Abstract
Objective
To identify cognitive phenotypes in children with new onset focal and generalized idiopathic epilepsies and determine their relationship with epilepsy syndrome, brain structure, neurodevelopmental history and family characteristics.
Methods
138 children with new onset epilepsy and 95 controls (age 8–18) underwent neuropsychological, clinical and quantitative MR evaluations. Control participants’ neuropsychological data were subjected to confirmatory factor analysis with resultant factor scores then applied to epilepsy participants and subjected to latent class analysis. Identified cognitive phenotypes were examined in relation to epilepsy syndrome, quantitative neuroimaging, familial and neurodevelopmental variables.
Results
Confirmatory factor analysis identified five cognitive factors (verbal, perceptual, speed, attention, executive) and latent class analysis identified three clusters of epilepsy participants: 1) average and comparable to controls, 2) mild impairment across multiple cognitive domains, and 3) impairment across all domains with severe attentional impairment, representing 44%, 44% and 12% of the epilepsy sample respectively. Cognitive phenotype membership was not associated with epilepsy syndrome but was associated with increasing abnormalities in brain structure, parental IQ and features of early developmental history.
Significance
Cognitive phenotypes are present in idiopathic childhood epilepsies that are unassociated with traditional epilepsy syndromes, but are associated with measures of brain structure, family history and neurodevelopmental features.
1. INTRODUCTION
Cognitive impairment is a major comorbidity of the epilepsies.1 A longstanding tradition in neuropsychological research has been to examine relationships between cognition and a range of factors that reflect core features of the epilepsies (epilepsy syndrome, EEG pathophysiology), medication treatment (type, dose, number), or clinical features that characterize epilepsy course and severity (age of onset, seizure frequency, duration of epilepsy).2,3 This work has led to a better understanding of the correlates of cognitive morbidity across the epilepsies with characterization of syndrome-specific modal cognitive profiles which inform the cognitive consequences associated with a particular epilepsy as well as the cognitive similarities and differences across discrete epilepsy syndromes (e.g., Nolan et al., 20034; Jackson et al., 20135).
Considerably less work has been devoted to identification of cognitive phenotypes that may exist within and across epilepsy syndromes. Here the issue is whether individuals with similar cognitive profiles can be identified, profiles that may range from indistinguishable from controls to focal impairment in specific cognitive domains to generally impaired cognition. This approach may not only yield a different view of the cognitive consequences of epilepsy, but may encourage the search for biomarkers and the broader meaning of identified phenotypic membership. We took this approach previously with a cohort of patients with chronic temporal lobe epilepsy (TLE) whose modal cognitive profile was characterized by impaired performance across all cognitive domains compared to controls6. However, latent class analysis deconstructed this modal profile into three subgroups including a TLE group whose neuropsychological status was comparable to controls; a primarily memory/executive function impaired group; and a globally impaired group with especially severe impairments in executive function and speed.7 These cognitive phenotypes were associated with unique quantitative MRI findings and prospective cognitive courses.7,8
In this investigation we apply a similar research philosophy and approach to children with diverse idiopathic epilepsies—many of which fall under the rubric of so-called “benign epilepsies”. The goal was to determine whether cognitive phenotypes could be identified and to ascertain their relationship to clinical epilepsy (epilepsy syndrome), neuroimaging (volumes of cortical and subcortical structures and cerebellum), family variables (parental IQ and education) and neurodevelopmental characteristics (pregnancy complications, birth weight). The hypotheses were as follows: a) discrete cognitive phenotypes exist and will range from unaffected and comparable to controls to varying degrees and types of cognitive compromise, b) phenotype membership will be independent of epilepsy syndrome, but c) will be associated with brain structure, familial and neurodevelopmental characteristics.
2. METHODS
Participants
Research participants consisted of 233 youth aged 8–18 years, including 138 with new and recent-onset epilepsy and 95 healthy first-degree cousin controls (see Table 1). Children with epilepsy were recruited from pediatric neurology clinics at three Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic) who met the following inclusion criteria: (i) diagnosis of epilepsy within the past 12 months; (ii) no other developmental disabilities (e.g. intellectual impairment, autism); (iii) no other neurological disorder, and (iv) normal clinical MRI. All children entered the study with active epilepsy diagnosed by their treating pediatric neurologists and confirmed by medical record review of the research study pediatric neurologist. We did not exclude children on the basis of psychiatric comorbidities (including ADHD) or learning disabilities. However, children with intellectual disability, autism, and/or other neurological disorders were excluded (see 9 for details). In general, we tried to stay true to the concept of “epilepsy only” as defined broadly in the literature: normal neurological exams, intelligence, and attendance at regular schools.10,11 Each child’s epilepsy syndrome (Genetic Generalized Epilepsy [GGE] or Focal Epilepsy [FE]) was defined in a research consensus meeting that included a research pediatric neurologist who reviewed all available clinical data (e.g., seizure description and phenomenology, EEG, clinical imaging, neurodevelopmental history) while blinded to all research data.
Table 1.
Study Participant Demographic and Clinical Characteristics
| Variable | Epilepsy (n=138) | Controls (n=95) |
|---|---|---|
| Age in years: M (SD) | 12.5 (3.1) | 12.5 (2.99) |
| Gender: Female: n (%) | 70 (50.7%) | 49 (51.6%) |
| Academic Grade: M (SD) | 6.5 (3.1) | 6.3 (2.8) |
| Full Scale IQ*: M (SD) | 102.8 (13.5) | 108.8 (10.9) |
| Academic Services: n (%)* | 66 (48.5%) | 17 (18.7%) |
| Epilepsy Syndrome: LRE1 / IGE2 | 69 / 69 | -- |
| Epilepsy Onset Age in Years: M (SD) | 11.7 (3.2) | -- |
| Antiepileptic Drugs: 0 / 1 / 2+ | 20 / 110 / 8 | -- |
Note.
p < 0.05
LRE: Localization-Related Epilepsy
IGE: Idiopathic Generalized Epilepsy
Ninety-five first-degree cousins were used as controls; exclusion criteria were as follows: (i) history of initial precipitating insult (e.g. simple or complex febrile seizures, cerebral infections, perinatal stroke); (ii) any seizure or seizure-like episode; (iii) diagnosed neurological disease; (iv) loss of consciousness for greater than 5 min; (v) history of a first-degree relative with epilepsy or febrile convulsions. We used cousin controls rather than siblings or other potential control groups for the following reasons: (i) first-degree cousins are more genetically distant from the participants with epilepsy and thus less pre-disposed than siblings to shared genetic factors that may contribute to anomalies in brain structure and cognition; (ii) a greater number of first-degree cousins are available than siblings in the target age range and (iii) the family link was anticipated to facilitate participant recruitment and especially retention over time (which was our intent) compared to more general control populations (e.g. unrelated school mates). The study protocol was reviewed and approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. Families and children gave written informed consent or assent, respectively, on the day of the study.
Parents participated in a structured clinical interview and completed questionnaires to provide information about gestation, delivery, neurodevelopment, and seizure history. All pertinent medical records were obtained after signed release of information was obtained from the parent. Parents were questioned through structured interview about their child’s school progress and, in particular, any specific educational services provided to address academic problems8. The parent interview was blinded to cognitive and behavioral results of the children’s’ assessments. Finally, at baseline, the participating parent (primary caregiver) of each child was administered the two-subtest form of the Wechsler Abbreviated Scale of Intelligence (WASI).12 We recognize that it would have been preferable to test both parents, but practical limitations (e.g., parent employment, staff limitations) prevented this from occurring.
Children with intellectual disability were not included in the sample. As defined by DSM-V, intellectual disability involves impairments in general mental abilities that impact adaptive functioning in three domains, or areas: a) the conceptual domain which includes skills in language, reading, writing, math, reasoning, knowledge, and memory, b) the social domain which refers to empathy, social judgment, interpersonal communication skills, the ability to make and retain friendships, and similar capacities, and c) the practical domain which centers on self-management in areas such as personal care, job responsibilities, money management, recreation, and organizing school and work tasks. Intellectual disability does not have a specific age requirement and an individual’s symptoms must begin during the developmental period and are diagnosed based on the severity of deficits in adaptive functioning. Children with specific learning disabilities were not excluded. As defined by DSM-V, learning disability is characterized by persistent difficulties in reading, writing, arithmetic, or mathematical reasoning skills during formal years of schooling with current academic skills well below the average range of scores in culturally and linguistically appropriate tests of reading, writing, or mathematics, the individual’s difficulties not better explained by developmental, neurological, sensory (vision or hearing), or motor disorders and must significantly interfere with academic achievement, occupational performance, or activities of daily living.
Participants with epilepsy and controls did not differ in age, sex, or grade level (Table 1). Compared to controls, children with epilepsy had lower though still average full-scale IQ (FSIQ) and exhibited more academic problems (e.g., need for school or parent based interventions to address academic performance problems).
Neuropsychological Assessment
All participants were administered a comprehensive test battery that included measures of intelligence, academic achievement, language, immediate and delayed verbal memory, executive function, and speeded fine motor dexterity (see Table 2, left column). Tests were selected for pertinence to the cognitive domains of interest and their applicability across the study’s age range (8–18), ensuring identical test items/task demands, thereby providing a uniform test protocol. Fifteen of the 17 measurements were age-adjusted norm-referenced scores provided by the instruments and 2 were raw scores (WAIS Digit Symbol-Coding and Grooved Pegboard-dominant hand). The 2 raw scores were regressed on age and the residuals were used in place of the raw scores. Z-scores were calculated using the healthy control mean and standard deviation for all 17 measurements.
Table 2.
Two-group Confirmatory Factor Analysis: Descriptive Statistics and Model Results
| Descriptive Statistics | Model Results3 | |||||
|---|---|---|---|---|---|---|
| Control1 | Epilepsy2 | Latent Factor | Path Coefficient | |||
| Test | Mean | Variance | Mean | Variance | ||
| WASI2 (Vocabulary)12 | 0.00 | 0.97 | −0.47 | 1.58 | Verbal | 0.77 |
| WASI (Similarities) | 0.00 | 0.97 | −0.20 | 0.83 | 0.53 | |
| Peabody Picture Vocabulary Test-III20 | 0.04 | 1.06 | −0.40 | 2.08 | 0.81 | |
| Expressive Vocabulary Test19 | −0.01 | 0.97 | −0.41 | 1.22 | 0.71 | |
| WRAT-3 (Spelling) | 0.04 | 1.08 | −0.28 | 1.12 | 0.52 | |
| WRAT-31 (Reading)17 | 0.02 | 0.99 | −0.29 | 1.41 | 0.69 | |
| WRAT-3 (Arithmetic) | 0.01 | 0.97 | −0.85 | 1.17 | 0.58 | |
| WASI (Block Design) | 0.01 | 0.98 | −0.56 | 1.34 | Perceptual | 0.64 |
| WASI (Matrix Reasoning) | 0.01 | 0.97 | −0.42 | 1.64 | 0.64 | |
| CPT-II (Omission Errors) | 0.01 | 0.98 | −0.68 | 4.97 | Attention | 0.85 |
| CPT-II (Hit Rate SE) | −0.01 | 0.98 | −0.70 | 1.20 | 0.42 | |
| CPT-II (Response Style) | −0.02 | 0.99 | −0.25 | 1.26 | 0.36 | |
| CPT-II (Reaction Time) | −0.03 | 1.05 | −0.58 | 1.24 | Speed | 0.30 |
| Grooved Pegboard | 0.01 | 0.98 | −0.84 | 2.04 | 0.79 | |
| WISC-III6 (Digit Symbol-Coding)25 | −0.01 | 0.98 | −0.53 | 0.79 | 0.48 | |
| D-KEFS Card Sorting (Confirmed Correct Sorts) | −0.03 | 1.05 | −0.79 | 1.77 | Executive | 1.02 |
| D-KEFS Card Sorting (Attempted Sorts) | −0.03 | 1.02 | −0.17 | 1.52 | 0.53 | |
Note.
Control group Z-scores were calculated using the Health Control mean and standard deviation
Epilepsy group Z-scores were calculated using the Health Control mean and standard deviation
The 2-group model has the following fit statistics:
| Fit Statistics | Overall | Control | Epilepsy |
|---|---|---|---|
| RMSEA (90% CI) | 0.12 (0.11, 0.13) | ||
| GFI | 0.78 | 0.74 | 0.80 |
| CFI | 0.74 | ||
| Chi-square (DF) | 2142.12 (272) | 666.10 (136) | 1476.01 (136) |
Neuroimaging
Images were obtained on a 1.5 T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, U.S.A.). Sequences acquired for each participant included (1) T1-weighted, three- dimensional spoiled gradient recall (SPGR) acquired with the following parameters: TE = 5 ms, TR = 24 ms, flip angle = 40 degrees, NEX = 1, slice thickness = 1.5 mm, slices = 124, plane = coronal, FOV = 200 mm, matrix = 256 × 256; (2) proton density (PD); and (3) T2-weighted images acquired with the following parameters: TE = 36 ms (for PD) or 96 ms (for T2), TR = 3,000 ms, NEX = 1, slice thickness = 3.0 mm, slices = 64, slice plane = coronal, FOV = 200 mm, matrix = 256 × 256. Images were transferred to a Mac OSX computer for processing with the FreeSurfer version 5.3 image analysis suite (http://surfer.nmr.mgh.harvard.edu). A brief technical presentation of these procedures as used in our laboratory is presented in Dabbs et al. (2012).13 Regions of interest (ROIs) included measures of global volume (total cortical matter, cortical white matter, cortical and subcortical gray matter), subcortical volumes (bilateral thalamus, caudate, putamen, and hippocampus) and cerebellar volumes.
Confirmatory Factor Analysis
The relationship of the 17 neuropsychological measurements to the five hypothesized underlying cognitive constructs (Verbal, Perceptual, Attention, Speed, Executive) was tested in the healthy control group by Confirmatory Factor Analysis (CFA). The final model was derived using data from the control participants and the same underlying structural equation model was then simultaneously fitted to both the control and epilepsy groups. In the healthy control group, the five latent factors were set to have mean 0 and variance 1; these parameters were estimated freely in the epilepsy group. This specification reflects the assumption that both groups share a common measurement model, i.e., that the path coefficients and residual variances for the manifest variables are invariant across groups. Any differences between the two groups arise due to different means, variances and correlations among the latent factors. This was implemented in SAS (version 9.4, Cary, NC) PROC CALIS with the GROUP statement for Multiple-Group Model and the FIML option to accommodate missing values. Using the 17 observed Z-scored measurements and the group-specific factor loadings, SAS PROC SCORES were used to estimate (extract) the 5 latent factor scores for each individual.
Cluster Analysis
To test the hypothesis that discrete cognitive phenotypes exist among the epilepsy participants the five extracted cognitive factor factors for each epilepsy participant were subjected to the K-means clustering method (http://web.stanford.edu/~hastie/Papers/gap.pdf) with the GAP statistic as implemented in the R “Cluster” package (https://stat.ethz.ch/R-manual/R-devel/library/cluster/html/clusGap.html)14–16. The GAP statistic reflects the goodness-of-clustering when comparing models with different numbers of clusters.
Neuroimaging Analysis
To control age and intracranial volume (ICV) effects, each brain volume measure was regressed on age and ICV in the control group to produce standardized age- and ICV-residualized volumes for each participant. The model from the controls was then used to standardize the epilepsy cases as well. Each score therefore represents age and ICV adjusted ROI volume relative to the control group. Regression-based Z-scores were then submitted to a one-way MANOVA with Group (Clusters 1–3 and controls) as the 4-level independent factor. Post-hoc tests were run for those variables with a significance value of <0.05 for the omnibus test of any differences among the four groups.
3. RESULTS
Confirmatory Factor Analysis
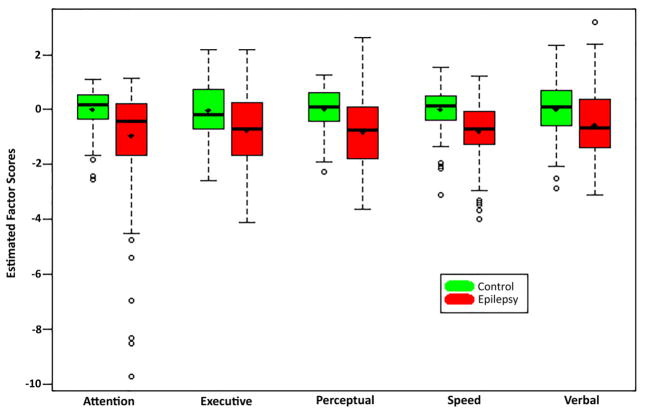
The relationships between the 17 cognitive test measures and the hypothesized underlying latent constructs (factors) were tested in the healthy control participants and a two-group structural equation model was fitted. Table 2 (columns 2–5) provide descriptive statistics for the 17 test measures for the epilepsy and control groups as well as the model results (columns 6–7) and fit statistics (bottom). Supplemental Table 1 provides the mean and covariance matrix estimation for the latent factors. Figure 1, a modal profile, shows that children with epilepsy exhibited poorer performance and had higher variability across all 5 cognitive domains.
Figure 1.
Extracted factor score distributions for Epilepsy versus Control Groups.
Notes: Control group factor scores were modeled to have mean 0 and variance 1, although realized distribution of extracted score distribution will differ somewhat from this ideal.
Cluster Analysis
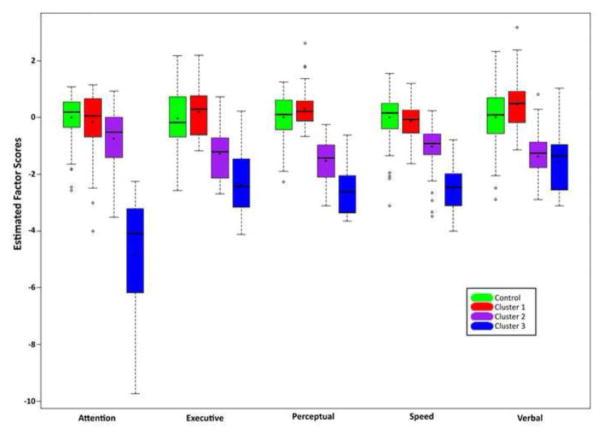
Based on the five cognitive factors, the best-fitting multi-cluster solution had 3 clusters of epilepsy participants (Figure 2). Cluster 1 participants (n=61) were the most intact across all five factors, differing from control participants only on the verbal (p = 0.003) and perceptual (p < 0.05) factor scores. Cluster 2 participants (n=61) exhibited more widespread impairment, differing from controls across all factors (p’s < 0.001), while participants in cluster 3 (n=16) had more severe deficits across all domains, with a strong attentional impairment (p’s < 0.001).
Figure 2.
Extracted factor score distributions for ContGroup and by cluster for Epilepsy Group.
Cognitive Phenotypes: Clinical, family, and developmental characteristics
Demographic and clinical characteristics of each cluster group are provided in Table 3. Across all clusters, there were no significant differences in gender, overall epilepsy syndrome (genetic generalized vs. focal epilepsy), X2 = 0.33, df=2, p=0.98, or specific syndromes (JME, absence, generalized NOS, BECTS, focal temporal or frontal, focal NOS), X2=10.8, df=6, p=.71. The various epilepsy syndromes were equally likely to be members of Clusters 1, 2, or 3. Earlier age of epilepsy onset was associated with membership in Cluster 3 (M = 10.5, SD = 3.7 years) compared to Cluster 1 (M = 12.3, SD = 3.2; p < 0.05), while Cluster 2 (M= 11.4, SD = 2.9) was between and not significantly different from the other clusters. Birth weight did not significantly differ across groups. Participants in Cluster 3 (25%) were most likely to have received developmental services before age 3 compared to participants in clusters 1 (0%) and 2 (11.5%); χ2 (2, N = 75) = 15.6 p = 0.001. Mother’s and father’s education (percent graduated from high school or received GED) were not significantly different across groups. Parent FSIQ was significantly higher in Cluster 1 (M = 115.7, SD = 11.4) than in Clusters 2 (M = 102.2, SD = 13.4) and 3 (M = 99.1, SD = 16.6); p’s <0.001.
Table 3.
Study Participant Demographic and Clinical Characteristics by Cluster Membership
| Variable | Cluster 1 (n = 61) | Cluster 2 (n=61) | Cluster 3 (n=16) |
|---|---|---|---|
| Gender: Female (n, %) | 27 (44.3%) | 35 (57.4%) | 8 (50.0%) |
| Epilepsy Syndrome: LRE1 / IGE2 | 30 / 31 | 31 / 30 | 8 / 8 |
| Epilepsy Onset Age in Years (M, SD) | 12.3 (3.2) | 11.4 (2.9) | 10.5 (3.7)** |
| Birthweight in Ounces (M, SD) | 119.1 (19.5) | 116.2 (21.0) | 117.2 (28.5) |
| Early Developmental Services3 (n, %)* | 0 (0%) | 7 (11.48%) | 4 (25.0%) |
| Parent’s Full Scale IQ | 115.7 (11.4) | 102.2 (13.4)** | 99.1 (16.6)** |
| Mother’s Education: HS/GED4 (n, %) | 59 (97%) | 58 (95%) | 14 (87%) |
| Father’s Education: HS/GED4 (n, %) | 60 (98%) | 54 (86%) | 13 (81%) |
Note.
Significant differences between all clusters, p ≤ 0.05
Significantly different from cluster 1, p ≤ 0.05
LRE: Localization-Related Epilepsy
IGE: Idiopathic Generalized Epilepsy
Developmental services received prior to the age of 3 years
General Education Diploma
Cluster membership and neuroimaging
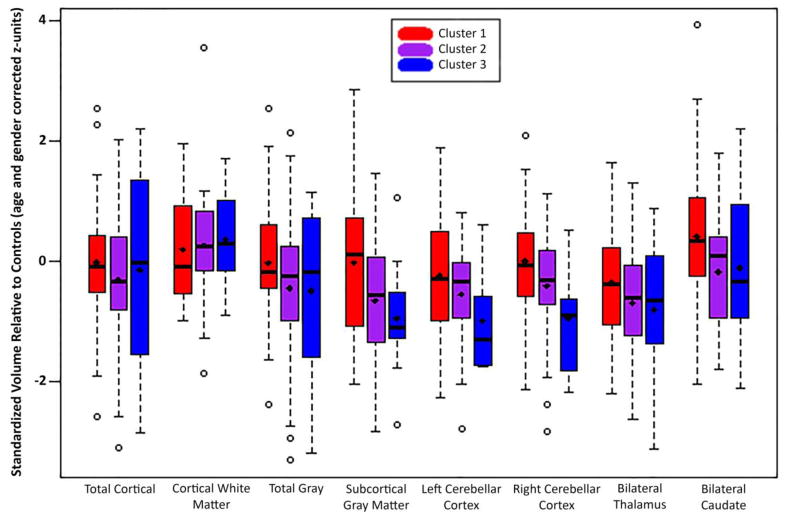
The MANOVA main effect for Group (Clusters 1–3, controls) across all brain volumes was significant (F(30, 358) = 1.60, p =0.025. Univariate tests were significant for multiple subcortical sites (left and right cerebellar cortex, bilateral thalamus and caudate) as well as for total subcortical grey volume. Post-hoc tests revealed that total subcortical grey volumes (univariate p < 0.05) for Clusters 2 and 3 were smaller than those of Controls (p < 0.05) and Cluster 1 (p < 0.05). Clusters 2 and 3 also had smaller left (univariate p < 0.05) and right (univariate p = 0.006) cerebellar cortices (p’s = 0.020, 0.005, 0.025, 0.003) and bilateral thalamus (univariate p < 0.05; post-hoc p’s = 0.003, 0.029) than Controls. Cluster 3 had smaller left and right cerebellar cortex relative to Cluster 1 (p’s = 0.028, 0.007). Finally, bilateral caudate (univariate p < 0.05) volumes were smaller in Cluster 2 relative to Controls (p < 0.05) and Cluster 1 (p < 0.05). See Figure 3 for volumetric means by cluster membership. Nonsignificant univariate p-values were returned for bilateral putamen and hippocampus, as well as for measures of cortical gray, white, and total volume.
Figure 3.
Standardized structural brain volume distributions by cluster for Epilepsy Group.
4. DISCUSSION
The three major findings that addressed our hypotheses were as follows. First, among children with new and recent onset idiopathic epilepsies, unique cognitive phenotypes were identified representing variations in the presence, type and degree of neuropsychological compromise. Second, these cognitive phenotypes, which address an important comorbidity of the childhood epilepsies, were independent of epilepsy syndrome. Third, the cognitive phenotypes were, however, associated with neurobiological measures of brain structure (quantitative volumetrics) and features of family environment and early neurodevelopment. These findings are discussed in further detail below.
Cognitive phenotypes
From a comprehensive battery of neuropsychological tests administered to healthy controls, five core dimensions were identified through confirmatory factor analysis including verbal ability, perceptual ability, executive function, cognitive/psychomotor speed, and attention. These factor scores were extrapolated to the epilepsy participants who, as a group, exhibited significantly poorer performance across all factor scores (Figure 1)—this being the unsurprising modal cognitive profile of a large group of children with epilepsy4–5. However, through latent class analysis this modal profile could be deconstructed into three cognitive phenotype groups (Figure 2). Cluster 1 (44% of the epilepsy group) was the most cognitively intact group, differing from control participants on only the verbal and perceptual factor scores and comparable to controls on the remaining factors (attention, executive, processing speed). Cluster 2 (44% of the total epilepsy group) showed mild but significant differences from the healthy controls across all factor scores assessed. Finally, Cluster 3 (12% of the total epilepsy group) was the most cognitively impaired group, differing from controls across all factor scores with especially adversely affected attention. While informative, it is clear that modal cognitive profiles do not reflect the substantial variation that exists within and across epilepsy syndromes4–8, and do not reflect the substantial proportion with essentially intact cognition.
Cognitive phenotypes and epilepsy syndromes
The current findings are also consistent with a growing literature indicating that neuropsychological status is not always closely associated with clinical epilepsy characteristics, particularly epilepsy syndrome2. Here there was no significant association between the cognitive identified cognitive phenotypes and epilepsy syndrome whether defined broadly i.e., focal versus generalized epilepsy) or defined by specific focal epilepsy syndromes (i.e., focal temporal, focal frontal, focal NOS; BECTS) or generalized epilepsies (i.e., absence, juvenile myoclonic epilepsy, generalized NOS). Indeed, there was representation of all three cognitive phenotype groups across all epilepsy syndrome groups, suggesting that identified cognitive phenotypes may not respect syndromic taxonomy and are driven by other factors2. Taxonomies of the comorbidities of the epilepsies themselves may have heuristic value in identifying groups at risk, the underlying causative factors and prospective course. That said, it is important to remember that children with epileptic encephalopathies were excluded from this sample and the lack of differences in cluster membership by epilepsy syndrome applies specifically to the idiopathic epilepsies
Cognitive phenotypes: brain, family and developmental characteristics
The identified cognitive phenotypes were associated with underlying brain structure (Figure 3). Cluster 1 deviated little from the healthy controls, consistent with their minimal deviation in cognitive performance. Cluster 3, the most cognitively impaired group, exhibited the most abnormal volumes compared to controls and Cluster 1, with smaller total subcortical gray volume and smaller left and right cerebellar cortex; and bilateral thalamus volume was also smaller relative to Cluster 1. These ROI’s showed a stepwise progression, as Cluster 2 volumes fell between Clusters 1 and 3. Bilateral caudate was smaller in Cluster 2 participants relative to those in Cluster 1 and controls. No significant volumetric differences were seen across the cognitive phenotype groups for volumes of bilateral putamen and hippocampus as well as for measures of cortical gray matter, white matter, and total brain volume.
Cluster membership was also associated with features of the home/family environment, early developmental history, and epilepsy onset age. Children in Cluster 3 had parents with lower IQ (though still average), increased history of being provided supportive services (e.g., speech, physical, or occupational therapy) before age three, and an earlier age of onset of epilepsy. Further exploration of paternal characteristics, family history of comorbidities, and other “non-epilepsy” factors may prove informative going forward. The other area for future research is to develop easily used clinical screening algorithms to identify a presenting child’s cognitive phenotype in order to efficiently facilitate appropriate early assessment and intervention. A broader characterization of additional behavioral, familial, social and other factors associated with cognitive phenotype membership will be useful in this regard as well.
In conclusion, compared to the typical modal profile of the cognitive consequences of epilepsy, discrete cognitive phenotypes can be identified which provide an alternative view of the cognitive complications of childhood epilepsy. These phenotypes help to demonstrate the variable risk of (and freedom from) comorbidities, provide a preliminary view of the wide and diverse range of associated “risk factors”, and perhaps provide an alternative conceptual approach to understanding the neurobehavioral comorbidities of epilepsy.
Supplementary Material
HIGHLIGHTS.
Cognition is variably affected in the idiopathic childhood epilepsies.
This variability was quantitated by identifying discrete cognitive phenotypes.
Identified phenotypes ranged from cognitively intact (44%) to very impaired (12%).
The cognitive phenotypes had no association with epilepsy syndrome.
They were associated with brain structure, parent IQ, and developmental history.
Acknowledgments
This study was supported by NIH 3RO1-44351. The authors have no financial disclosures to report related to this investigation. The funder had no role in study design, data collection, data analysis, manuscript preparation and/or publication decisions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine. Epilepsy across the spectrum: promoting health and understanding. Washington, DC: The National Academy Press; 2012. [Google Scholar]
- 2.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380:1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmstaedter C, Witt JA. Clinical neuropsychology in epilepsy: theoretical and practical issues. Handbook of clinical neurology. 2012;107:437–59. doi: 10.1016/B978-0-444-52898-8.00036-7. [DOI] [PubMed] [Google Scholar]
- 4.Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, Bleasel AF, Bye AM. Intelligence in childhood epilepsy syndromes. Epilepsy research. 2003;53:139–50. doi: 10.1016/s0920-1211(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DC, Dabbs K, Walker NM, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP. The neuropsychological and academic substrate of new/recent-onset epilepsies. The Journal of pediatrics. 2013;162:1047–53. e1. doi: 10.1016/j.jpeds.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–42. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 7.Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13:12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- 8.Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy & behavior: E&B. 2009;15:445–51. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–19. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 10.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A Dutch Study Group of Epilepsy in C. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–44. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 11.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. The New England journal of medicine. 1998;338:1715–22. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 13.Dabbs K, Becker T, Jones J, Rutecki P, Seidenberg M, Hermann B. Brain structure and aging in chronic temporal lobe epilepsy. Epilepsia. 2012;53:1033–43. doi: 10.1111/j.1528-1167.2012.03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broberg Per. SAGx: Statistical Analysis of the GeneChip. R package version 1.9.7. http://home.swipnet.se/pibroberg/expression_hemsida1.html.
- 15.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a dataset via the Gap statistic. Stanford University; 2000. [Google Scholar]
- 16.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J Roy Stat Soc B. 2001;63:411–23. [Google Scholar]
- 17.Wilkinson GS. Wide Range Achievement Test: Manual. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 18.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 19.Williams KT. Expressive Vocabulary Test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 20.Dunn L, Dunn L, WIlliams KT. Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 21.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 22.Cohen MJ. Children’s Memory Scale. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 23.Conners CK. The Connors’ Continuous Performance Test. Toronto, Canada: Multi-Heath Systems; 1995. [Google Scholar]
- 24.Trites RL. Neuropsychological Test Manual. Ottawa, Ontario, Canada: Royal Ottawa Hospital; 1977. [Google Scholar]
- 25.Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: The Psycholocal Corporation; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.