Abstract
The association between dementia and the risk of death after ischemic stroke was investigated. Neurological, neuropsychological and functional assessments were evaluated in 619 patients with acute ischemic stroke. Dementia was diagnosed at admission and at three months after stroke onset. The patients were scheduled for a two-year follow-up after the index stroke. The Kaplan-Meier survival and Cox proportional hazards regression analyses were used to estimate the cumulative proportion of survival, and the association between dementia and risk of death after stroke. In total, 146 patients (23.6%) were diagnosed with dementia after stroke. The cumulative proportion of surviving cases was 49.3% in patients with dementia after a median follow-up of 21.2±5.6 months, and 92.5% in patients without dementia. Multivariate analysis revealed that dementia (HR, 7.21; 95% CI, 3.85–13.49) was associated with death, independent of age, atrial fibrillation, previous stroke and NIH stroke scale. In conclusion, the mortality rate is increased in stroke patients with dementia. Dementia is an important risk factor for death after stroke, independent of age, atrial fibrillation, previous stroke, and the severity of the stroke.
Keywords: stroke, mortality, dementia
Introduction
Epidemiological studies have consistently demonstrated that there is a high incidence rate of stroke and mortality (11,663/100,000 per year) in China (1). Poor outcomes after stroke have been reported in numerous countries worldwide (2–5). The risk factors of death after stroke include age, stroke severity, history, previous stroke and atrial fibrillation. Previous studies have focused on the association between death after stroke and the decline of cognitive function or dementia caused by stroke (6–8). The results suggested that dementia is a potentially important factor influencing survival after stroke (7,9–12).
With a population of 1.3 billion in China, the prevalence of patients with ischemic stroke is extremely high (1). Owing to racial differences and economic lifestyle, the mortality rate and its risk factors in Chinese patients after ischemic stroke may differ from that in western countries (13). At present, few studies have focused on the relationship between dementia and survival after stroke in China.
In the present study, we studied a large cohort of patients in Chongqing, China with acute ischemic stroke, to investigate the association of mortality with dementia post-stroke.
Subjects and methods
Subjects
From January 2005 to July 2008, patients with ischemic stroke who were admitted to Daping Hospital (Chongqing, China) were registered. Eligibility requirements included: i) acute onset of ischemic stroke within 48 h; and ii) aged ≥55 years. The diagnosis of ischemic stroke was confirmed when there were focal signs of cerebral dysfunction of acute onset lasting for >24 h, brain CT scan or MRI.
In total, 727 patients were admitted during the study period, and 95 declined to participate. Thus, 619 patients were enrolled into the present study.
Clinical assessment
During admission, the patients underwent structured demographic data, medical history, neurological and head imaging examinations. The data collected were: age, gender, educational level (<6 or ≥6 years), cigarette smoking (current, past and non-smoking) and alcohol intake (daily, weekly, monthly and non-alcohol intake), heart diseases (myocardial infarction, atrial fibrillation, and heart failure, diagnosed and treated previously or during admission), hypertension (previously diagnosed and treated or systolic pressure >160 mmHg and/or diastolic pressure >90 mmHg persistently observed during admission after the acute phase), diabetes mellitus (previously diagnosed and treated or fasting glucose >7 mmol/l in two blood samples after the acute phase), stroke severity (NIH stroke scale), neurological sign, and stroke features (location, type of lesion, and stroke mechanism).
Neuropsychological evaluation
During admission, the dementia before index stroke was assessed by inquiring close relatives of the patients using a Chinese version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), previously validated in the Chinese population (14). Functional status prior to stroke was assessed with the activity of daily living (ADL) (15).
At three months after the index stroke, the subjects were administered with a battery of neuropsychological tests developed for use in epidemiologic studies of dementia (16,17). This neuropsychological battery of tests included: the Chinese Mini-Mental State Examination (CMMS) (18), ADL (19,20), instrumental ADL (15), Pfeiffer's Outpatient Disability Questionnaire (POD) (21), the Fuld Object-Memory Evaluation (FOM) (22), rapid verbal retrieval (RVR) (23), revised Wechsler Adult Intelligence Scale (DS and BD subtests) (24), and the Hamilton Rating Scale for Depression. The normative data for these tests were previously established in a control group of healthy elderly volunteers living in the same urban area and with the same age, gender distribution, and cultural background (16). The score ≤1 SD of normative value was judged as unnormative.
The diagnosis of dementia was performed by a group of senior neurologists and psychiatrists based on criteria modified from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (25).
Statistical analyses
Participants who were lost or declined for follow-up were considered as censored data. As the onset time of dementia was not exactly observed, we defined the time to an event as the time of a diagnosis of dementia.
The univariate analyses were taken to compare the data of the death and survival groups, including demographic data, smoking, drinking, comorbidities, stroke severity and characteristics. Categorical variables were compared using the Pearson Chi-square test, and quantitative variables were compared using an independent samples t-test or the Mann-Whitney U test in appreciation.
The cumulative proportion of patients surviving in the groups with and without dementia was determined using the Kaplan-Meier survival analyses (26). In the multivariate analysis, we studied the association between dementia and risk of death using the Cox proportional hazards regression analysis with a backward procedure and with p>0.05 as the criterion for exclusion to estimate hazard ratios (HRs), with 95% confidence intervals (CIs). These analyses were performed using SPSS for Windows, version 10.0 (SPSS Inc., Chicago, IL, USA).
Results
Follow-up, prevalence and incidence of dementia and mortality
Of the 619 patients, 39 (6.3%) were diagnosed with dementia during admission and 107 (17.3%) three months after index stroke. Thus, 146 (23.6%) patients were diagnosed with dementia.
After a median follow-up of 10.3±5.7 months, 112 patients (16.5%) succumbed during the study. A total of 29 patients (4.7%) were lost during follow-up. The median follow-up time was 9.1±5.3 months and four of 29 patients were diagnosed as having dementia.
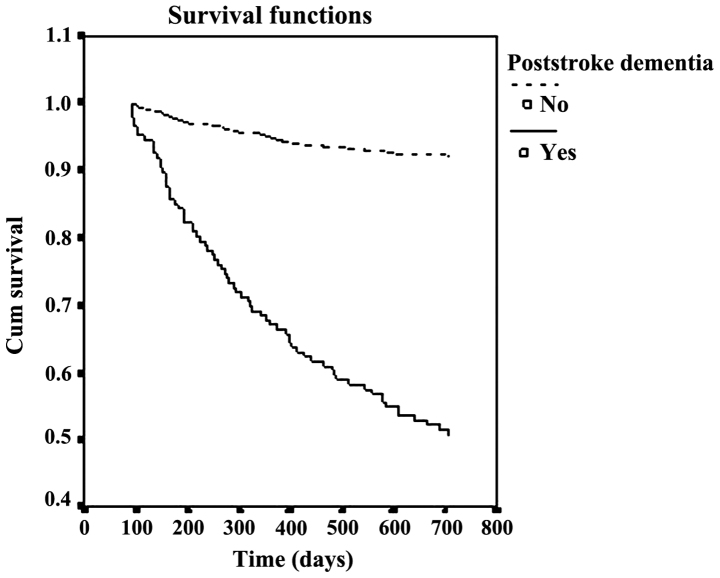
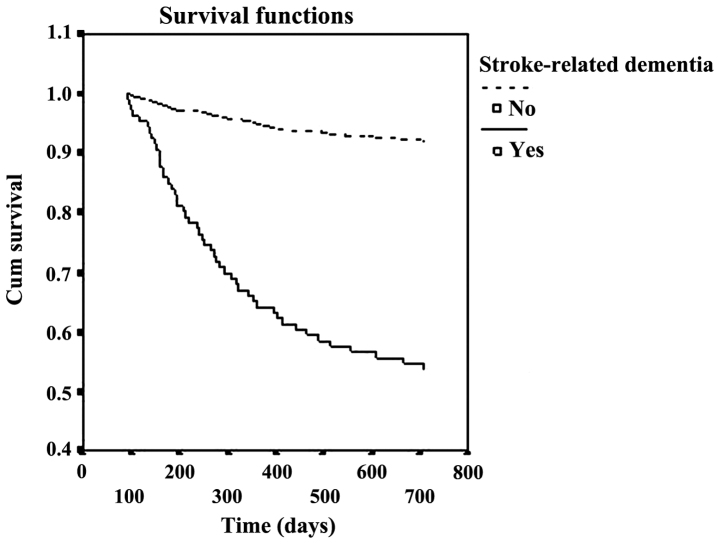
After a median follow-up of 21.2±5.6 months, the cumulative proportion of case survival was 49.3 and 92.5% in patients with and without dementia, respectively. The survival curves were significantly different (p<0.001) between the two groups (Figs. 1 and 2).
Figure 1.
Relationship between probability of survival and time.
Figure 2.
Relationship between probability of survival and time.
Demographic characteristics, smoking, drinking, comorbidities, dementia and stroke features of patients according to death
Table I indicates the demographic characteristics, smoking and drinking status, and comorbidities of deceased and surviving patients. Deceased patients were older (74.6±11.2 vs. 65.2±9.3, p<0.001), less frequent with diabetes mellitus [odds ratio (OR), 0.93; 95% CI, 0.63–1.40], and more frequent in female (OR, 1.04; 95% CI, 0.72–1.48), hypertension (OR, 1.13; 95% CI, 0.75–1.70), myocardial infarction (OR, 1.26; 95% CI, 0.67–2.39), heart failure (OR, 1.22; 95% CI, 0.61–2.43), atrial fibrillation (OR, 2.24; 95% CI, 1.41–3.53), prior stroke (OR, 2.28, 95% CI, 1.40–3.70), current smoking (OR, 1.16; 95% CI, 0.76–1.79), daily alcohol intake (OR, 1.36; 95% CI, 0.83–2.21), and dementia (OR, 12.01; 95% CI, 7.35–19.64). Only differences in age (p<0.001), atrial fibrillation (p<0.001), prior stroke (p=0.001) and dementia (p<0.001) reached statistical significance.
Table I.
Demographic characteristics, smoking, drinking and comorbidities according to death after stroke.
| Variable | Death (n=112) | Survival (n=507) | P-value or OR (95% CI)a |
|---|---|---|---|
| Age (years), mean ± SD | 74.6±11.2 | 65.2±9.3 | p<0.001 |
| Lower education (≤6 y), n (%) | 38 (33.9) | 187 (36.9) | 0.881 (0.543–1.429) |
| Female, n (%) | 57 (50.9) | 249 (49.1) | 1.04 (0.72–1.48) |
| Hypertension, n (%) | 63 (56.3) | 271 (53.5) | 1.13 (0.75–1.70) |
| Diabetes mellitus, n (%) | 34 (30.3) | 158 (31.1) | 0.93 (0.63–1.40) |
| Myocardial infarction | 12 (10.7) | 43 (8.5) | 1.26 (0.67–2.39) |
| Heart failure, n (%) | 15 (13.4) | 59 (11.6) | 1.22 (0.61–2.43) |
| Atrial fibrillation, n (%) | 39 (34.8) | 97 (19.1) | 2.24 (1.41–3.53) |
| Prior stroke, n (%) | 28 (25.0) | 65 (12.8) | 2.28 (1.40–3.70) |
| Current smoking, n (%) | 47 (41.9) | 192 (37.1) | 1.16 (0.76–1.79) |
| Daily alcohol intake, n (%) | 39 (34.8) | 149 (28.8) | 1.36 (0.83–2.21) |
| Post-stroke dementia, n (%) | 74 (66.1) | 72 (14.2) | 12.01 (7.35–19.64) |
OR was adjusted for age, educational level and gender. OR, odds ratio; CI, confidence interval; SD, standard deviation.
Table II shows the stroke features according to death. Stroke features associated with death included multiple stroke lesions (OR, 2.15; 95% CI, 1.49–3.11), and embolism (OR, 4.21; 95% CI, 2.36–7.53), and NIH stroke scale score (p<0.001).
Table II.
Stroke features according to death after stroke.
| Variable | Death) (n=112 | Survival (n=507) | P-value or OR (95% CI) |
|---|---|---|---|
| Location, n (%) | |||
| Left hemisphere | 47 (41.9) | 206 (40.1) | 1.48 (0.95–2.28)a |
| Right hemisphere | 49 (43.8) | 145 (28.6) | |
| Vertebrobasilar | 16 (14.3) | 156 (30.8) | |
| Type of lesion, n (%) | 2.15 (1.49–3.11)b | ||
| Single | 66 (58.9) | 386 (76.9) | |
| Multiple | 46 (41.1) | 116 (23.1) | |
| Stroke mechanism, n (%) | 4.21 (2.36–7.53)c | ||
| Thrombotic | 86 (76.8) | 461 (90.9) | |
| Embolic | 23 (20.5) | 30 (5.9) | |
| Others | 3 (2.7) | 16 (3.2) | |
| NIH stroke scale (SD) | 9.34 (6.7) | 6.81 (4.7) | p<0.001 |
OR was adjusted for age, educational level and gender.
Right vs. left
multiple vs. single
embolic vs. thrombotic; OR, odds ratio; CI, confidence interval.
Multivariate analysis of death after ischemic stroke in patients with dementia
Independent predictors for death in patients with ischemic stroke were age (HR, 1.06; 95% CI, 1.02–1.10), atrial fibrillation (HR, 1.78; 95% CI, 1.32–2.40), previous stroke (HR, 2.14; 95% CI, 1.49–3.08), NIH stroke scale score (HR, 1.95; 95% CI, 1.04–1.27), and dementia (HR, 7.21; 95% CI, 3.85–13.49) in the Cox's regression model (Table III).
Table III.
Cox proportional hazards regression analysis according to dementia.
| Variable | SE OR (95% CI) |
|---|---|
| Age (years) | 1.06 (1.02–1.10) |
| Atrial fibrillation | 1.78 (1.32–2.40) |
| Previous stroke | 2.14 (1.49–3.08) |
| NIH stroke scale | 1.15 (1.04–1.27) |
| Dementia | 7.21 (3.85–13.49) |
Values are expressed as HR (95% CI). HR, hazard ratio; CI, confidence interval.
Discussion
Chongqing, the largest city in southwest China, has an urban population of five million individuals, of whom 93.6% are Han, and have similar lifestyles (27). Furthermore, the social and economic characteristics of the city is a miniature version of China today, and can be a good sample to study disease features. In the present study, the Kaplan-Meier analysis revealed that the cumulative proportion of survival cases was 49.3 and 92.5% in patients with and without dementia, after median follow-up of 21.2±5.6 months. The mortality rate of patients with dementia was >4-fold that of patients without dementia.
The results of the present study with regard to the cumulative proportion of survival of dementia after stroke str consistent with other studies in western countries. Previous findings on vascular dementia suggested that patients with vascular dementia had a higher risk for mortality compared to the control subjects (28,29). A study in Finland showed dementia is a significant predictor of poor long-term survival and death from brain-associated causes in patients with acute stroke (30). Barba et al (31) also found that dementia increased the risk for mortality in stroke patients. A study demonstrated that the mortality rate was 15.90 deaths/100 person-years in dementia patients with ischemic stroke and 5.37 deaths/100 person-years in non-dementia patients with ischemic stroke during a period of up to 10 years of follow-up (32). Another study suggested that the cumulative proportion surviving after a median follow-up of 58.6 months was 38.9±0.08% for those with dementia and 74.5±0.04% for those without dementia (12). There are discrepancies between our findings and those of the abovementioned studies. The reason may be because the duration gap and outpatient treatment is different.
Consistent with other studies (9,23–38), age, atrial fibrillation, NIH stroke scale, and prior stroke were found to be associated with death after stroke in the present study. We also found that dementia was associated with long-term survival of stroke patients. This association was independent of other predictors of post-stroke death such as older age, higher stroke severity, presence of atrial fibrillation and previous stroke. In a prospective study on the association between mortality and dementia after stroke, dementia was demonstrated to adversely influence long-term survival after stroke, even after adjusting for other commonly accepted predictors of stroke mortality (12). In studies of cognitive decline and death after first-ever stroke, dementia was also proven to be an important predictor (9,39). Dementia was considered to have prognostic implications in stroke patients, i.e., stroke prior to the index stroke and dementia developed after stroke may determine a significant reduction in survival and were among the most important risk factors of mortality in these patients (31). Dementia was identified to be a significant independent risk factor for reduced survival after ischemic stroke, after adjusting for other recognized predictors of mortality in a 10-year follow-up study (32).
Four explanations may be proposed for the mechanism of the elevated risk of death among patients with dementia after ischemic stroke (6,10,12,32,40,41). First, patients with dementia after stroke have an increased burden of cerebrovascular disease, which later may in return increase their risk of death. Second, patients with dementia tend to be treated less aggressively for stroke prophylaxis as well as other medical conditions for their weakened social abilities. Third, patients with dementia may be less compliant with prescribed treatment regiments. Fourth, dementia, either degenerative or vascular, tends to appear in an already weak individual and constitutes by itself a general deleterious condition.
In conclusion, mortality is increased in Chinese stroke patients with dementia. Dementia is a risk factor for death after stroke, independent of other factors including age, stroke severity, atrial fibrillation and previous stroke. Prevention and management of the dementia after stroke is critical to reduce the mortality after stroke.
Acknowledgements
The present study was funded by contract no. 2001-54-23 from the Science and Technology Committee of Chongqing, China.
References
- 1.Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: Epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 2.Bonita R, Ford MA, Stewart AW. Predicting survival after stroke: a three-year follow-up. Stroke. 1988;19:669–673. doi: 10.1161/01.STR.19.6.669. [DOI] [PubMed] [Google Scholar]
- 3.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker-Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A. Reducing attention deficits after stroke using attention process training: a randomized controlled trial. Stroke. 2009;40:3293–3298. doi: 10.1161/STROKEAHA.109.558239. [DOI] [PubMed] [Google Scholar]
- 5.Arac A, Blanchard V, Lee M, Steinberg GK. Assessment of outcome following decompressive craniectomy for malignant middle cerebral artery infarction in patients older than 60 years of age. Neurosurg Focus. 2009;26:E3. doi: 10.3171/2009.3.FOCUS0958. [DOI] [PubMed] [Google Scholar]
- 6.Barba R, Martínez-Espinosa S, Rodríguez-García E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia : clinical features and risk factors. Stroke. 2000;31:1494–1501. doi: 10.1161/01.STR.31.7.1494. [DOI] [PubMed] [Google Scholar]
- 7.Melkas S, Oksala NK, Jokinen H, Pohjasvaara T, Vataja R, Oksala A, Kaste M, Karhunen PJ, Erkinjuntti T. Poststroke dementia predicts poor survival in long-term follow-up: influence of prestroke cognitive decline and previous stroke. J Neurol Neurosurg Psychiatry. 2009;80:865–870. doi: 10.1136/jnnp.2008.166603. [DOI] [PubMed] [Google Scholar]
- 8.Shipley BA, Der G, Taylor MD, Deary IJ. Association between mortality and cognitive change over 7 years in a large representative sample of UK residents. Psychosom Med. 2007;69:640–650. doi: 10.1097/PSY.0b013e31814c3e7c. [DOI] [PubMed] [Google Scholar]
- 9.Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003;34:122–126. doi: 10.1161/01.STR.0000047852.05842.3C. [DOI] [PubMed] [Google Scholar]
- 10.Moroney JT, Bagiella E, Tatemichi TK, Paik MC, Stern Y, Desmond DW. Dementia after stroke increases the risk of long-term stroke recurrence. Neurology. 1997;48:1317–1325. doi: 10.1212/WNL.48.5.1317. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood K, Wentzel C, Hachinski V, Hogan DB, MacKnight C, McDowell I. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging: Prevalence and outcomes of vascular cognitive impairment. Neurology. 2000;54:447–451. doi: 10.1212/WNL.54.2.447. [DOI] [PubMed] [Google Scholar]
- 12.Tatemichi TK, Paik M, Bagiella E, Desmond DW, Pirro M, Hanzawa LK. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25:1915–1919. doi: 10.1161/01.STR.25.10.1915. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhou L, Zhang Y, Yi D, Liu L, Rao W, Wu Y, Ma D, Liu X, Zhou XH, Lin H, Cheng D, Yi D. Risk factors of stroke in Western and Asian countries: A systematic review and meta-analysis of prospective cohort studies. BMC Public Health. 2014;14:776. doi: 10.1186/1471-2458-14-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuh JL, Teng EL, Lin KN, Larson EB, Wang SJ, Liu CY, Chou P, Kuo BI, Liu HC. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening tool for dementia for a predominantly illiterate Chinese population. Neurology. 1995;45:92–96. doi: 10.1212/WNL.45.1.92. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Yu ES, Zhang M, Liu WT, Hill R, Katzman R. ADL dependence and medical conditions in Chinese older persons: a population-based survey in Shanghai, China. J Am Geriatr Soc. 1995;43:378–383. doi: 10.1111/j.1532-5415.1995.tb05811.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Deng J, Li J, Wang Y, Zhang M, He H. Study of the relationship between cigarette smoking, alcohol drinking and cognitive impairment among elderly people in China. Age Ageing. 2003;32:205–210. doi: 10.1093/ageing/32.2.205. [DOI] [PubMed] [Google Scholar]
- 17.Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004;251:421–427. doi: 10.1007/s00415-004-0337-z. [DOI] [PubMed] [Google Scholar]
- 18.Katzman R, Zhang MY, Ouang-Ya-Qu Wang ZY, Liu WT, Yu E, Wong SC, Salmon DP, Grant I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41:971–978. doi: 10.1016/0895-4356(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_Part_1.20. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Fuld PA. The Fuld Object-Memory Evaluation. Stoelting Instrument Co.; Chicago, IL: 1981. [Google Scholar]
- 23.Zhang M. Prevalence study on dementia and Alzheimer disease. Zhonghua Yi Xue Za Zhi. 1990;70(30):424–428. (In Chinese) [PubMed] [Google Scholar]
- 24.Zhou B, Hong Z, Huang M. Prevalence of dementia in Shanghai urban and rural area. Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:368–371. (In Chinese) [PubMed] [Google Scholar]
- 25.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 26.Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38:921–932. doi: 10.2307/2529872. [DOI] [PubMed] [Google Scholar]
- 27.Gao BW, Wang W, Zou DC. Effects of change in urban-rural statistical definition in two censuses on urbanization process of Chongqing. Popul Res. 2002;26:10–14. (In Chinese) [Google Scholar]
- 28.Koedam EL, Pijnenburg YA, Deeg DJ, Baak MM, van der Vlies AE, Scheltens P, van der Flier WM. Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord. 2008;26:147–152. doi: 10.1159/000149585. [DOI] [PubMed] [Google Scholar]
- 29.Knopman DS, Rocca WA, Cha RH, Edland SD, Kokmen E. Survival study of vascular dementia in Rochester, MN. Arch Neurol. 2003;60:85–90. doi: 10.1001/archneur.60.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Melkas S, Laurila JV, Vataja R, Oksala N, Jokinen H, Pohjasvaara T, Leppavuori A, Kaste M, Karhunen PJ, Erkinjuntti T. Post-stroke delirium in relation to dementia and long-term mortality. Int J Geriatr Psychiatry. 2012;27:401–408. doi: 10.1002/gps.2733. [DOI] [PubMed] [Google Scholar]
- 31.Barba R, Morin MD, Cemillán C, Delgado C, Domingo J, Del Ser T. Previous and incident dementia as risk factors for mortality in stroke patients. Stroke. 2002;33:1993–1998. doi: 10.1161/01.STR.0000017285.73172.91. [DOI] [PubMed] [Google Scholar]
- 32.Desmond DW, Moroney JT, Sano M, Stern Y. Mortality in patients with dementia after ischemic stroke. Neurology. 2002;59:537–543. doi: 10.1212/WNL.59.4.537. [DOI] [PubMed] [Google Scholar]
- 33.Kimura K, Minematsu K, Yamaguchi T. Japan Multicenter Stroke Investigators' Collaboration (J-MUSIC): Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:679–683. doi: 10.1136/jnnp.2004.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vemmos KN, Bots ML, Tsibouris PK, Zis VP, Takis CE, Grobbee DE, Stamatelopoulos S. Prognosis of stroke in the south of Greece: 1 year mortality, functional outcome and its determinants: the Arcadia Stroke Registry. J Neurol Neurosurg Psychiatry. 2000;69:595–600. doi: 10.1136/jnnp.69.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vohra EA, Ahmed WU, Ali M. Aetiology and prognostic factors of patients admitted for stroke. J Pak Med Assoc. 2000;50:234–236. [PubMed] [Google Scholar]
- 36.Rabkin SW, Mathewson FA, Tate RB. The relation of blood pressure to stroke prognosis. Ann Intern Med. 1978;89:15–20. doi: 10.7326/0003-4819-89-1-15. [DOI] [PubMed] [Google Scholar]
- 37.Woo J, Kay R, Yuen YK, Nicholls MG. Factors influencing long-term survival and disability among three-month stroke survivors. Neuroepidemiology. 1992;11:143–150. doi: 10.1159/000110924. [DOI] [PubMed] [Google Scholar]
- 38.Baptista MV, van Melle G, Bogousslavsky J. Prediction of in-hospital mortality after first-ever stroke: the Lausanne Stroke Registry. J Neurol Sci. 1999;166:107–114. doi: 10.1016/S0022-510X(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 39.Srikanth VK, Quinn SJ, Donnan GA, Saling MM, Thrift AG. Long-term cognitive transitions, rates of cognitive change, and predictors of incident dementia in a population-based first-ever stroke cohort. Stroke. 2006;37:2479–2483. doi: 10.1161/01.STR.0000239666.46828.d7. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann M, Schmitt F, Bromley E. Comprehensive cognitive neurological assessment in stroke. Acta Neurol Scand. 2009;119:162–171. doi: 10.1111/j.1600-0404.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 41.Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing rates of dementia at time of declining mortality from stroke. Stroke. 2006;37:1155–1159. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]