Abstract
Neurodegenerative disorders are characterized by progressive degeneration and loss of neurons in the brain. Oxidative stress is implicated in the pathogenesis of neurological disorders, although the pathological mechanism remains unelucidated. Daphnetin, an active ingredient extracted from Changbai daphne (Daphne Korean Nakai), exhibits various pharmacological effects, including anti-inflammatory, anti-oxidative and anti-tumor effects. However, the neuroprotective effects, as well as the specific mechanisms of daphnetin, remain unclear. Neuronal-like rat pheochromocytoma PC12 cells were pretreated with daphnetin for 2 h, then treated with or without H2O2 for various times. Cell morphology was detected using an inverted microscope, the apoptotic ratio was determined by Annexin V fluorescein isothiocyanate/propidium iodide assay, nuclear morphology was observed and photographed using a fluorescence microscope following 4′,6-diamidino-2-phenylindole staining. The levels of pro-caspase 3, cleavage of poly ADP-ribose polymerase and caspase 3 were detected by western blotting. In addition, the activation of mitogen-activated protein kinase (MAPK) signal pathway and the expression of HSP70 were detected by western blotting. The present study demonstrated that daphnetin attenuated hydrogen peroxide (H2O2)-induced apoptosis in a concentration-dependent manner, reduced the cleavage of poly ADP ribose polymerase and caspase 3, and inhibited the phosphorylation of p38 MAPK and c-Jun N-terminal kinases (JNK) in H2O2-induced PC12 cells. In addition, daphnetin induced the expression of HSP70 in a dose- and time-dependent manner, and daphnetin-induced HSP70 expression was reduced by extracellular signal-regulated kinase (ERK) 1/2 inhibitor U0126 in PC12 cells. Therefore, the present results indicate that daphnetin protects PC12 cells against oxidative stress injury by regulating p38 MAPK and JNK signaling and increasing the expression of HSP70 via ERK signaling. This suggests that daphnetin may have the potential to treat certain neurodegenerative diseases. The present results not only provide insight into the potential use of daphnetin in H2O2-induced PC12 cell apoptosis, but also highlight the potential role of HSP70 in neuroprotection.
Keywords: daphnetin, oxidative stress, apoptosis, MAPK, HSP70
Introduction
Oxidative stress is associated with the pathogenesis of neurological disorders, including dysautonomia, Alzheimer's disease and Parkinson's disease (1,2). Hydrogen peroxide (H2O2), which is one of the major reactive oxygen species, is considered as a major cause of neuronal cell death (3). Therefore, pharmacological approaches for intervening in oxidative stress may be potential therapeutic strategies for neurodegenerative disorders (4).
Natural antioxidants with neuroprotective potential are being considered as a promising approach to prevent or slow the effects of neurological illness, due to their low toxicity and absence of clear side effects. It has been reported that numerous natural antioxidants, including resveratrol, celastrol and salidroside, may protect neurons from oxidative stress injury (5–7).
Daphnetin (7,8-dihydroxycoumarin), an active ingredient extracted from Changbai daphne (Daphne Korean Nakai), exhibits various pharmacological effects, including anti-inflammatory, anti-oxidative and anti-tumor effects (8,9). However, whether daphnetin exerts neuroprotection against H2O2-induced neuronal-like rat pheochromocytoma PC12 cell apoptosis, and the mechanisms responsible for this effect, remains unclear.
Inducible heat shock protein (HSP) 70, a member of the HSP superfamily, is an important protective protein induced by various stimuli that prevents cell apoptosis (10,11). A previous study has suggested that HSP70 is protective in neurodegenerative diseases, including Parkinson's disease, through its chaperone and direct antiapoptotic role (11). It has also been reported that natural antioxidants, including celastrol, protect nerve cell damage by inducing the expression of HSP70 (12).
The present study investigated the activity of daphnetin in neuronal apoptosis and the underlying mechanisms of this effect. The present study demonstrated that daphnetin dose-dependently attenuated H2O2-induced PC12 cell apoptosis via suppression of p38 and c-Jun N-terminal kinases (JNK) phosphorylation. In addition, the present study revealed that HSP70 expression was elevated in daphnetin-treated PC12 cells, and HSP70 expression was regulated by extracellular signal-regulated kinase (ERK) signaling. Overall, the present study concluded that daphnetin attenuates p38 and JNK activation and upregulates HSP70 expression in H2O2-treated PC12 cells. These two mechanisms reduce H2O2-induced PC12 apoptosis, and are protective in oxidative stress-induced neuronal injury.
Materials and methods
Antibodies and reagents
Daphnetin (purity >98%) was obtained from Sigma-Aldrich (St. Louis, MO, USA), and the ERK inhibitor U0126 was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). H2O2 (30%) was purchased from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit monoclonal antibodies against β-actin (catalog no., 4970S), Akt, phospho (p)-Akt (Ser 473; catalog no., 9272S), p38 mitogen-activated protein kinase (MAPK; catalog no., 8690S), p-p38 MAPK (Thr180/Tyr182; catalog no., 4511S), ERK (catalog no., 4695S), p-ERK (Thr202/Tyr204; catalog no., 4376S), JNK/stress-activated protein kinase (SAPK; catalog no., 9258S), p-JNK/SAPK (Thr183/Tyr185; catalog no., 4668S), poly ADP-ribose polymerase (PARP; catalog no., 9532S), cleaved-caspase 3 (catalog no., 9664S; 1:500), pro-caspase 3 (catalog no., 9665S) and HSP70 (catalog no., 4872S) were all purchased from Cell Signaling Technology, Inc and used at 1:1,000 dilution, unless otherwise specified. Rabbit polyclonal antibody against glyceraldehyde 3-phosphate dehydrogenase (catalog no., AP0063; 1:1,000) was purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA). Secondary antibodies coupled to IRDye800 fluorophore (catalog no., 926-32211; dilution, 1:5,000) for use with the Odyssey® Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) were purchased from LI-COR Biosciences.
Cell culture
Rat pheochromocytoma PC12 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (Invitrogen™; Thermo Fisher, Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) heat-inactivated horse serum, 5% fetal bovine serum and 1% antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) (Hyclone™; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in 5% CO2/95% air humidified atmosphere. Culture medium was changed every 2–3 days.
Cell viability assay
Cell viability was detected using Cell Counting Kit-8 (CCK-8; KeyGen Biotech Corp., Ltd., Nanjing, China). Briefly, PC12 cells were plated onto 96-well plates at a density of 2×104 cells per well 24 h prior to treatment. Cells were treated with various concentrations of daphnetin (0, 2.5, 5.0, 10.0 and 20.0 µM) for 2 h, then stimulated with H2O2 (200 µΜ) for 24 h, followed by incubation with 10 µl CCK-8 working solution at 37% for 4 h. The absorbance of each well at 450 nm was measured using a Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific, Inc.). Three repeats were performed for each of the different treatments.
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay
The apoptotic ratio was analyzed using Annexin-V/PI Double Staining Assay (KeyGen Biotech Corp., Ltd.), according to the manufacturer's protocol. Briefly, the cells were washed, trypsinized and resuspended with 500 µl binding buffer, then stained with Annexin V-FITC (5 µl) and PI (5 µl). The stained cells were visualized directly using a Guava EasyCyte™ System (EMD Millipore, Billerica, MA, USA), and the data were analyzed using Guava TUNEL ExpressPro version 8.0 software (EMD Millipore).
Western blotting
Cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and lysed on ice in a lysis buffer containing 20 mM Tris (pH 7.5), 2 mM EDTA, 135 mM NaCl, 2 mM DTT, 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 10 mM NaF, 10 µg/ml leupeptin, 10 µg/ml aprotinin and 1 mM PMSF, supplemented with a complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) for 30 min. Lysates were centrifuged at 12,500 × g for 15 min at 4°C. Equal amounts of proteins were subjected to 12% SDS-PAGE, and then transferred onto nitrocellulose membranes (GE Healthcare Life Sciences, Chalfont, UK). The membrane was blocked with 5% skimmed milk for 1 h at room temperature, washed with Tris-buffered saline with Tween 20 three times, then incubated with the indicated primary antibodies at 4°C overnight. Subsequently, the membranes were incubated with secondary antibodies for 1 h at room temperature. The antibody-antigen complexes were visualized by the Odyssey Infrared Imaging System using IRDye800 fluorophore-conjugated antibodies (LI-CORBiosciences).
Morphological observation
PC12 cells were pretreated with various concentrations of daphnetin (5, 10 and 20 µM) for 2 h, then stimulated with H2O2 (200 µΜ) for 24 h. Subsequently, cell morphology was observed using an inverted microscope (DP72; Olympus Corporation, Tokyo, Japan).
Immunofluorescence microscopy and 4′,6-diamidino-2-phenylindole (DAPI) staining
Cells were pre-incubated with daphnetin for 2 h, then stimulated with H2O2 for 24 h. Cells were fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized in 0.2% Triton X-100 and then incubated with DAPI (1 µg/ml) for 5 min in the dark. Subsequent to washing with PBS, nuclear morphology was observed and photographed using a fluorescence microscope (DP72; Olympus Corporation).
Statistical analysis
Data are expressed as the mean ± standard deviation. One-way analysis of variance was used to determine the significance of the difference between two groups. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Effect of daphnetin on H2O2-induced cell viability
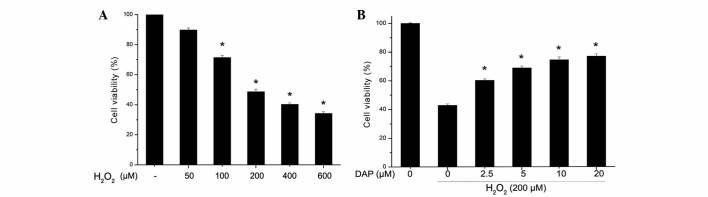
PC12 cells were treated with various concentrations of H2O2 (50, 100, 200, 400 and 600 µM) for 24 h, and subsequently cell viability was determined using CCK-8. As shown in Fig. 1A, H2O2 reduced cell viability in a dose-dependent manner (P<0.05 vs. control). For the cells treated with 200 µM H2O2, the cell viability was 48.75±1.63%. Since, the cell viability was reduced by ~50% with 200 µM H2O2 at 24 h, in subsequent experiments these variables were selected as the standard dose and time point for the induction of PC12 cell apoptosis. In PC12 cells treated with various doses of daphnetin and then stimulated with 200 µM H2O2, cell viability was increased in a daphnetin dose-dependent manner (P<0.05 vs. H2O2-treated group; Fig. 1B).
Figure 1.
DAP enhanced H2O2-induced cell viability. (A) Rat pheochromocytoma PC12 cells were treated with various concentrations of H2O2 for 24 h, (B) PC12 cells were pretreated with various doses of DAP for 2 h, then stimulated with H2O2 (200 µM) for 24 h. Cell viability was determined by Cell Counting Kit-8. Experiments were independently repeated three times. (A) *P<0.05 vs. control; (B) *P<0.05 vs. H2O2-treated group. H2O2, hydrogen peroxide; DAP, daphnetin.
Daphnetin attenuated H2O2-induced PC12 cell apoptosis
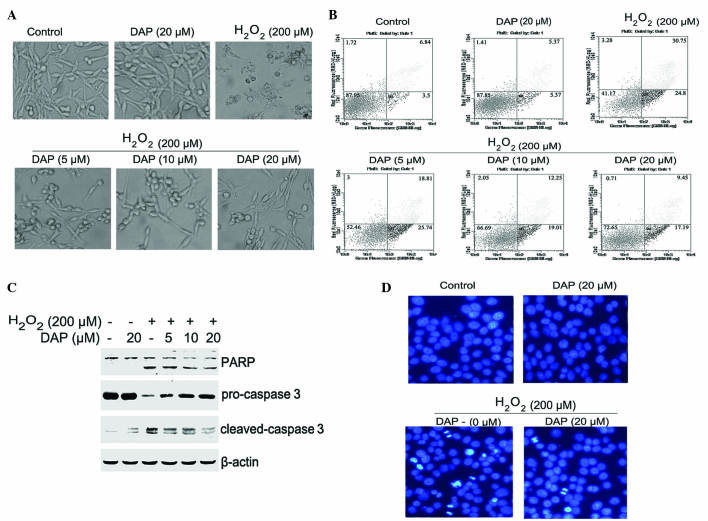
Observation of cell morphology revealed that untreated PC12 cells exhibited typical long fusiform-like morphology. By contrast, H2O2-treated (200 µM; 24 h) PC12 cells were shrunken, scattered and floating. Pretreating cells with daphnetin significantly reversed the apoptotic morphological alterations observed with H2O2-treated cells (Fig. 2A). To further determine the role of daphnetin in suppressing H2O2-induced PC12 cell apoptosis, the apoptotic ratio was determined by Annexin V-FITC/PI assay. As shown in Fig. 2B, cells exposed to H2O2 for 24 h had an apoptotic ratio of 55.6%. However, the number of apoptotic cells, when cells were pretreated with various concentrations of daphnetin, was significantly reduced; the apoptotic ratio was 34.6, 31.3, 26.6%, for 5, 10 and 20 µM daphnetin, respectively.
Figure 2.
DAP attenuated H2O2-induced apoptosis in rat pheochromocytoma PC12 cells. PC12 cells were pretreated with various concentrations of daphnetin for 2 h, then stimulated with H2O2 (200 µΜ) for 24 h. (A) Cell morphology was detected using an inverted microscope. (B) The apoptotic ratio was determined by Annexin V-FITC/PI assay. (C) Cleavage of PARP (89/116 kDa) and caspase 3 (17/19 kDa), and the level of pro-caspase 3 (35 kDa) were detected by western blotting (β-actin, 45 kDa). (D) Cells were stained with 4′,6-diamidino-2-phenylindole, and nuclear morphology was observed and photographed using a fluorescence microscope. H2O2, hydrogen peroxide; DAP, daphnetin; FITC, fluorescein isothiocyanate; PI, propidium iodide; PARP, poly ADP-ribose polymerase.
The activation of apoptosis-associated proteins was detected using western blotting to confirm the occurrence of apoptosis. Daphnetin pretreated cells clearly attenuated the H2O2-induced cleavage of PARP and caspase 3, and enhanced the level of pro-caspase 3 (Fig. 2C) compared with cells not pretreated with daphnetin. DAPI staining was used to determine the apoptotic status of the PC12 cells. Staining with DAPI revealed that the round nuclei of normal cells was homogeneous, and when exposed to 200 µM H2O2 for 24 h cells underwent nuclear condensation and fragmentation. However, these alterations in nuclear morphology were significantly attenuated by pretreatment with daphnetin (Fig. 2D). Overall, these results suggest that daphnetin has a protective effect in H2O2-induced PC12 cell apoptosis.
Daphnetin reduced the activation of p38 MAPK and JNK induced by H2O2
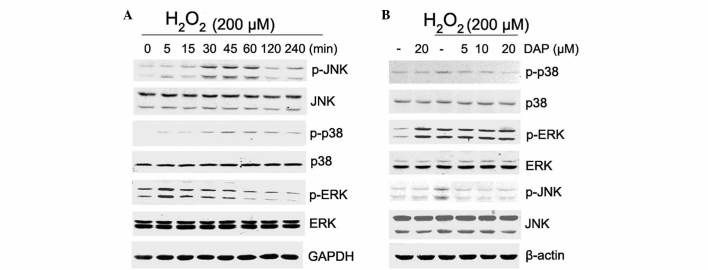
To determine the mechanism by which daphnetin inhibits H2O2-induced apoptosis, the phosphorylation levels of MAPKs in PC12 cells were investigated. PC12 cells were stimulated with 200 µM H2O2 for various times, and the phosphorylation levels of p38, ERK and JNK were detected by western blotting. The results showed that stimulation of PC12 cells with H2O2 resulted in an increase in phosphorylation of JNK, p38 and ERK (Fig. 3A). The enhanced phosphorylation of JNK and p38 was significantly attenuated by daphnetin pretreatment in a dose-dependent manner (Fig. 3B). However in the presence of daphnetin, increased ERK phosphorylation observed with H2O2 stimulation was not attenuated. In addition, with daphnetin stimulation, phosphorylation of ERK was clearly elevated, but ERK activation had no additive effect on daphnetin and H2O2 stimulation (Fig. 3B).
Figure 3.
DAP reduced H2O2-induced p38 mitogen-activated protein kinase and JNK activation. (A and B) The phosphorylation level of p38 (43 kDa), ERK (42/44 kDa) and JNK (46/54 kDa) was detected by western blotting in (A) rat pheochromocytoma PC12 cells stimulated with 200 µM H2O2 for various times and (B) PC12 cells pretreated with DAP (5, 10, 20 µM) for 2 h, then stimulated with H2O2 (200 µΜ) for 30 min. Equal protein loading was confirmed by GAPDH (37 kDa) and β-actin (45 kDa). H2O2, hydrogen peroxide; DAP, daphnetin; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinase; p-phospho; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Daphnetin induced HSP70 expression in PC12 cells
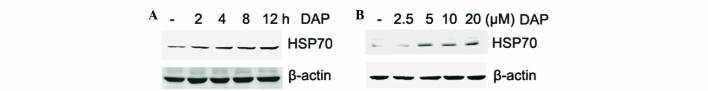
In order to determine other mediators in daphnetin neuroprotection, HSP70, a well-known chaperone with cytoprotective effects, was investigated. PC12 cells were stimulated with daphnetin, and total cellular protein was extracted and subjected to western blotting with an anti-HSP70 antibody. The present results revealed that the level of HSP70 was increased upon daphnetin stimulation in a dose- and time-dependent manner (Fig. 4A and B). The level of HSP70 peaked at 12 h.
Figure 4.
DAP induced HSP70 expression in rat pheochromocytoma PC12 cells. (A and B) PC12 cells were stimulated with (A) 20 µM DAP for 2, 4, 8, 12 h or (B) various concentrations of DAP for 12 h. Total cellular protein were extracted and subjected to western blotting probed with an anti-HSP70 (70 kDa) antibody. Equal protein loading was confirmed by β-actin (45 kDa). DAP, daphnetin; HSP, heat shock protein.
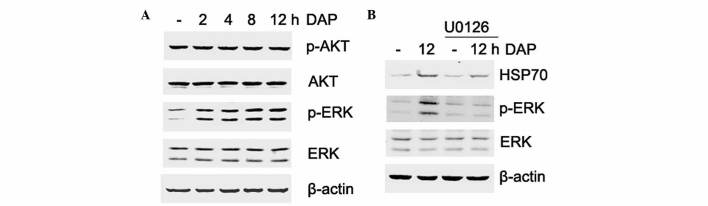
ERK signaling is involved in daphnetin-induced HSP70 expression
The phosphorylation of upstream kinases was examined to investigate the possible signal transduction pathways involved in daphnetin-induced HSP70 expression. PC12 cells were treated with daphnetin (20 µM) for various times and the phosphorylation level of ERK and Akt was detected by western blotting. As shown in Fig. 5A, the activation of ERK was enhanced in a time-dependent manner following daphnetin stimulation. ERK activation peaked at 12 h, which is consistent with the expression of HSP70. By contrast, Akt phosphorylation was not significantly different compared with unstimulated PC12 cells. Overall, these observations suggested that ERK signaling activation may be involved in daphnetin-induced HSP70 expression.
Figure 5.
ERK signaling is involved in DAP-induced HSP70 expression. (A) Rat pheochromocytoma PC12 cells were stimulated with 20 µM daphnetin for 2, 4, 8 and 12 h, and total cellular protein was extracted and subjected to western blotting with p-Akt (60 kDa), Akt (60 kDa), p-ERK (42/44 kDa) and ERK (42/44 kDa) antibodies. (B) PC12 cells were pretreated with U0126 for 2 h, then treated with daphnetin for 12 h. Cell lysates were prepared and subjected to western blotting using HSP70 (70 kDa), p-ERK and ERK antibodies. Equal protein loading was confirmed by β-actin (45 kDa). DAP, daphnetin; HSP, heat shock protein; ERK, extracellular signal-regulated kinase; p, phospho.
To test this hypothesis, U0126, a specific inhibitor of ERK, was used to verify the activation of ERK in daphnetin-induced HSP70 expression. PC12 cells were pretreated with 20 µM U0126 for 2 h, and then exposed to 20 µM daphnetin for a further 12 h, and the expression of HSP70 and phosphorylated ERK were detected using western blotting. As shown in Fig. 5B, attenuation of ERK activation by U0126 clearly reduced the protein expression level of HSP70. Therefore, the present data suggest that HSP70 is involved in daphnetin-mediated cytoprotection via ERK signaling.
Discussion
Oxidative stress has been widely implicated in neuronal cell death, and is associated with a variety of chronic neurodegenerative diseases, including Parkinson's, Alzheimer's and Huntington's disease. H2O2 is a strong oxidant, which reduces cell viability and increases cell apoptosis (13). Currently, great effort is being made to identify potent natural antioxidants with neuroprotective potential, due to their low toxicity and absence of clear side effects. Daphnetin, a natural antioxidant, exhibits a variety of biological effects, including anti-inflammatory and antitumor effects (14). Recently, studies have demonstrated that daphnetin exhibits a neurotrophic effect on peripheral nerve regeneration by suppressing nuclear factor-κB expression (15), provides a neuroprotective effect against glutamate-induced toxicity in immortalized mouse hippocampal HT22 cells and ischemic brain injury (16), and prevents excitotoxicity by inhibiting the N-methyl D-aspartate receptor subtype 2B -containing receptors and the subsequent calcium overload in cultured cortical neurons (17). These studies suggest that daphnetin has a neuroprotective effect, which is consistent with the present findings. The present study confirmed that treating PC12 cells with H2O2 resulted in suppression of cell viability in a dose-dependent manner; however, pretreatment of these cells with various concentrations (5, 10, 20 µM) of daphnetin significantly increased PC12 cell viability (Fig. 1). The present study further investigated whether daphnetin has protective effects against H2O2-induced nerve cell apoptosis. Cell morphology analysis revealed that untreated PC12 cells exhibited typical long fusiform-like morphology, while treatment with H2O2 (200 µM) for 24 h resulted in shrunken, scattered and floating cells. However, pre-incubation with daphnetin clearly reversed the morphology induced by H2O2 (Fig. 2A). The cell apoptotic ratio was detected using flow cytometry, and the present data revealed that the number of apoptotic cells was significantly reduced in daphnetin-pretreated PC12 cells compared with cells stimulated with H2O2 (Fig. 2B). The present study also examined the activation of apoptotic proteins by immunoblotting, in order to confirm the occurrence of apoptosis. Upon H2O2 stimulation, cleavage of PARP and caspase 3 were clearly enhanced in PC12 cells; however, pretreatment with daphnetin significantly decreased the cleavage of PARP and caspase 3 in a concentration-dependent manner (Fig. 2C). DAPI staining of H2O2-treated cells revealed typical apoptotic morphology. However, pre-incubation with daphnetin clearly attenuated these morphological alterations (Fig. 2D). These results suggest that daphnetin exerts its neuroprotective effect via inhibition of PC12 cell apoptosis.
Certain studies have revealed that oxidative stress-induced toxicity is associated with intracellular signaling, including the activation of JNK and p38 signaling pathways (7,13). In addition, it has been reported that the activation of ERK may protect neurons from oxidative stress-induced cell death (18). The present study hypothesized that daphnetin may protect PC12 cells against H2O2-induced apoptosis via the MAPK signaling pathway. Western blotting revealed that p38, ERK and JNK were phosphorylated upon H2O2 stimulation, and ERK was activated earlier than p38 MAPK and JNK activation. Additionally, the enhanced phosphorylation of JNK and p38 observed following stimulation with H2O2 was clearly attenuated by daphnetin pre-treatment in a dose-dependent manner. However, phosphorylation of ERK upon H2O2 stimulation was not attenuated by daphnetin treatment (Fig. 3). Furthermore, daphnetin was demonstrated to induce ERK activation, and ERK activation had no cumulative effect in PC12 cells stimulated with daphnetin and H2O2. Therefore, the present study hypothesized that ERK activation reached the maximum when PC12 cells were treated with daphnetin. These results suggest that daphnetin protects PC12 cells by suppressing p38 MAPK and JNK, and enhances ERK signaling.
HSP70 is induced in cells in response to a wide variety of chemical and physiological stresses, and its expression provides protection against cell death (10,19,20). A previous study also suggested that HSP70 is protective in nervous system diseases (21), and HSP70 protects against neuronal, apoptosis at least in part, by inhibiting caspase-dependent and caspase-independent programmed cell-death pathways (22). Therefore, the present study investigated the effect of daphnetin on the expression of HSP70 in PC12 cells. The present data revealed that daphnetin clearly upregulates HSP70 expression in a dose- and time-dependent manner, which reached its maximum at 12 h (Fig. 4A and B).
To further determine the possible signal transduction pathway involved in daphnetin-induced HSP70 expression, the present study investigated pro-survival signaling protein activation, including Akt and ERK, upon daphnetin treatment. The activation of ERK was enhanced time-dependently upon daphnetin stimulation (Fig. 5A). By contrast, Akt phosphorylation did not exhibit a significant difference compared with unstimulated PC12 cells. Furthermore, a specific inhibitor of ERK revealed that ERK significantly suppressed HSP70 protein expression (Fig. 5B). These data confirmed that ERK, but not Akt, signaling is involved in daphnetin-mediated HSP70 expression.
In conclusion, the present study elucidated that daphnetin protects PC12 cells against H2O2-induced apoptosis by suppressing p38 MAPK and JNK signaling activation and increasing HSP70 expression via ERK signaling. Overall, the present results suggest that daphnetin may be a potential candidate for the treatment of neurodegenerative diseases. However, daphnetin should be further tested in animal models mimicking neurodegenerative diseases prior to being considered as a candidate for a clinical trial to prevent neurodegenerative disease progression in humans.
Acknowledgements
The present study was supported by the Natural Science Research Project of Anhui Colleges and Universities (grant no. KJ2016SD59), Outstanding Young Talent Support Program Key Projects in Anhui Colleges and Universities (grant no. gxyqZD2016173), National Nature Science Foundation of China (grant no. 31301171), Natural Science Research Project of Anhui Provincial Education Department (grant no. KJ2013B311) and Anhui Province Key Laboratory of Active Biological Macro-molecules (grant no. 1306C083008).
Glossary
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- H2O2
hydrogen peroxide
- HSP
heat shock protein
References
- 1.Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: Possible role of hydrogen sulfide. J Alzheimers Dis. 2011;24(Suppl 2):S173–S182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 2.Pan LL, Liu XH, Jia YL, Wu D, Xiong QH, Gong QH, Wang Y, Zhu YZ. A novel compound derived from danshensu inhibits apoptosis via upregulation of heme oxygenase-1 expression in SH-SY5Y cells. Biochim Biophys Acta. 2013;1830:2861–2871. doi: 10.1016/j.bbagen.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Asaithambi A, Kanthasamy A, Saminathan H, Anantharam V, Kanthasamy AG. Protein kinase D1 (PKD1) activation mediates a compensatory protective response during early stages of oxidative stress-induced neuronal degeneration. Mol Neurodegener. 2011;6:43. doi: 10.1186/1750-1326-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karuppagounder SS, Pinto JT, Xu H, Chen H-L, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin W, Zhang X, Shi X, Li Y. Curcumin protects SH-SY5Y cells from oxidative stress by up-regulating HO-1 via Phosphatidylinositol 3 Kinase/Akt/Nrf-2 and down-regulating HO-2. Mol Neurodegener. 2012;7(Suppl 1):S14. doi: 10.1186/1750-1326-7-S1-S14. [DOI] [Google Scholar]
- 7.Zhang L, Ding W, Sun H, Zhou Q, Huang J, Li X, Xie Y, Chen J. Salidroside protects PC12 cells from MPP+-induced apoptosis via activation of the PI3K/Akt pathway. Food Chem Toxicol. 2012;50:2591–2597. doi: 10.1016/j.fct.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang X, Yu T. Effect of Daphnetin on the expression of β-APP after the ischemia in the front brain of the mouse. Zhonghua Naoxueguanbing Zazhi. 2012;6 (In Chinese) [Google Scholar]
- 9.Kuang NZ, Fu YY, Gong SQ, Zeng ZP, Zhang ZQ, Chen ZY. Effects of Daphnetin extract on the expression of Bcl-2 and Bax mRNA during the apoptosis of SMMC-7721 cells. Guangdong Yiyao Zazhi. 2012;33:1374–1377. (In Chinese) [Google Scholar]
- 10.Gao Y, Han C, Huang H, Xin Y, Xu Y, Luo L, Yin Z. Heat shock protein 70 together with its co-chaperone CHIP inhibits TNF-alpha induced apoptosis by promoting proteasomal degradation of apoptosis signal-regulating kinase1. Apoptosis. 2010;15:822–833. doi: 10.1007/s10495-010-0495-7. [DOI] [PubMed] [Google Scholar]
- 11.Sabirzhanov B, Stoica BA, Hanscom M, Piao CS, Faden AI. Over-expression of HSP70 attenuates caspase-dependent and caspase-independent pathways and inhibits neuronal apoptosis. J Neurochem. 2012;123:542–554. doi: 10.1111/j.1471-4159.2012.07927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow AM, Tang DW, Hanif A, Brown IR. Induction of heat shock proteins in cerebral cortical cultures by celastrol. Cell Stress Chaperones. 2013;18:155–160. doi: 10.1007/s12192-012-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kou X, Shen K, An Y, Qi S, Dai WX, Yin Z. Ampelopsin inhibits H2O2-induced apoptosis by ERK and Akt signaling pathways and up-regulation of heme oxygenase-1. Phytother Res. 2012;26:988–994. doi: 10.1002/ptr.3671. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li CF, Pan LM, Gao ZL. 7,8-Dihydroxycoumarin inhibits A549 human lung adenocarcinoma cell proliferation by inducing apoptosis via suppression of Akt/NF-kB signaling. Exp Ther Med. 2013;5:1770–1774. doi: 10.3892/etm.2013.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du JS, Zhao Q, Zhang YL, Wang Y, Ma M. 7,8-dihydroxycoumarin may promote sciatic nerve regeneration by suppressing NF-kB expression in mice. Mol Med Rep. 2013;8:1525–1530. doi: 10.3892/mmr.2013.1682. [DOI] [PubMed] [Google Scholar]
- 16.Du G, Tu H, Li X, Pei A, Chen J, Miao Z, Li J, Wang C, Xie H, Xu X, Zhao H. Daphnetin, a natural coumarin derivative, provides the neuroprotection against glutamate-induced toxicity in HT22 cells and ischemic brain injury. Neurochem Res. 2014;39:269–275. doi: 10.1007/s11064-013-1218-6. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Yang Q, Zhang K, Li YJ, Wu YM, Liu SB, Zheng LH, Zhao MG. Neuroprotective effects of daphnetin against NMDA receptor-mediated excitotoxicity. Molecules. 2014;19:14542–14555. doi: 10.3390/molecules190914542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu S, Shen Y, Liu J, Ding F. Involvement of ERK1/2 pathway in neuroprotection by salidroside against hydrogen peroxide-induced apoptotic cell death. J Mol Neurosci. 2010;40:321–331. doi: 10.1007/s12031-009-9292-6. [DOI] [PubMed] [Google Scholar]
- 19.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Han C, Huang H, Xin Y, Xu Y, Luo L, Yin Z. Heat shock protein 70 together with its co-chaperone CHIP inhibits TNF-alpha induced apoptosis by promoting proteasomal degradation of apoptosis signal-regulating kinase1. Apoptosis. 2010;15:822–833. doi: 10.1007/s10495-010-0495-7. [DOI] [PubMed] [Google Scholar]
- 21.Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int. 2011;2011:618127. doi: 10.1155/2011/618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabirzhanov B, Stoica BA, Hanscom M, Piao CS, Faden AI. Over-expression of HSP70 attenuates caspase-dependent and caspase-independent pathways and inhibits neuronal apoptosis. J Neurochem. 2012;123:542–554. doi: 10.1111/j.1471-4159.2012.07927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]