Abstract
Advances approaches in the treatment of Parkinson's disease are needed. The study was aimed to evaluate the therapeutic value of the new dopamine receptor agonist pramipexole. The effects of pramipexole on serum exosomes were investigated, and the possible mechanisms of action of the drug were explored. Initially, 68 patients were included in the study, of whom 3 cases did not complete the study. The remaining 65 patients were administered pramipexole at increasing doses starting at 0.25 mg twice a day for the 1st week, and reaching 1.5 mg three times daily at the 8th week. The doses were tapered during the course of the following 4 weeks. The total scores of the motor examination of the unified Parkinson's disease rating scale III (UPDRS III) and the total scores of the daily life activity in UPDRS II were compared before and after treatment. The relative expression amounts of α-synuclein in serum exosomes were then calculated by western blot analysis. Scores of UPDRS III and UPDRS II following treatment were significantly lower than the scores prior to treatment, and the difference was statistically significant (P<0.05). The relative expression of α-synuclein in serum exosomes was also found to be significantly lower after treatment (P<0.05). The relative expression of α-synuclein in the effective treatment group was significantly lower than that in the ineffective treatment group, and the difference was statistically significant (P<0.05). The relative expression of α-synuclein in serum exosomes was significantly correlated with treatment effects (P<0.05). In conclusion, pramipexole was effective and safe as a treatment for Parkinson's disease. The therapeutic effect of pramipexole may be associated with its reducing effect on the relative expression of α-synuclein in serum exosomes.
Keywords: pramipexole, Parkinson disease, serum exosomes, α-synuclein, unified Parkinson's disease rating scale
Introduction
Parkinson's disease, also known as tremor paralysis, is the second largest neurodegenerative disease, with morbidity rates second only to Alzheimer's disease (1). The etiology and pathogenesis of Parkinson's disease are not clear. The most important pathological changes are degeneration and loss of dopaminergic neurons in the substantia nigra of the midbrain, and the formation of α-synuclein-based acidophilic inclusion bodies (lewy bodies) (2) in the remaining dopaminergic neurons. In 2010, it was reported that α-synuclein was secreted out of the cells through exosomes in a calcium-dependent mechanism (3). The phenomenon was confirmed by a group of independent researchers (4). The finding of that study emphasized the importance of exosomes in the transcellular transport of α-synuclein, and it strengthened the correlation between the amount of secreted exosomes and the pathogenesis of Parkinson's disease. It has also laid a theoretical basis for the early diagnosis and targeted treatment of Parkinson's disease.
In the present study, the therapeutic effects on Parkinson's disease of the new dopamine receptor agonist pramipexole and its relationship with serum exosomes and inclusion bodies were examined. The possible underlying therapeutic mechanisms were also investigated.
Patients and methods
Patients
Initially, 68 patients diagnosed with primary Parkinson's disease were selected from Xianyang Central Hospital during the period October, 2012 to October, 2014. The diagnoses were confirmed according to the UK Brain Banks Network. The inclusion criteria for the study were: i) Age, ≥18 and <75 years. ii) First time diagnosis. Absence of history of Parkinson's disease drug use. iii) Patients with normal intelligence, good compliance and complete clinical data. The exclusion criteria for the study were: i) Symptomatic Parkinson's disease or Parkinson's plus a syndrome with severe peak dose dyskinesia. History of brain surgery and trauma, or malignant tumor. ii) Patients required to stay in bed for long periods of time. Patients with severe heart, liver and kidney dysfunctions. Patients allergic to pramipexole. iii) Pregnant and lactating women, patients with mental disorders, and any patients that refused to participate.
Three patients did not complete the study, while the remaining 65 cases successfully completed the study. There were 38 men and 27 women, with an age range of 57–74 years, and an average age of 67.8±9.2 years. The course of disease at the time of diagnosis ranged from 1 week to 3 months, with an average of 1.2±0.3 months.
Methods
After obtaining the approval of the ethics committee of the Xianyang Central Hospital and the consent of patients and their relatives, the patients were administered oral pramipexole using the following dosing schedule: 0.25 mg twice a day for the 1st week, followed by 0.25 mg 3 times daily for the 2nd week. From the 3rd to 8th week, the doses were increased gradually starting at 0.25 mg 3 times daily and adding 0.25 mg to each dose every 3 days until 1,5 mg 3 times daily was achieved and until the end of the 8th week. Subsequently, the doses were tapered, and were reduced by 1.125 mg/week, during the course of the following 4 weeks. Liver and kidney function, and electrocardiograph indices were periodically checked and the patients' clinical symptoms were evaluated often.
Observation indices
Total scores of motor examination of the unified Parkinson's disease rating scale III (UPDRS III) and total scores of the daily life activity in UPDRS II, before and after treatment, were compared. To evaluate the result of the treatment, a reduction of ≥50% in the score of UPDRS II/III was defined as effective.
The exosomes were separated and purified from sera using an ExoQuick-TC kit (System Biosciences, Mountain View, CA, USA). The morphology of the exosomes was detected using a transmission electron microscope (JEOL, Tokyo, Japan). The presence of α-synuclein in the exosomes was confirmed by western blot analysis of the relative expressions.
Detection methods
Isolation of serum exosomes
Elbow venous blood (5 ml) was drawn and placed into a serum separation gel tube (Becton-Dickinson and Company, Franklin Lakes, NJ, USA). After mixing, the tubes were maintained at at 37°C for 30 min. Centrifugation was performed at 2,000 × g at 4°C for 15 min to achieve separation of the contents of each tube into three layers from top to bottom: serum, separation and blood cell layers. The serum layer was placed on ice and then stored in 500 µl aliquots in 1.5 ml Eppendorf tubes (Becton-Dickinson and Company) at −80°C. An ExoQuick-TC kit was used to purify the exosomes according to the manufacturer's instructions.
Morphology of exosomes under transmission electron microscope
Serum samples were thawed and mixed with 200 µl phosphate-buffered saline (PBS) solution. The electron microscope copper mesh grid was dipped into the suspension and kept there for ≥20 min. Then, 2–3 PBS solution washes (50 µl each) lasting 1 min were performed. The suspension was fixed to the copper mesh by the addition of 10 µl 4% glutaraldehyde for 5 min, followed by filter paper seeping and a PBS solution rinse. After drying at room temperature, the rinsed copper mesh was placed under a Hitachi-7500 transmission electron microscope (Lincoln, NE, USA) and images were captured.
Western blot analysis
Total proteins were extracted from exosomes. The protein concentration was quantified by the bicinchoninic acid (BCA) method (Bio-Rad, Berkeley, CA, USA). Electrophoresis was run on SDS-PAGE gels. Western blotting was performed using rabbit anti-CD63 polyclonal antibody (dilution, 1:1000) (LifeSpan Biosciences, Seattle, WA, USA; cat. no.: LS-C387289) and rabbit anti-CD9 polyclonal antibody (dilution, 1:1000) (LifeSpan Biosciences; cat. no.: LS-C382578), rabbit polyclonal anti-GAPDH antibody (dilution, 1:2000) (Abcam, Cambridge, MA, USA; cat. no.: ab8245), rabbit anti-α-synuclein polyclonal antibody (dilution, 1:1000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, cat. no.: sc-10717), and horseradish peroxidase-labeled goat anti-rabbit secondary antibody (dilution, 1:5000) (Sigma, St. Louis, MO, USA, cat. no.: A0545).
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used for statistical analysis. Data were presented as mean ± standard deviation, the t-test was used for inter-group comparisons, and enumeration data were presented as a percentage (%), Spearman correlation analysis was used for α-synuclein in exosomes and curative effects. P<0.05 was considered to indicate a statistically significant difference.
Results
General data
Of the initial 68 patients, 3 cases did not complete the study (2 cases had aggravated symptoms and required change of medication or a combination of several drugs, and 1 case with severe liver and kidney damage). The remaining 65 cases successfully completed the study. There were 38 men and 27 women, with an age range of 57–74 years, and an average of 67.8±9.2 years. The course of disease at the time of diagnosis ranged from 1 week to 3 months, with an average of 1.2±0.3 months.
Identification of exosomes
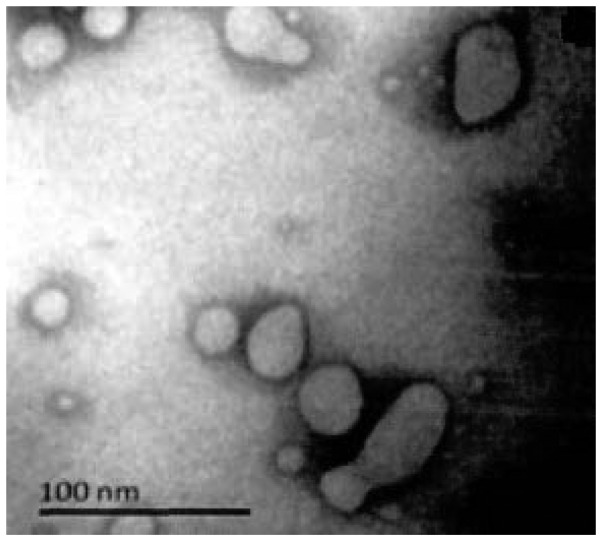
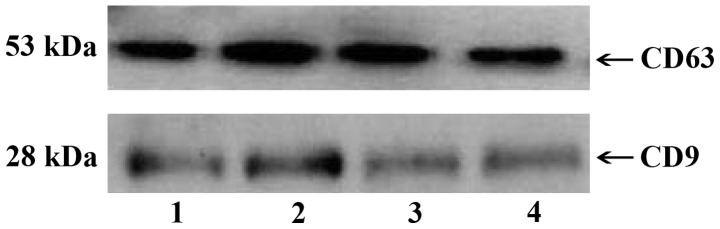
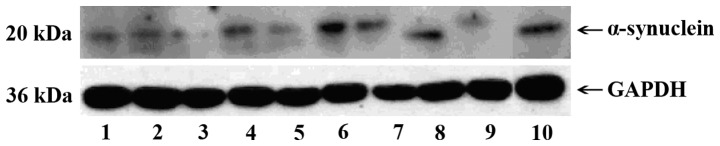
Several vesicle-like structures of uniform size, with an average diameter of ~50 nm were observed under electron microscopy (Fig. 1). Exosome-labeled proteins CD63 and CD9 were detected by western blotting in 4 samples (Fig. 2). Furthermore, α-synuclein was expressed in exosomes isolated from 10 samples (Fig. 3).
Figure 1.
Electron microscope image showing vesicle-like structures consistent with isolated exosomes.
Figure 2.
Western blotting showing exosome-labeled proteins CD63 and CD9 in 4 samples.
Figure 3.
Western blotting showing α-synuclein expressed in exosomes isolated from 10 samples.
UPDRS III and UPDRS II scores before and after treatment
After treatment, UPDRS III and UPDRS II scores were significantly lower than those before treatment, and the difference was statistically significant (P<0.05; Table I).
Table I.
UPDRS III and UPDRS II scores before and after treatment.
| Items | Pre-treatment | Post-treatment | t | P-value |
|---|---|---|---|---|
| UPDRS II | 12.6±3.3 | 7.7±1.5 | 5.624 | 0.034 |
| UPDRS III | 30.8±4.4 | 19.5±2.1 | 5.768 | 0.032 |
UPDRS, unified Parkinson's disease rating scale.
Relative expression of α-synuclein in serum exosomes before and after treatment
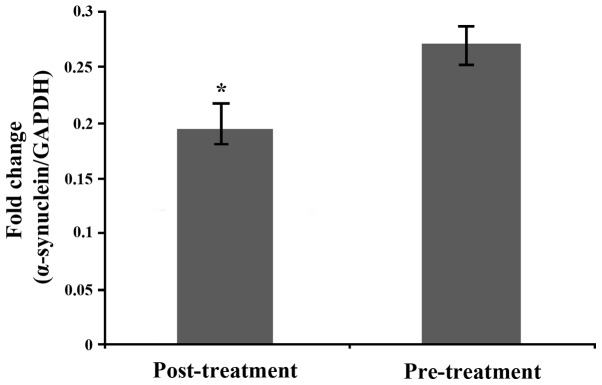
The relative expression of α-synuclein in serum exosomes after treatment was significantly lower than that before treatment, and the difference was statistically significant [(0.27±0.04), t=−0.523, P=0.017] (Fig. 4).
Figure 4.
Relative expression of α-synuclein in serum exosomes before and after treatment. *P<0.05.
Correlation between the relative expression of α-synuclein in serum exosomes and therapeutic effects
From the 65 cases studied, 52 cases had what was considered effective treatment and 13 cases had an ineffective one. The relative expression of α-synuclein in the effective treatment group was significantly lower than that in the ineffective treatment group, and the difference was statistically significant [(0.15±0.02 vs. 0.22±0.05), t=−0.603, P<0.01]. The relative expression of α-synuclein in serum exosomes was significantly correlated with treatment effects (r=0.426, P=0.029).
Discussion
Exosomes are a membranous microvesicle with a 30–100 nm diameter and 1.13–1.19 g/ml of density (5). They are revealed by a concave disk or cup mouth shape under the electron microscope (6). As a type of nanomembrane vesicle, it was firstly identified in the late 1980s in the culture and differentiation process of sheep reticulocytes (7), In 2005, Caby et al (8) identified the existence of exosomes in healthy human blood, indicating exosomes could act as a molecular medium and promote the exchange of information between cells or organs. Exosomes can be secreted from live cells cultured in vitro and in vivo (9). This phenomenon has been observed in neurons (10) and microglia (11) in nerve cells, and lymphocyte and mononuclear cells in non-neuronal cells. Exosomes also exist in various body fluid environments, such as serum (12). Detailed information for the biosynthetic pathway of exosomes has been previously published, including a useful graph diagram (13).
Exosomes play an important role in nerve development and regeneration as well as in the deformability of the cortex and hippocampal neuron synapses (14). In the case of pathological stimulation or loss, exosomes have immunostimulatory functions, activating and spreading the inflammatory response (12). This inflammatory response has been demonstrated to play an important role in the pathogenesis of Parkinson's disease. It has been confirmed to be able to accelerate the degenerative changes of the substantia nigra (15).
The identification of exosomes requires morphological observation and protein analysis. In the present study, the ExoQuick reagent method was used to separate and purify exosomes. In our experience, compared with the differential centrifugation method, the output of exosomes obtained from ExoQuick reagent method was higher and also purer. The transmembrane 4 superfamily CD63 and CD9 are expressed in exosomes but not in cytoplasmic membranes; they are, therefore, reliable exosome markers (16). CD63 and CD9 were abundant in exosome extractions.
α-synuclein is widely expressed in the neuronal presynaptic membrane and cytoplasm. Mutations on the gene encoding α-synuclein are closely related to familial Parkinson's disease. The pathological abnormal aggregation of the protein is thought to adversely affect synaptic function and signaling, and to promote neuronal degeneration, playing an important role in the pathogenesis of Parkinson's disease. At first, α-synuclein was only thought to be a form of cytoplasmic protein involved in the dopamine metabolism pathway. However, α-synuclein was also found in extracellular environments, such as serum (17), cerebrospinal fluid (18) and neuron cell culture (19). In the early onset of Parkinson's disease, where there is an α-synuclein genic mutation, this protein was found to play a role in the pathogenesis of the disease. In 2012, it was confirmed (20) that an α-synuclein oligomer exists in the exosomes of neuron cells and that the oligomer inside exosomes exhibits higher toxicity than the sporadic α-synuclein oligomer outside of exosomes. α-synuclein can be transported outside cells through the exosome channel. Such discoveries lead to the proposal that exosomes can transmit potential toxic proteins and cause disease in neighboring cells. Therefore, exosomes were dubbed the ‘Trojan Horse’ of neurodegenerative disease (21).
Dopamine receptor agonists are the first choice of treatment for the early functional decompensation seen in Parkinson's disease. As the disease progresses and drug doses are increased, the efficacy of levodopa decreased, and other medications are needed in the case of motor affectation. Pramipexole (2,6-diamino-4,5,6,7-tetrahydrobenzothiazole) is a non-ergotine D2 receptor agonist with unique pharmacological effects and exhibits high affinity for the D3 receptor sub-type (22). In the present study, scores of UPDRS III and UPDRS II after treatment were significantly lower than the scores prior to treatment, and the relative expression of α-synuclein in serum exosomes was also significantly lower than that prior to treatment, and differences were statistically significant. The effective therapeutic rate was 80% (52/65), and there were no serious complications of the treatment. The relative expression of α-synuclein in the effective therapy group was significantly lower than that in the ineffective therapy group with statistically significant differences. Furthermore, the relative expression of α-synuclein in serum exosomes was significantly correlated with treatment effects.
In conclusion, pramipexole was effective and safe in treating patients with Parkinson's disease. Results obtained in the present study indicate that pramipexole reduces the relative expression level of α-synuclein in serum exosomes.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Shulman JM, De Jager PL, Feany MB. Parkinson's disease: Genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 3.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 6.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supematants and biological fluids. Curr Protoc Cell Biol Chapter. 2006;3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 8.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 9.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 10.Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: Metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- 12.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 13.Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Front Physiol. 2012;3:145. doi: 10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Panaro MA, Cianciulli A. Current opinions and perspectives on the role of immune system in the pathogenesis of Parkinson's disease. Curr Pharm Des. 2012;18:200–208. doi: 10.2174/138161212799040574. [DOI] [PubMed] [Google Scholar]
- 16.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 17.El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 18.Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, Ishigami N, Tamaoka A, Nakagawa M, El-Agnaf OM. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766–1772. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 19.Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghidoni R, Benussi L, Binetti G. Exosomes: The Trojan horses of neurodegeneration. Med Hypotheses. 2008;70:1226–1227. doi: 10.1016/j.mehy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Mierau J, Schingnitz G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine D2 receptor agonist. Eur J Pharmacol. 1992;215:161–170. doi: 10.1016/0014-2999(92)90024-X. [DOI] [PubMed] [Google Scholar]