials (in 33% after the 1st treatment cycle).35 The onset in our patients however, ranged widely (3 weeks to 2 years), which may be indicative of both an acute and delayed immunological reaction to these drugs, as seen with many other drug-induced skin eruptions. The peripheral blood eosinophil counts were normal, which is in contrast to the experience with CTLA-4 blockade (ipilimumab).34,36
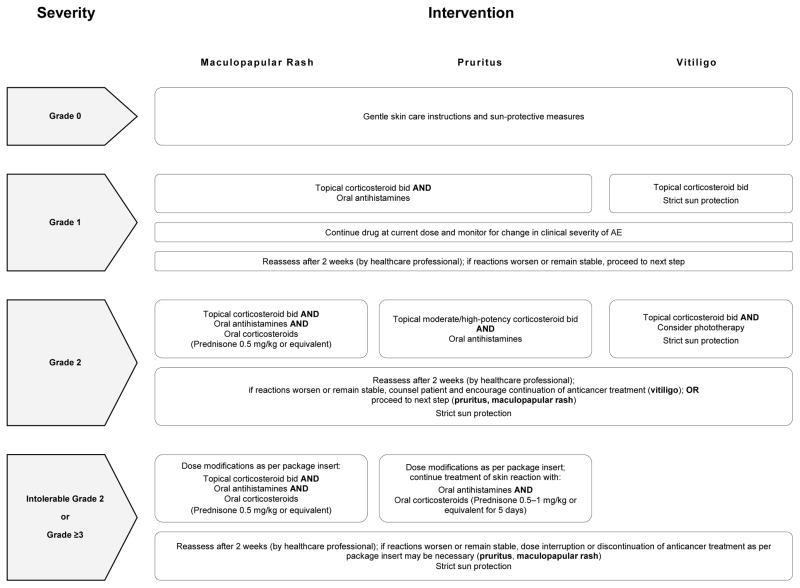
On histology, a lichenoid tissue reaction/interface dermatitis was frequently observed (Fig. 4A–D), similar to a case-series of 3 patients who developed lichenoid dermatitis during treatment with pembrolizumab.37 This reaction pattern may be due to the nonspecific activation of T-cells (as a result of PD-1 receptor blockade), which may be targeting antigen (drug)-presenting keratinocytes in susceptible individuals.38 An instance of psoriasiform eruption has also been documented.39 In our patients, mostly medium-to-high potency topical (and sometimes oral) corticosteroids sufficed for the treatment of rashes due to either drug, although this could vary depending upon the clinical severity. In this regard, we have proposed an algorithm for the management of these AEs occurring due to nonspecific immunologic enhancement (Fig. 5).
Fig 5.
Treatment algorithm for the management of anti-PD-1 inhibitor-induced dermatologic AEs.
In general, the dermatologic AEs from targeted therapies (erlotinib, sunitinib, sorafenib) have frequently been associated with efficacy, higher response rates and survival,40,41 as is the case with IL-2,42 but with ipilimumab-induced irAEs it is unclear whether they are associated with prognostically favorable outcomes.43–45 Despite the anti-PD-1 receptor inhibitors being relatively new, early studies do in fact show associations with some dermatologic AEs and positive outcomes. With pembrolizumab, patients who developed dermatologic AEs had more favorable outcomes i.e. longer progression-free intervals as compared to those who did not.35 Thus, it was suggested that the development of these AEs (especially hypopigmentation in melanoma patients) could serve as a surrogate marker for treatment response. Similarly, with nivolumab, both rash and vitiligo were associated with prolonged PFS and overall survival.46 Interestingly, a previous meta-analysis found that the development of vitiligo-like depigmentation in immunotherapy-treated advanced melanoma patients was associated with a lower risk (two-fold) of disease progression, along with a lower risk (four-fold) of death, contrary to those not developing it.47
Our study has some limitations. First, the AE reporting can vary in clinical trials,48,49 and moreover, the safety data is representative of summary results (not subject-level data). Second, the use of accurate terms for skin eruptions in oncology reports varies and/or overlaps (e.g. macular, maculopapular, rash, dermatitis, eczema), and it may be subject to investigator expertise—therefore, we only included instances of “rash” for consistency (although the excluded data was negligible). Third, clinical trials only report AEs above a certain threshold (e.g. >5% or 10%). Fourth, the relative risk calculations cannot be used to compare/contrast results between the drugs since the control arms varied across the studies. Lastly, the impact of dose modification/termination could not be ascertained. Therefore, our findings may be an under-estimation of the true incidence and severity of these AEs.
In summary, the safety profiles and clinicomorphological features of immune-checkpoint inhibitor (anti-CTLA-4, anti-PD-1)-induced AEs appear to be similar and manageable, however, direct head-to-head trials are needed for precise characterization. With newer anti-PD-1 agents in the pipeline, and the likelihood of combination regimens (with chemotherapy, radiotherapy, targeted therapy, immunotherapy) increasing, it is imperative that oncologists and dermatologists be cognizant of the emerging pattern of dermatologic AEs with these agents and their management. This is critical for optimal care, maintaining dose intensity, and satisfactory health-related quality of life in cancer patients and survivors.
HIGHLIGHTS.
This is the first meta-analysis to ascertain the incidence and risk of developing dermatologic adverse events (AEs) during treatment with the recently approved PD-1 inhibitors, pembrolizumab and nivolumab.
Skin rash, pruritus, and vitiligo are the most commonly reported AEs, although they appear to be primarily low-grade and manageable.
Among the PD-1 inhibitor-induced skin rashes, the macular-papular morphology is most frequent, often portraying a lichenoid reaction/interface dermatitis pattern on histology.
Acknowledgments
Funding Support: This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
We thank the Information Systems’ data delivery group (DataLine) at the Memorial Sloan-Kettering Cancer Center, especially Galina Yusim (Data Administrator) and Stuart Gardos (Project Manager), for their swift assistance with the complex task of medical data acquisition and electronic medical record search. We also thank Bernadette Murphy (Medical Photographer) for the clinical photographs.
ABBREVIATIONS/ACRONYMS USED
- AE
adverse event
- ASCO
American Society of Clinical Oncology
- BRAF
B-rapidly accelerated fibrosarcoma proto-oncogene serine–threonine-protein kinase
- CD
cluster of differentiation
- CI
confidence interval
- CTCAE
Common Terminology Criteria for Adverse Events
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- ESMO
European Society for Medical Oncology
- HRQoL
health-related quality of life
- mAb
monoclonal antibody
- MSKCC
Memorial Sloan Kettering Cancer Center
- NCI
National Cancer Institute
- NRCT
non-randomized controlled trial
- NSCLC
non-small-cell lung cancer
- PFS
progression-free survival
- ORR
overall response rate
- RR
relative risk
- USFDA
United States Food and Drug Administration
- WNL
within normal limits
Footnotes
IRB Status: The clinical and histopathological data was collected utilizing an IRB-approved waiver.
Submission Declaration: The contents of this manuscript have not been presented earlier, and are not under consideration for publication elsewhere.
CONFLICT OF INTEREST STATEMENT
VRB, BB, MDH, NHS, RJM, and KJB have no conflicts of interest to declare.
MAP has had a consulting or advisory role with Amgen and Bristol-Myers Squibb, and receives research support from Bristol-Myers Squibb and Novartis (Inst).
AML has a consulting or advisory role with Bristol-Myers Squibb, Janssen, Aduro, Efranat, Jounce, and Novartis, and receives research funding from Bristol-Myers Squibb (Inst), Janssen (Inst.), and Genentech (Inst).
SW has a speaking arrangement with Novartis, Bayer-Onyx, Pfizer, and Mediavation.
JDW has a consulting or advisory role with Bristol-Myers Squibb, Merck, MedImmune, Ziopharm, Polynoma, Polaris, Jounce, GlaxoSmithKline; receives honoraria from EMD Serono, Janssen Oncology, and research funding from Bristol-Myers Squibb (Inst), MedImmune (Inst), GlaxoSmithKline (Inst), Merck (Inst).
MEL has consulted for BMS and Merck, and received research support from Bristol-Myers Squibb (Inst).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 5.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11:91–9. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–8. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pembrolizumab [package insert] Merck & Co., Inc; Whitehouse Station, NJ: 2015. [on Dec 18, 2015]. accessed from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125514s005lbl.pdf. [Google Scholar]
- 11.Nivolumab [package insert] [on Dec 18, 2015];Princeton, NJ: Bristol-Myers Squibb Company. 2015 accessed from: http://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 12.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161–9. doi: 10.1016/j.jaad.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute [Internet] Cancer Therapy Evaluation Program: Protocol Development. Bethesda: The Organization; [webpage last updated 2015 Jun 8; cited 2015 Dec 18] Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 16.Borenstein M, Hedges L, Higgins J, et al. Comprehensive Meta-analysis Version 2. Biostat; Englewood NJ: 2005. [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–8. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamanishi J, Mandai M, Ikeda T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33:4015–22. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 23.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 25.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 26.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 28.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 29.Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21:4286–93. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 30.Perdon KM, Kim DW, Balmes GC, et al. A single-institution experience with the pembrolizumab (PEM) Expanded Access Program (EAP) ASCO Meeting Abstracts. 2015;33(15_suppl):e20088. [Google Scholar]
- 31.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33:1430–7. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 34.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166–72. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 35.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–12. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 37.Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18–22. doi: 10.1158/2326-6066.CIR-14-0134. [DOI] [PubMed] [Google Scholar]
- 38.Okiyama N, Fujimoto M. Clinical perspectives and murine models of lichenoid tissue reaction/interface dermatitis. J Dermatol Sci. 2015;78:167–72. doi: 10.1016/j.jdermsci.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Ohtsuka M, Miura T, Mori T, et al. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatology. 2015;151:797–9. doi: 10.1001/jamadermatol.2015.0249. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non—small-cell lung cancer. Journal of Clinical Oncology. 2004;22:3238–47. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 41.Poprach A, Pavlik T, Melichar B, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol. 2012;23:3137–43. doi: 10.1093/annonc/mds145. [DOI] [PubMed] [Google Scholar]
- 42.Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–82. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 43.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman-Keller ML, Weber JS. Nivolumab in resected and unresectable melanoma: Immune-related adverse events and association with survival outcomes. ASCO Meeting Abstracts. 2015;33(15_suppl):9028. [Google Scholar]
- 47.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–81. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 48.Pitrou I, Boutron I, Ahmad N, et al. Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med. 2009;169:1756–61. doi: 10.1001/archinternmed.2009.306. [DOI] [PubMed] [Google Scholar]
- 49.Seruga B, Sterling L, Wang L, et al. Reporting of serious adverse drug reactions of targeted anticancer agents in pivotal phase III clinical trials. J Clin Oncol. 2011;29:174–85. doi: 10.1200/JCO.2010.31.9624. [DOI] [PubMed] [Google Scholar]