Abstract
The antimicrobial peptide LL-37 not only contributes to the host defence against microbial invasion but also regulates immune activity, angiogenesis and cell proliferation. Studies have shown that LL-37 participates in the development of a variety of tumours, such as lung cancer, ovarian cancer, breast cancer and melanoma. However, the role of LL-37 in the development of skin squamous cell carcinoma (SCC) is not clear. The present study used immunohistochemistry to confirm that the expression of human DNA-binding protein A (dbpA) was increased in SCC tissues. After stimulating SCC A341 cells, LL-37 was shown promote the proliferation, migration and invasion of these malignant cells. LL-37 also promoted the upregulation of dbpA mRNA and protein expression. In addition, after using small interfering RNA to silence the normal dbpA expression in these malignant cells, the proliferation and invasion of the tumor cells were significantly reduced. When the NF-κB inhibitor PDTC was used to inhibit the process of LL-37-stimulated cells, it was found that the original upregulated expression of dbpA was downregulated. Overall, the present demonstrated that by upregulating the expression of dbpA, LL-37 can promote the proliferation and invasion of tumour cells, and that this process depends on the NF-κB signalling pathway.
Keywords: LL-37, squamous cell carcinoma, Y-box, DNA-binding protein A, epidermal growth factor receptor, nuclear factor-κB
Introduction
Squamous cell carcinoma (SCC), derived from epidermal keratinocytes of the skin, is one of the most common and malignant skin cancers (1), accounting for 20% of all skin cancers. The disease usually originates from certain types of skin or precancerous lesions. Currently, there are ~1,000,000 new cases every year, and the number of mortalities from this disease is gradually increasing (2,3).
Human DNA-binding protein A (dbpA) is a multifunctional protein containing a cold shock domain, and it participates in gene transcription and translation by directly or indirectly binding the target gene sequence (4,5). DbpA is primarily expressed in the nucleus and the cytoplasm, and it acts on the transcription, shear action and translation processes of target genes (6,7). Previous studies have shown that dbpA is upregulated in numerous tumour cells, and that this upregulation is associated with the growth of the tumour cells and their resistance to chemotherapy. Therefore, dbpA is considered a cancer prognosis marker. The overexpression of dbpA in tumours usually indicates that the proliferation and invasion of tumour cells is increasing (8–10). Additionally, previous studies have found that by regulating the expression of cyclin D1 and upregulating the cell nuclear antigen, dbpA promotes tumour cell proliferation, and that the increase in dbpA is closely associated with the increase in the transcription factor, E2F transcription factor 1 (E2F1) (10,11). These results demonstrated that dbpA expression is closely associated with tumour development and progression.
The effect of human anti-microbial protein 18 (hCAP-18) on tumour progression has drawn much attention (12). LL-37 is a cationic peptide composed of 37-amino acid residues in the hCAP-18 C-terminal, and it is the only cathelin family member in the human body. LL-37 is also an important component of the innate immune system, which is mediated by neutrophils (12–14). In addition to its role against bacterial infection, LL-37 also regulates immune activity, angiogenesis and cell proliferation (13,15). Studies have shown that LL-37 is not only upregulated in a variety of solid tumours, but that it is also involved in the progression mechanisms of a variety of tumours by promoting the proliferation, migration and invasion of lung cancer, ovarian cancer, breast cancer, prostate cancer and melanoma tumour cells (16–21). Additionally, several studies have reported that these features may be associated with formyl peptide receptor-like 1 (FPRL1), epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor (14,22–24). It is unclear how LL-37 affects the proliferation and invasion of skin squamous cells. DbpA is associated with tumour proliferation and invasion. However, LL-37 stimulates dbpA expression in skin squamous cells, and its effect on tumour cells is a focus of interest.
In the present study, the effects of LL-37 on dbpA expression and the changes in the dbpA concentration during the proliferation and invasion of skin squamous cells were investigated. The results showed that LL-37 may promote the occurrence and development of skin squamous cells by upregulating dbpA expression and that this process is mediated by the nuclear factor-κB (NF-κB) signalling pathway.
Materials and methods
Tissue collection
Fresh skin SCC and adjacent normal tissues were obtained from 18 patients who underwent surgery at the Department of Dermatological Surgery, Second Affiliated Hospital of Xi'an Jiaotong University (Xi'an, Shaanxi, China). A total of 18 samples of normal tissues and 18 samples of SCC were obtained during surgery. All tissues were embedded in liquid paraffin to form tissue blocks. All skin SCC cases were clinically and pathologically verified. Standard protocols established by the Hospital's Protection of Human Subjects Committee were followed in this study. Written informed consent was obtained from all patients for publication of this study and the study was approved by the Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University.
Immunohistochemistry
The normal skin tissues, the uninvolved tissue of the SCC and the lesions of the SCC were analysed via immunohistochemistry. First, the paraffin-embedded blocks were sliced into 4-µm sections and dewaxed in xylene, followed by re-hydration in an alcohol gradient. Next, the tissues were incubated on slides with rabbit anti-human dbpA polyclonal antibody (catalog no. ab48952; Abcam, Cambridge, UK) at a 1:50 dilution. The slides were placed in a humid environment at 4°C overnight, washed twice with phosphate-buffered saline (PBS) and incubated in goat anti-rabbit antibody (Abcam) at 37°C for 1 h. The slides were then washed twice with PBS, stained with 3,3′-diaminobenzidine (Sigma-Aldrich, Munich, Germany) and observed using a microscope. Next, the slides were rinsed with PBS and re-stained with haematoxylin for ~2 min. After mounting, the results were observed using light microscopy. Normal skin tissue samples were used as the negative controls.
Cell culture
A human SCC cell line (A431) was cultured in F12 medium (Gibco, Karlsruhe, Germany) containing 10% foetal bovine serum (FBS; Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich). The cells were cultured in a 37°C environment containing 5% CO2.
Inhibiting the dbpA expression using small interfering (si)RNA
The siRNA oligonucleotide sequence was synthesized by Shanghai GenePharma (Shanghai, China) as follows: DbpA siRNA 1, 5′-GUCCUUGGCACUGUCAAAUTT-3′ (sense) and 5′-AUUUGACAGUGCCAAGGACTT-3′ (antisense); dbpA siRNA 2, 5′-GAGAGGCUGAAGAUAAAGATT-3′ (sense) and 5′-UCUUUAUCUUCAGCCUCUCTT-3′ (antisense); and dbpA siRNA 3, 5′-CUGCCAUCAAGAAGAAUAATT-3′ (sense) and 5′-UUAUUCUUCUUGAUGGCAGTT-3′ (antisense). The negative control duplexes of siRNA (siRNA-NC) were random sequences and did not target any known mammalian gene according to Genbank searches. The cells had a density of 1×105 cells/well and were seeded in 6-well plates. When the fusion reached 70–80% ~24 h later, the cells were treated with serum-free medium according to the manufacturer's instructions (Lipofectamine 2000; Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA). Next, the cells were cultured for 48 h, and the dbpA inhibition rate was analysed using western blotting.
Proliferation and invasion assay of the transfected cells
The transfected cells were seeded at a density of 3×103 cells/well in 96-well plates. After 12 h, the medium was replaced with serum-free medium and the cells were cultured for another 24 h. Subsequently, 10 µl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/ml; Sigma-Aldrich) was added to each well and incubated for an additional 4 h. The supernatant was discarded and 150 µl dimethyl sulphoxide (Sigma-Aldrich) was added. The absorbance of each well was measured at 490 nm using a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). The invasion assay was performed using Transwell chambers (Costar; Corning Inc., Corning, NY, USA) pre-coated with Matrigel (BD Biosciences, Heidelberg, Germany). The transfected cells were cultured without serum for 12 h and then resuspended in serum-free medium, with the density adjusted to 2.5×105 cells/ml. Next, 200 µl of cell suspension was added to each Transwell chamber, and 500 µl of culture medium with 10% FBS was added to the lower chamber. After culturing for 24 h, the residual cells on the surface were gently wiped away using a cotton swab. The cells that invaded the lower chamber were stained with a staining solution (0.1% crystal violet ethanol). Under a microscope (×200 magnification), three representative fields were randomly selected and the average number of invaded cells was calculated.
Cell proliferation assay
The cells were seeded at a density of 3×103 cells/well in 96-well plates. After 12 h, the medium was replaced with serum-free medium and the cells were cultured for an additional 24 h. The LL-37 (Sigma-Aldrich, Munich, Germany) was used to stimulate the cells at 10 mg/ml for 24, 48 and 72 h. Next, the aforementioned MTT assay was used to evaluate the degree of cell proliferation.
Migration and invasion assay after LL-37 stimulation
The aforementioned Transwell assays were used to test cell migration and invasion abilities after LL-37 stimulation. In the upper chamber, a specific concentration (0.05, 0.5 or 5 µg/ml) of LL-37 was added. The chambers with pre-coated Matrigel were used for invasion assays or without Matrigel for migration assays.
Total RNA extraction and quantitative polymerase chain reaction (qPCR)
TRIzol reagent (Sigma-Aldrich) was used to extract total RNA from the A431 cells after stimulation by LL-37. The total RNA (3 µg) was reverse transcribed to cDNA in a total volume of 20 µl using a reverse transcription reaction kit (Promega Corporation, Madison, WI, USA). qPCR was performed using an Mx 3000P Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific Inc.) according to the manufacturer's instructions. SYBR Premix Ex Taq II (Takara Biotechnologies Co., Ltd., Dalian, China) was used as a DNA-specific fluorescent dye. PCR was performed for 50 cycles of 95°C for 10 sec and 60°C for 30 sec. Primer sequences for the detection of mRNA expression were synthesized as follows: DbpA specific primers, 5-CTCTACAGTTTCTCCATCTCCTAC-3 (forward) and 5-TTCTCGCCACCAAAGTCCT-3 (reverse); and human β-actin primers, 5-TTCCATATCGTCCCAGTTGGT-3 (forward) and 5-CCAGGGCGTTATGGTAGGCA-3 (reverse). The dbpA transcriptional level was corrected based on the corresponding level of β-actin transcription. All the values are from the results of at least three independent experiments.
Immunofluorescence staining
Once a monolayer of cells had been placed on the climbing film, LL-37 of the appropriate concentration (0.05, 0.5 or 5 µg/ml) was added for 48 h. The cells were then fixed with 4% paraformaldehyde at room temperature for 10 min, followed by permeabilisation with 5% Triton X100 (Sigma-Aldrich) for 15 min. The cells were then blocked with 2% goat serum for 30 min. The cells were incubated with a rabbit anti-human dbpA polyclonal antibody diluted at 1:50 (Abcam) overnight at 4°C, and washed 3 times with PBS. The cells were incubated with fluorescein isothiocyanate-labelled goat anti-rabbit antibody (Abcam) at 37°C for 1 h, washed with PBS 3 times and finally stained with DAPI (Sigma, Munich, Germany) for 1 min. The cells were rinsed with PBS and the staining intensity was observed using an inverted fluorescence microscope (LSM 700; Zeiss GmbH, Jena, Germany).
Protein extraction and western blot analysis
Total protein was extracted following LL-37 stimulation. Protein isolates (~10 µg) from each sample were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% skimmed milk and a 0.1% Tween-20 phosphate-buffered solution at room temperature for 2 h, and then treated with rabbit polyclonal anti-human dbpA antibody diluted at 1:500 (Abcam) at 4°C overnight. Next, it was hybridized with secondary antibody (1:5,000, Abcam) for 1 h. The results were detected by exposing the film to enhanced chemiluminescence colour detection solutions (EMD Millipore, Billerica, MA, USA).
Analysis of the signal transduction pathways of the dbpA induction by LL-37
The SCC cells were seeded at 1×105 cells/well in 6-well plates. First, the cells were treated with the mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor, PD98059 (10 µM; Abcam), the p38/MAPK inhibitor, SB203580 (10 µM; Abcam) and the NF-κB inhibitor, PDTC (1 µM; Abcam), for 30 min. Next, LL-37 (0.5 µM) was added for 24 h. The protein was then extracted for western blot analysis.
Statistical analysis
All data are presented as the mean ± standard deviation. Pearson's χ2 test was used for the immunohistochemical analysis. Student's t-test was used for comparisons between groups and an analysis of variance was used for three or more groups. All analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, IL, USA). Results were considered statistically significant at P<0.05.
Results
DbpA is upregulated in SCC
In the normal skin tissues, the dbpA protein was strongly expressed in the granular layers and weakly expressed in the upper layers of the stratum spinosum (Fig. 1A), and the positive rate in the stratum spinosum was 20.0% (4/20). In the uninvolved epidermis of SCC, dbpA was strongly expressed in the granular layers and in the upper layers of the stratum spinosum (Fig. 1B), and the positive rate in the stratum spinosum was 27.8% (5/18). However, in SCC, dbpA was detected in significant quantities in nearly all of the tumour cells (Fig. 1C), and the positive rate was 83.3% (15/18). The dbpA expression in SCC was stronger than that in the normal skin tissues (χ2=15.5; P<0.01) or the uninvolved epidermis (χ2=10.7; P<0.01).
Figure 1.
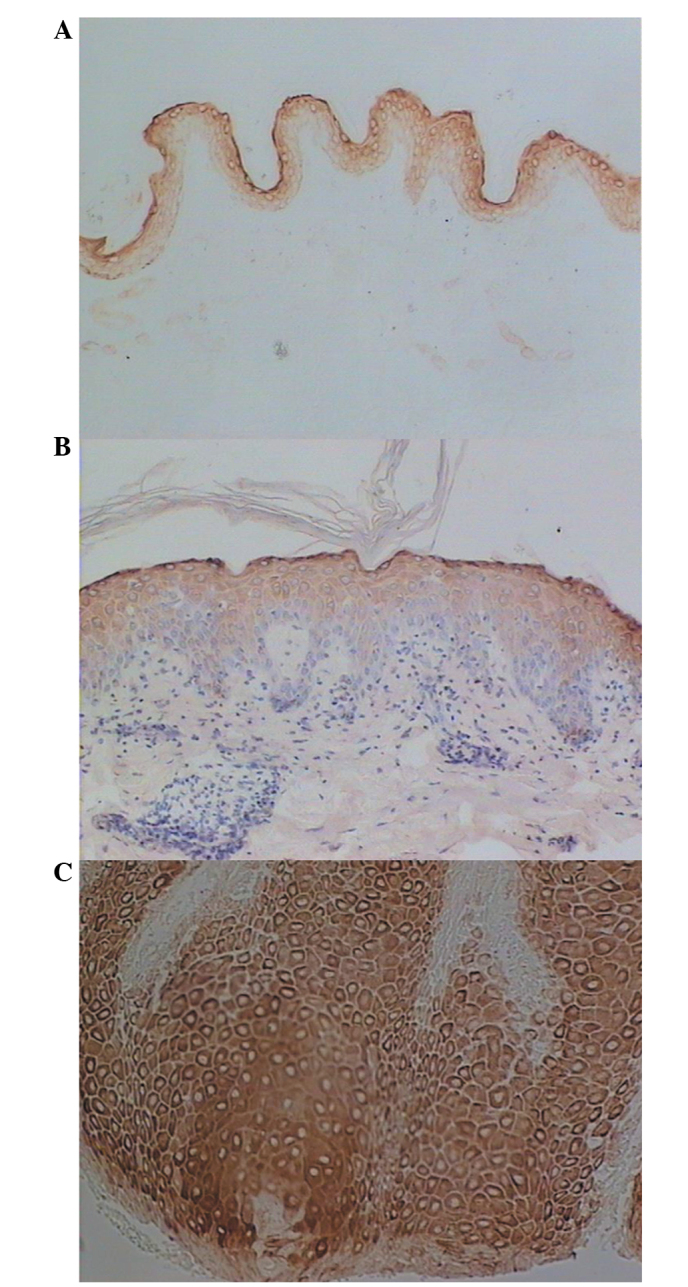
Immunohistochemical analysis of dbpA expression in (A) normal skin, (B) in tissue surrounding the SCC and (C) in SCC. In SCC, dbpA expression was stronger than in normal skin tissue. Magnification: (A) ×100, (B) ×200 and (C) ×200. SCC, squamous cell carcinoma; dbpA, DNA-binding protein A.
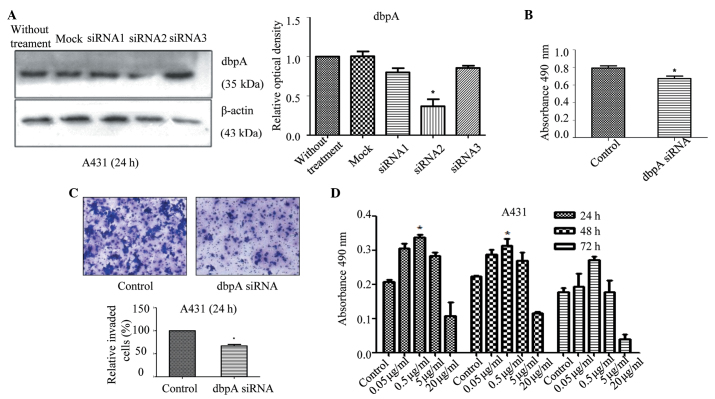
dbpA siRNA decreases the protein levels of dbpA, and reduces the proliferation and invasion of the SCC cells
After 48 h of culture, total protein was extracted from the transfected cells and analysed using western blotting. Compared with the control group, the protein expression of dbpA in the dbpA siRNA 2 group was significantly reduced (P=0.008; Fig. 2A); thus, dbpA siRNA 2 was chosen for the subsequent experiments. Compared with the control group, cell proliferation in the presence of the siRNA decreased after 24 h, indicating that the dbpA expression was correlated with the proliferation of the tumour cells (P=0.028; Fig. 2B). Transwell chambers coated with Matrigel were used to study the invasion ability of the A431 cells after inhibiting the dbpA expression. Compared with the control group, the number of invading cells that crossed the membranes diminished after 24 h, indicating that dbpA was correlated with the invasiveness of the tumour cells (P<0.034; Fig. 2C). Thus, the inhibition of dbpA may reduce the proliferation and invasiveness of A431 cells.
Figure 2.
(A) Expression of dbpA in A431 cells after treatment with dbpA siRNA. Western blot analysis of dbpA and β-actin expression in the dbpA siRNA(1–3) and control siRNA groups. (B) DbpA siRNA reduced the proliferation ability in the A431 cells. After treatment with dbpA siRNA for 24 h, an MTT assay was performed to analyse cell proliferation by reading absorbance at 490 nm in each well using an automatic microplate reader and (C) the invasion ability of the cells was also assessed (magnification, ×200). (D) The proliferation of the A431 cells was promoted by culturing with 0.05, 0.5, 5 or 20 µg/ml LL-37 for 24, 48 or 72 h. Cell proliferation levels were also analysed by MTT assay. The results from three independent experiments are shown as the mean ± standard deviation. n=5 samples in each group. *P<0.05 vs. control. siRNA, small interfering RNA; dbpA, DNA-binding protein A; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
LL-37 promotes the proliferation of SCC cells
The A431 cells were stimulated using different concentrations of LL-37 and its effect on the cell proliferation was observed at various time intervals. Compared with the control group, stimulation with LL-37 at different times and concentrations increased the proliferation of the A431 cells (Fig. 2D), and these increases were significant for 24 or 48 h of culture with 0.5 µg/ml of LL-37 (P=0.028).
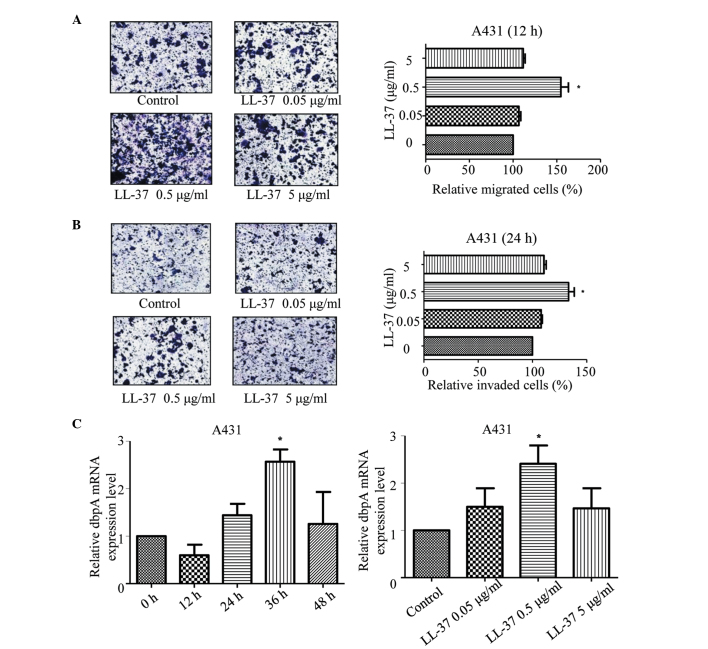
LL-37 promotes the migration and invasion of SCC cells
Compared with the control group, different concentrations of LL-37 enhanced the migration of the A431 cells after 12 h of culture (P=0.001; Fig. 3A) and the invasiveness of the A431 cells after 24 h of culture (Fig. 3B). The most effective concentration was 0.5 µg/ml (P=0.002).
Figure 3.
(A) LL-37 induces migration in A431 cells. Serum-starved A431 were treated with LL-37 at 0.05, 0.5 and 5 µg/ml of LL-37 for 12 h, and the cell migration was analysed. (B) LL-37 induces invasion of A431 cells. Serum-starved A431 cells were treated with LL-37 at 0.05, 0.5 and 5 µg/ml of LL-37 for 24 h, and the cell invasion was analysed. Magnification, ×200. The results from three random fields are shown and presented as the mean ± standard deviation. (C) LL-37 promotes the upregulation of dbpA mRNA in A431 cells. Time effect: A431 cells were stimulated with 0.5 µg/ml LL-37 for the specified durations. Dose effects: A431 cells were stimulated with the specified doses of LL-37 for 24 h. DbpA mRNA levels were determined by quantitative polymerase chain reaction. The figure shows the ratio of dbpA mRNA/β-actin mRNA. *P<0.05 vs. control, 0 h or 0 µg/ml. siRNA, small interfering RNA; dbpA, DNA-binding protein A.
LL-37 promotes the mRNA and protein expression of dbpA in SCC cells
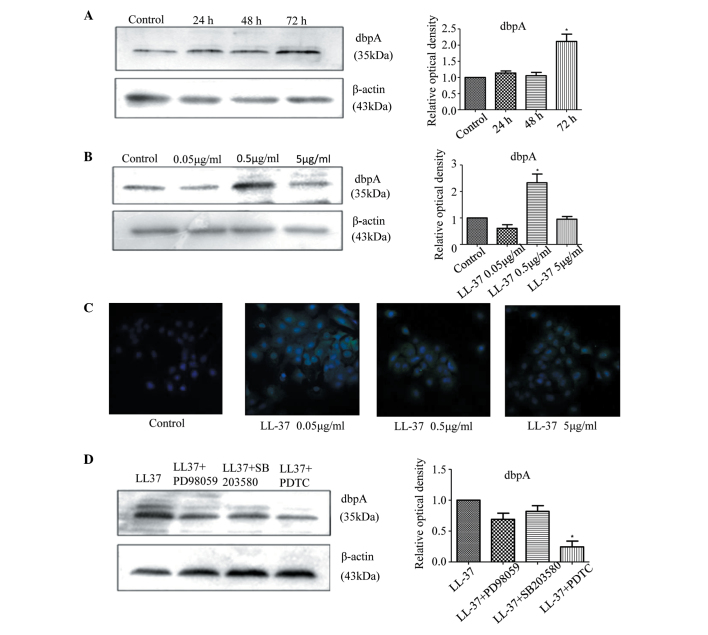
Compared with the control group, stimulation of the A431 cells with LL-37 for 36 h increased the mRNA expression of dbpA (P=0.004), with the most significant increase observed for 0.5 µg/ml LL-37 (P=0.003; Fig. 3C). Western blot showed that after stimulating the A431 cells with LL-37 for 72 h, the protein expression of dbpA increased (P=0.041; Fig. 4A) and again, the most significant increase was observed for 0.5 µg/ml LL-37 (P=0.029; Fig. 4B). Moreover, stimulating the A431 cells with various concentrations of LL-37 increased the fluorescence intensity of dbpA immunostaining, and 0.5 µg/ml was the most effective concentration (Fig. 4C). Thus, LL-37 promoted the expression of dbpA in the A431 cells.
Figure 4.
(A and B) LL-37 promotes the upregulation of dbpA protein in A431 cells. Time effect: A431 cells were stimulated with 0.5 µg/ml LL-37 for the specified durations. Dose effect: A431 cells were stimulated with the specified doses of LL-37 for 48 h. DbpA protein levels were determined by western blotting. The figure shows the ratio of dbpA protein/β-actin protein. The results from three independent experiments are shown as the mean ± standard deviation. (C) LL-37 promotes the upregulation of dbpA protein in A431 cells. The A431 cells were stimulated with the specified dose of LL-37 for 48 h, and dbpA protein expression levels were determined by immunofluorescence. The figures show dbpA protein fluorescence intensity after stimulation by different concentrations of LL-37. Magnification, ×400. (D) The NF-κB signalling pathway is involved in the upregulation of dbpA stimulated by LL-37 in A431 cells. The A431 cells were pretreated for 30 min with MAPK kinase-extracellular signal-regulated kinase inhibitor (PD98059; 10 µM), MAPK inhibitors (SB203580, 10 µM) or NF-κB inhibitor (PDTC; 1 µM), followed by treatment with 0.5 µg/ml LL-37 for 48 h. The protein levels of dbpA were determined by western blotting. The figure shows the ratio of dbpA protein/β-actin protein. The results from three independent experiments are shown as the mean ± standard deviation. *P<0.05 vs. control. MAPK, mitogen-activated protein kinase kinase; dbpA, DNA-binding protein A; NF-κB, nuclear factor-κB.
An NF-κB inhibitor inhibits dbpA expression induced by LL-37 in SCC cells
To study the signalling pathway downstream of LL-37 that induced the expression of dbpA, inhibition experiments were performed. The A431 cells were pretreated with the MEK inhibitor, PD98059, the p38/MAPK inhibitor, SB203580, and the NF-κB inhibitor, PDTC, to inhibit the effect of LL-37 on the induction of dbpA expression. PDTC significantly inhibited the LL-37-induced expression of dbpA in the A431 cells (P=0.011; Fig. 4D), indicating that the increased dbpA expression that was stimulated by LL-37 may occur via an NF-κB signalling pathway.
Discussion
The present results indicated that dbpA was not expressed in the basal layer in normal skin tissue, but its expression increased in SCC as it was expressed in almost all of the tumour cells. When stimulated by LL-37, the protein level of dbpA increased in the cutaneous SCC cells in a time- and concentration-dependent manner. The inhibition of the NF-κB signalling pathway led to a lower amount of LL-37-induced dbpA protein expression in the A431 cells. This indicated that LL-37 could regulate the expression of dbpA in the A431 cells and that this process may occur via the activation of the NF-κB signalling pathway.
LL-37 is a member of the antibacterial peptide family and is correlated with the proliferation of epidermal cells (13,14). Previous studies have shown that LL-37 can promote malignant tumours, including lung cancer, ovarian cancer, melanoma, prostate cancer and oral SCC, and this process is primarily associated with the upregulation of EGFR and the receptor tyrosine kinase ErbB2 (25). Through the induction of the membrane-associated protein kinase, EGFRs cleave the matrix metalloproteinase (MMP), and LL-37 activates EGFR. This process may be dependent on the G protein-coupled receptors (GPCRs) present in different cell types (14,22–24). In lung SCC, LL-37 stimulates the proliferation and invasion of tumour cells, accompanied by its mitogenic effect of EGFR phosphorylation and the subsequent activation of the Ras/MAPK cascade. EGFR signalling in lung cancer cells plays a direct role in proliferation, anti-apoptosis, angiogenesis generation, invasion and metastasis (14,21). The majority of the EGFR ligands, such as transforming growth factor and heparin-binding epidermal growth factor, are expressed as soluble transmembrane precursors that are released after cleavage by a protease. These precursors can diffuse freely and bind and activate EGFR. Thus, the oncogenic effect of LL-37 in certain tissues occurs via the activation of the EGFR-mediated transcriptional mechanism (23,26,27). In breast cancer, LL-37 promotes tumour progression via the ErbB-mediated pathway, upregulating the expression of ErbB2 or EGFR to enhance ErbB signalling, thereby promoting growth and metastasis (19). Additionally, formyl peptide receptor 2 (FPR2) may be involved in this process. LL-37 stimulates the activation of MAPK and Janus kinase/signal transducer and activator of transcription and undergoes a biochemical cascade with transcription factor signalling, thereby leading to significant activation of several transcription factors. This process may be dependent on FPR2 or may be independent of it (22,24,28). In ovarian cancer, the fact that LL-37 may stimulate cell proliferation has been considered not dependent of the GPCR. However, LL-37 enhances the invasiveness of ovarian cancer cells via the upregulation of tissue remodelling enzymes, such as MMP-2, and this enhancement is GPCR-mediated. FPR2 in ovarian cancer cells can increase the expression of MMP-2, thus inhibiting or blocking the invasive ability of the receptors of LL-37 and thereby promoting the invasiveness of tumour cells (20,23,26,27). Previous studies have shown that the Y box binding protein (Y-BOX) family member of dbpA could regulate the proliferation of epithelial cells and is abnormally expressed in liver cancer, stomach cancer and other tumours (29–32). In transgenic mice, the increase in FPR2 can promote the expression of dbpA mRNA (11,33). Studies have also suggested that proteins of the Y-BOX family regulate the expression of EGFR and ErbB2. By binding to the EGFR receptor, Y-BOX proteins regulate the transcription of these receptors by binding to the enhancer region of the EGFR gene and to the promoter region of the ErbB2 (HER-2/neu) gene. EGFR and ErbB2 are considered to be associated with the proliferation and invasion of epidermal tumours to increase the degree of malignancy (6,8). A previous study showed that the upregulation of dbpA during cell proliferation is due to the upregulation of E2F1 activity. The E2F1 activity in cell proliferation and apoptosis is extremely important. Therefore, dbpA is a downstream target of E2F1 that promotes cell proliferation and transformation (10,34).
The results of the present study showed that the expression of dbpA is increased in SCC. siRNA was used to inhibit the expression of dbpA in A431 cells, and in vitro MTT and Transwell invasion assays confirmed that the reduced expression of dbpA inhibits the proliferation and invasion of A431 cells. The A431 cells were stimulated with LL-37, and it was found that dbpA mRNA expression increased as a function of time and concentration. Immunofluorescence and western-blot analyses were used to detect the dbpA cell protein expression changes in the A431 cells and dbpA protein expression was also found to increase as a function of time and concentration. These results suggested that LL-37 can upregulate dbpA expression in A431 cells. A previous study (35) reported that LL-37 increases the degree of malignancy of tumour cells, and that this is associated with the Ras/MAPK signalling cascade and the NF-κB pathway. Therefore, inhibitors of the extracellular signal-regulated kinase, MAPK and NF-κB signalling pathways were tested and it was found that the increase in dbpA protein expression that was induced by LL-37 could be blocked by the NF-κB inhibitor. This result indicated that LL-37 upregulates dbpA expression via the NF-κB signalling pathway. Through the induction of EGFR to cleave MMP-2, LL-37 activates EGFR and ErbB2, accompanied by the phosphorylation of EGFR and the activation of the downstream Ras/MAPK cascade. This enhances the ErbB signal and promotes the expression of MMP-2 via FPR2. Thus, EGFR, ErbB2 and FPR2 are involved in the regulation of dbpA expression, and the overexpression of these factors may increase the proliferation and invasion of tumour cells, indicating that that LL-37 promotes the proliferation and invasion of A431 cells by upregulating dbpA expression. NF-κB is a transcription factor that is known to regulate the expression of multiple genes and is involved in a wide range of cellular responses. When found in tumour cells, LL-37 can increase the levels of NF-κB p65, which can regulate the expression of genes, such cyclin D1, to promote cell growth. The activation of NF-κB has a significant role in promoting metastasis, and inhibiting NF-κB can prevent the apoptotic process in tumour cells (36). The present study showed that the inhibition of the NF-κB signalling pathway suppressed the upregulation of dbpA that was induced by the LL-37 in A431 cells, indicating that this process is associated with the NF-κB signalling pathway.
In conclusion, the present study confirms that the expression of dbpA is increased in SCC, and that it can be a marker for the degree of malignancy. The antimicrobial peptide LL-37 upregulates dbpA expression and promotes proliferation and invasion in A431 cells. This process may be regulated by the activation of the NF-κB signalling pathway. This study introduces a novel perspective on the association between LL-37 and dbpA in SCC, and provides a possible strategy for clinical drug development.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos. 81071299, 81371732 and 81573055) and was partially supported by the Fundamental Research Funds for the Central Universities and for Changjiang Scholars and Innovative Research Team in University (grant no. PCSIRT:1171).
References
- 1.Knackstedt TJ, Brennick JB, Perry AE, Li Z, Quatrano NA, Samie FH. Frequency of squamous cell carcinoma (SCC) invasion in transected SCC in situ referred for Mohs surgery: The Dartmouth-Hitchcock experience. Int J Dermatol. 2015;54:830–833. doi: 10.1111/ijd.12867. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 3.Sapijaszko M, Zloty D, Bourcier M, Poulin Y, Janiszewski P, Ashkenas J. Canadian Non-melanoma Skin Cancer Guidelines Committee: Non-melanoma skin cancer in Canada chapter 5: Management of squamous cell carcinoma. J Cutan Med Surg. 2015;19:249–259. doi: 10.1177/1203475415582318. [DOI] [PubMed] [Google Scholar]
- 4.Sakura H, Maekawa T, Imamoto F, Yasuda K, Ishii S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 1988;73:499–507. doi: 10.1016/0378-1119(88)90514-8. [DOI] [PubMed] [Google Scholar]
- 5.Kudo S, Mattei MG, Fukuda M. Characterization of the gene for dbpA, a family member of the nucleic-acid-binding proteins containing a cold-shock domain. Eur J Biochem. 1995;231:72–82. doi: 10.1111/j.1432-1033.1995.tb20672.x. [DOI] [PubMed] [Google Scholar]
- 6.Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolffe AP. Structural and functional properties of the evolutionarily ancient Y-box family of nucleic acid binding proteins. Bioessays. 1994;16:245–251. doi: 10.1002/bies.950160407. [DOI] [PubMed] [Google Scholar]
- 8.Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- 9.Uramoto H, Izumi H, Ise T, Tada M, Uchiumi T, Kuwano M, Yasumoto K, Funa K, Kohno K. p73 Interacts with c-Myc to regulate Y-box-binding protein-1 expression. J Biol Chem. 2002;277:31694–31702. doi: 10.1074/jbc.M200266200. [DOI] [PubMed] [Google Scholar]
- 10.Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–2398. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobita H, Kajino K, Inami K, Kano S, Yasen M, Imamura O, Kinoshita Y, Hino O. Gene expression profile of DNA binding protein A transgenic mice. Int J Oncol. 2006;29:673–679. [PubMed] [Google Scholar]
- 12.Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Bucki R, Leszczynska K, Namiot A, Sokolowski W. Cathelicidin LL-37: A multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz) 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 14.Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D, Wu K, Yu J, Sung JJ, Cho CH. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer. 2010;127:1741–1747. doi: 10.1002/ijc.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s) Cancer Res. 2008;68:6482–6485. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensel JA, Chanda D, Kumar S, Sawant A, Grizzle WE, Siegal GP, Ponnazhagan S. LL-37 as a therapeutic target for late stage prostate cancer. Prostate. 2011;71:659–670. doi: 10.1002/pros.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill K, Mohanti BK, Singh AK, Mishra B, Dey S. The over expression of cathelicidin peptide LL37 in head and neck squamous cell carcinoma: The peptide marker for the prognosis of cancer. Cancer Biomark. 2011;10:125–134. doi: 10.3233/CBM-2012-0238. 2012. [DOI] [PubMed] [Google Scholar]
- 18.Kim JE, Kim HJ, Choi JM, Lee KH, Kim TY, Cho BK, Jung JY, Chung KY, Cho D, Park HJ. The antimicrobial peptide human cationic antimicrobial protein-18/cathelicidin LL-37 as a putative growth factor for malignant melanoma. Br J Dermatol. 2010;163:959–967. doi: 10.1111/j.1365-2133.2010.09957.x. [DOI] [PubMed] [Google Scholar]
- 19.Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, Tham E, Sørensen OE, Weber G, Ståhle M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–719. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 20.Coffelt SB, Waterman RS, Florez L, Höner zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES, Morris CA, Scandurro AB. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 21.von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, Wiewrodt R, Biesterfeld S, Aigner A, Czubayko F, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Mol Cancer Res. 2009;7:907–915. doi: 10.1158/1541-7786.MCR-08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K, Danka ES, Henkle SL, Scandurro AB. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girnita A, Zheng H, Grönberg A, Girnita L, Ståhle M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene. 2012;31:352–365. doi: 10.1038/onc.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Choi KY, Napper S, Mookherjee N. Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation. Immunology. 2014;143:68–80. doi: 10.1111/imm.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang CM, Monie A, Wu A, Mao CP, Hung CF. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther. 2009;20:303–313. doi: 10.1089/hum.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS One. 2013;8:e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kittaka M, Shiba H, Kajiya M, Ouhara K, Takeda K, Kanbara K, Fujita T, Kawaguchi H, Komatsuzawa H, Kurihara H. Antimicrobial peptide LL37 promotes vascular endothelial growth factor-A expression in human periodontal ligament cells. J Periodontal Res. 2013;48:228–234. doi: 10.1111/j.1600-0765.2012.01524.x. [DOI] [PubMed] [Google Scholar]
- 29.Yasen M, Kajino K, Kano S, Tobita H, Yamamoto J, Uchiumi T, Kon S, Maeda M, Obulhasim G, Arii S, Hino O. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res. 2005;11:7354–7361. doi: 10.1158/1078-0432.CCR-05-1027. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LL, He DL, Li X, Li L, Zhu GD, Zhang D, Wang XY. Overexpression of coxsackie and adenovirus receptor inhibit growth of human bladder cancer cell in vitro and in vivo. Acta Pharmacol Sin. 2007;28:895–900. doi: 10.1111/j.1745-7254.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 31.Guay D, Garand C, Reddy S, Schmutte C, Lebel M. The human endonuclease III enzyme is a relevant target to potentiate cisplatin cytotoxicity in Y-box-binding protein-1 overexpressing tumor cells. Cancer Sci. 2008;99:762–769. doi: 10.1111/j.1349-7006.2008.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GR, Zheng Y, Che XM, Wang XY, Zhao JH, Wu KJ, Zeng J, Pan CE, He DL. Upregulation of human DNA binding protein A (dbpA) in gastric cancer cells. Acta Pharmacol Sin. 2009;30:1436–1442. doi: 10.1038/aps.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koike K, Uchiumi T, Ohga T, Toh S, Wada M, Kohno K, Kuwano M. Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett. 1997;417:390–394. doi: 10.1016/S0014-5793(97)01296-9. [DOI] [PubMed] [Google Scholar]
- 34.Arakawa Y, Kajino K, Kano S, Tobita H, Hayashi J, Yasen M, Moriyama M, Arakawa Y, Hino O. Transcription of dbpA, a Y box binding protein, is positively regulated by E2F1: Implications in hepatocarcinogenesis. Biochem Biophys Res Commun. 2004;322:297–302. doi: 10.1016/j.bbrc.2004.04.208. [DOI] [PubMed] [Google Scholar]
- 35.Bandurska K, Berdowska A, Barczyńska-Felusiak R, Krupa P. Unique features of human cathelicidin LL-37. Biofactors. 2015;41:289–300. doi: 10.1002/biof.1225. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Du L. PERK pathway is involved in oxygen-glucose-serum deprivation-induced NF-κB activation via ROS generation in spinal cord astrocytes. Biochem Biophys Res Commun. 2015;467:197–203. doi: 10.1016/j.bbrc.2015.10.007. [DOI] [PubMed] [Google Scholar]