Abstract
Background
There is controversy in medical literature over the outcome of patients with lupus nephritis (LN) class II. The aim of this study was to explore the risk of histological transformation (HT) and possible factors related to negative response to treatment in patients with mesangial LN class II.
Methods
A retrospective and multicenter study was carried out that includes patients who had received a diagnosis of LN class II on their first renal biopsy. Creatinine, urine sediment, and proteinuria were recorded at the time of the first biopsy, 6 months, and 1, 2, and 5 years after the first biopsy. Response to treatment, HT, and long-term outcome were evaluated.
Results
Forty-one patients were included. The manifestation at first biopsy was proteinuria greater than 0.5 g/d in 28 patients (68.29%; 8 [28.57%] of 28 patients had nephrotic syndrome), hematuria in 18 patients (43.90%), and deterioration of renal function in 3 patients (7.31%). During the follow-up (median, 8 years; range, 1–35 years), a new biopsy was performed in 18 patients (43.90%), and in 17 patients (17/18 [94.44%]), there was HT. Median time at rebiopsy was 32 months (range, 11–305 months). Of the 18 patients who had a second biopsy, 10 (55.55%) were on hydroxychloroquine versus 100% (19/19) of patients who did not undergo the procedure (P = 0.001). A year after the first renal biopsy, there are data available from 34 patients; of them, 24 patients (70.58%) had achieved response, and 10 patients (29.41%) had no response (NR) (missing data in 7). A higher 24-hour urinary protein at 6 months was predictor of worse outcome at 1 year, with statistical significance difference for the nonresponder group (median proteinuria, 2.3 g/d [range, 0–4.7 g/d]) compared with responders (median proteinuria, 0.28 g/d [range, 0–1.7 g/d]) (P = 0.0133).
In the long-term follow-up (5 years), HT was the main cause of unfavorable outcome and was measured in 78.57% of patients (11/14 patients).
Conclusions
This series shows a high rate of HT in long-term follow-up. Proteinuria at 6 months made it possible to set aside patients who will have an unfavorable outcome in the long term and who will thus benefit from a more aggressive treatment. The results suggest that hydroxychloroquine had a nephroprotective effect.
Key Words: histological transformation, long-term outcome, lupus nephritis class II, mesangial proliferative lupus nephritis
Renal involvement due to systemic lupus erythematosus (SLE) is frequent; it has been reported that more than half of patients with SLE will develop lupus nephritis (LN), which is a strong predictor of morbidity and mortality.1 Mesangial LN (class II) accounts for 4.9% to 22% of LN cases.2–5 Reports have generally shown a favorable outcome for LN class II.5–7 However, in recent years, series of cases of LN class II have been published that suggest otherwise. Tam et al.2 studied 19 patients, 10 of whom required a second biopsy, and 9 presented histological transformation (HT). Lee et al.8 evaluated 15 patients, 5 of whom required a second biopsy, and all of them presented transformation into histological class III or IV. Arévalo-Martinez et al.9 have evaluated 20 patients, 10 of whom had a second biopsy, and 5 presented HT. Rubio-Rivas et al.10 evaluated 27 patients; 5 required a second biopsy, and 4 presented HT. We were unable to find studies about LN class II in relation to the Argentine population.
The goal was to explore the risk of HT into proliferative and membranous classes and possible factors related to negative response to treatment in patients with mesangial LN (class II). For this purpose, a multicenter study was carried out in Argentina.
PATIENTS AND METHODS
A multicenter, retrospective study was performed. Histology and medical records were reviewed, and data were collected from patients who met the 1997 revised American College Rheumatology classification criteria for SLE11 and who had a biopsy-proven mesangial LN in accordance with the classification criteria of the World Health Organization 1982 classification12 and/or the International Society of Nephrology/Renal Pathology Society 2003 classification13 in their first renal biopsy performed between 1975 and 2014. Patients with less than 6 months’ follow-up after biopsy were excluded from the study.
The biopsies were evaluated by pathologists with experience in LN. Biopsies performed during the period 1975–1982 were reviewed and classified according to the International Society of Nephrology/Renal Pathology Society classification.14 There were no histological findings compatible with the antiphospholipid syndrome in any of the biopsies.
The following data at the time of the first biopsy were analyzed: demographic data: patient’s age at SLE diagnosis, sex, and ethnic group (in accordance with the Grupo Latinoamericano de Estudio del Lupus classification)15; and clinical data: clinical presentation of the nephropathy and cause for biopsy (proteinuria/hematuria/deterioration of renal function). It was considered that there was deterioration of renal function when the values were greater than the highest value of the detection method range. Time between SLE diagnosis and first signs of nephropathy, time between SLE diagnosis and the first renal biopsy, and time between the first signs of nephropathy and the first renal biopsy were recorded: Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC) (SLICC basal)15,16; comorbidities: hypertension, diabetes, dyslipidemia, and antiphospholipid syndrome; and immunology laboratory: anti–double-stranded DNA, anticardiolipin antibodies, and complement (C3 and C4) levels. It was considered that there was hypocomplementemia when the result was less than these method reference levels. For the remaining parameters, measurement results were considered positive if the corresponding antibody was present according to each detection method.
Renal laboratory data (creatinine, urine sediment, and proteinuria) were registered at various time points including the time of the first biopsy; at 6 months; at 1, 2, and 5 years; and once every 5 years after the first renal biopsy.
The SLICC was analyzed at the last evaluation or at the time of documented transformation in HT patients (SLICC final).
Treatments carried out at first renal biopsy and after it were recorded: nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), hydroxychloroquine (HCQ), glucocorticoids, immunosuppressants (ISs), and dialysis.
Patients were classified according to their response to treatment at various time points including 6 months, 1 year, 2 years, and 5 years after the first biopsy. Taking into account the American College of Rheumatology renal response criteria,17 the following categories were defined: (1) complete response (CR): inactive sediment, proteinuria less than 0.2 g/d, normal creatinine; (2) partial response (PR): inactive sediment, proteinuria greater than 0.2 g/d and less than 2 g/d, a rise in creatinine levels of not more than 25% from the basal level; and (3) NR: active sediment, proteinuria greater than 2 g/d, a rise in creatinine of more than 25% from the basal level. Patients were considered responders when they presented CR or PR criteria and nonresponders when they met NR criteria.
The following data were collected from patients who underwent more than 1 biopsy: number of biopsies; time between the first biopsy and a rebiopsy; HT into classes III, IV, or V in the repeated biopsy; and reason reported by the doctors treating each patient for repeated biopsy including (1) flare: a flare occurs when patients having reached PR or CR were NR in a subsequent evaluation and (2) persistent nonresponders: defined as patients who never achieved CR or PR.
Outcome was considered favorable if the patient remained a CR or a PR at the last evaluation and unfavorable if the patient remained a nonresponder at the last evaluation, presented end-stage renal disease or HT at any time during the follow-up, or died.
Statistical Analysis
Continuous variables are expressed as mean (SD) or median and range, as appropriate. Categorical variables are expressed as numbers and percentages (%). The characteristics of patients in the 2 groups were compared using t test for independent data, Mann-Whitney U test, or Fisher exact test. Statistical analysis was performed with the STATA 11.0 package (StataCorp, College Station, TX).
RESULTS
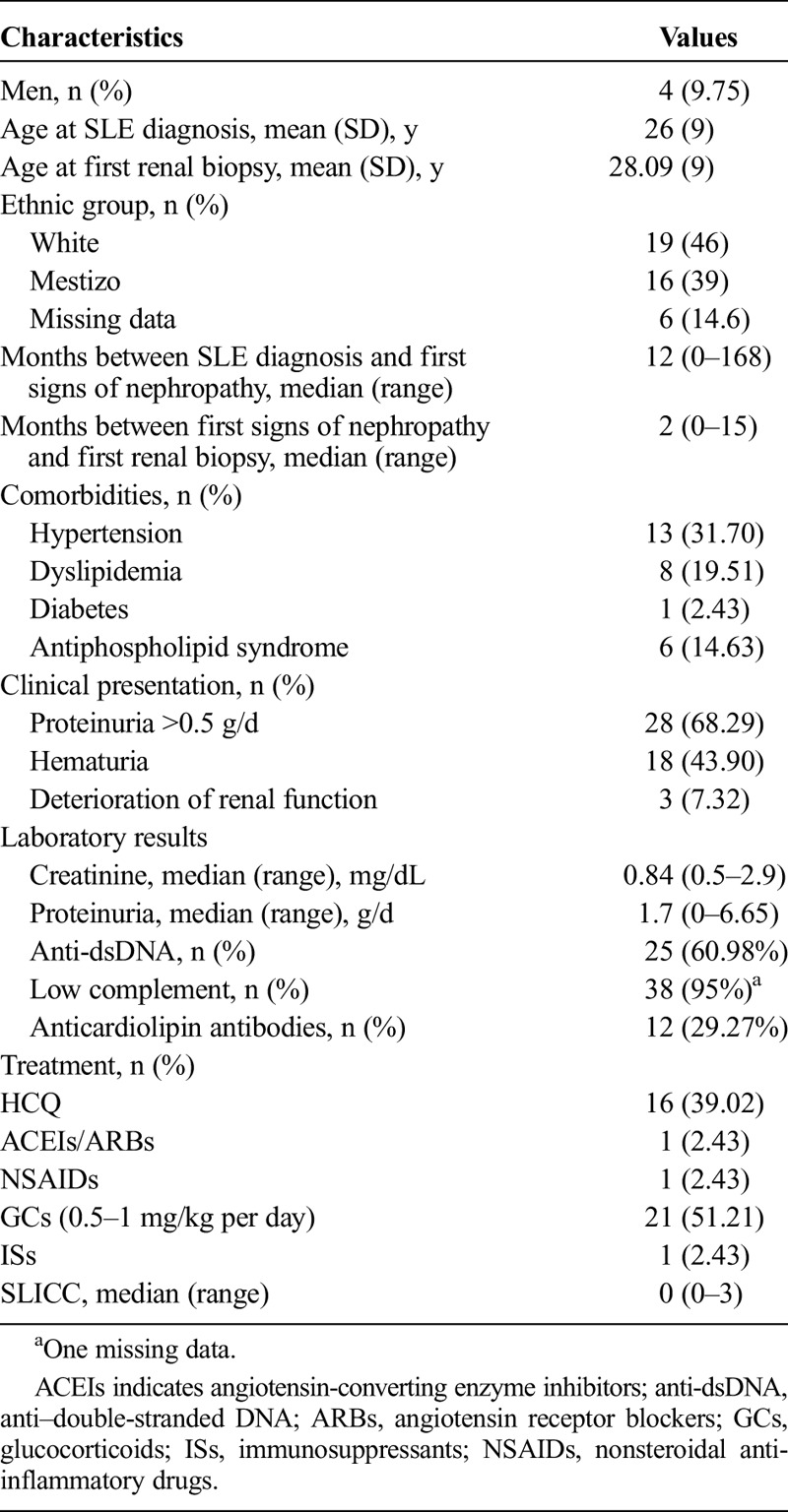
Data from 41 patients with LN class II confirmed by a first renal biopsy between 1975 and 2013 were reviewed. The median time between first signs of nephropathy and first renal biopsy was 2 months (range, 0–15 months). The main manifestation at first biopsy was proteinuria greater than 0.5 g/d in 28 patients (68.29%), including, of 28, 8 patients (28.57%) with nephrotic syndrome. The median creatinine level at the time of the first biopsy was 0.84 mg/dL (range, 0.5–2.9 mg/dL), and the median proteinuria level was 1.7 g/d (range, 0–6.65 g/d). Of 41 patients, 25 (60.98%) showed positive anti-dsDNA, and 95% (38/40) showed hypocomplementemia (1 with missing data). Of 41 patients, 16 (39.02%) were being treated with HCQ at the time of the first renal biopsy, 51.21% (21/41) with corticosteroids and 2.43% (1/41) with ISs (cyclophosphamide for neuropsychiatric manifestation). The details of the sample are shown in Table 1.
TABLE 1.
Description of the Sample of 41 Patients With LN Class II, at the Moment of the First Renal Biopsy
Rebiopsies
Within a median of 8 years (range, 1–35 years) of follow-up after the first renal biopsy, 18 (43.9%) of 41 patients had a subsequent biopsy performed. The reasons for a rebiopsy were a renal flare in 16 patients and persistently no response in 2 patients. Three patients who required a rebiopsy (2 because of renal flare and 1 because of persistently no response) did not undergo the procedure. The remaining 20 patients did not meet the rebiopsy criteria. The median time between the first and second biopsy was 40 months (range, 11–120 months). In the 18 patients who had a second biopsy, the median age was 27.5 years (range, 15–46 years), the median creatinine level was 1.06 mg/dL (range, 0.69–1.9 mg/dL), and the median level of proteinuria was 3.08 g/d (range, 0–8 g/d) at the time of the second biopsy.
Of the 18 patients who required and had a second biopsy performed, 2 patients remained in class II; we missed the follow-up of 1 patient, and the other presented a renal flare that required a third biopsy 21 years after the second biopsy (LN class IV).
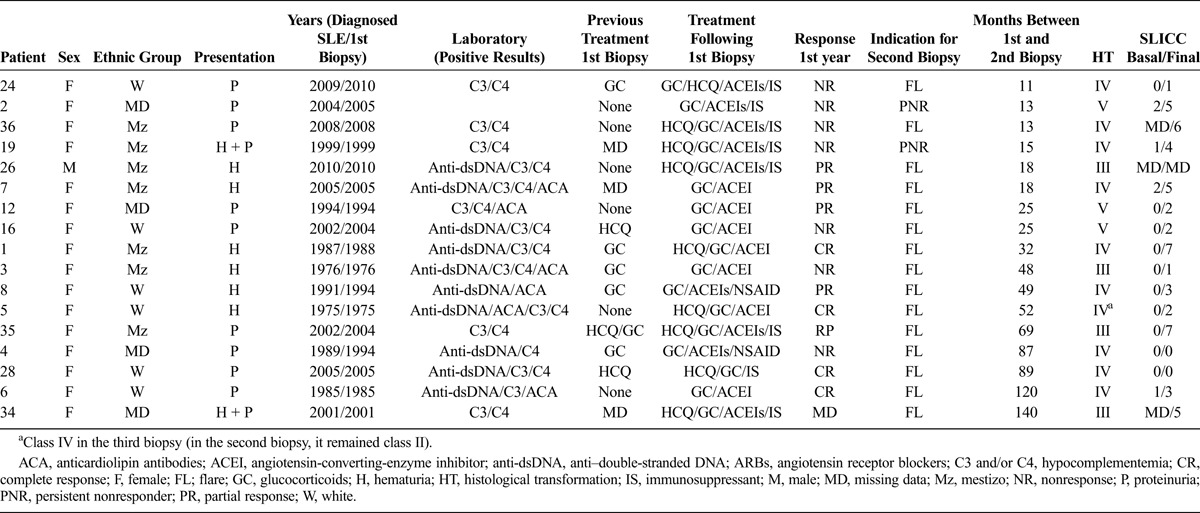
Of the 18 patients who had a second biopsy, 17 showed HT. The most frequent HT was class IV (10/17 = 58.82%), 4 (23.52%) progressed into class III, and 3 (17.64%) into class V. Table 2 displays data for patients who presented HT. The median time to HT was 32 months (range, 11–305 months).
TABLE 2.
Information About the 17 Patients With LN Class II Who Showed Histological Transformation
Comparison of Patients Who Had a Subsequent Biopsy Versus Patients Who Did Not Undergo This Procedure
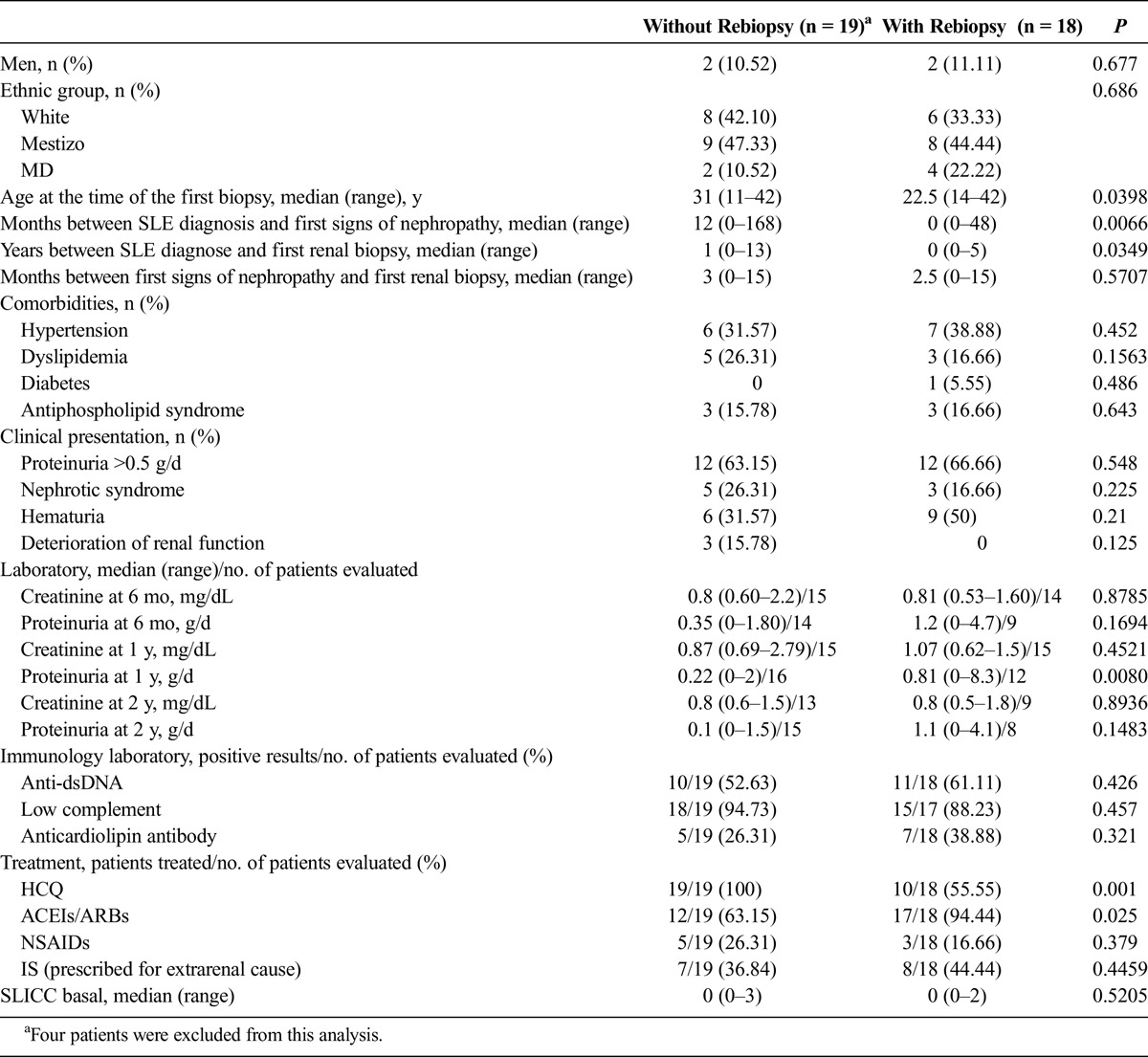
The group of patients who had a subsequent biopsy was compared with the group of patients who did not undergo this procedure. Because in this series of cases the first subsequent biopsy was performed at 11 months, patients with less than a year of follow-up (4 patients) were excluded from this analysis. The median follow-up time from SLE diagnosis to the second biopsy was 4 years (range, 1–12 years) among the 18 patients who underwent subsequent biopsies, and the median follow-up time from SLE diagnosis to the last evaluation was 8 years (range, 4–23 years) in the 19 patients who did not undergo subsequent biopsies. The patients in the group that underwent subsequent biopsies were younger at the time the disease was diagnosed and showed a significantly shorter median time between SLE diagnosis and the first biopsy versus patients who did not undergo subsequent biopsies. The group that did not have a second biopsy showed a significantly higher median proteinuria level at 1 year versus the group that did not have a second biopsy (0.81 g/d [range, 0–8.3 g/d] vs 0.22 g/d [range, 0–2 g/d]; P = 0.0080). All patients who did not have a second biopsy were treated with HCQ after the first biopsy, contrary to the 55.55% (10/18) of patients who had a second biopsy (P = 0.001). Twelve patients (63.15%) of the total of 19 patients who did not have a second biopsy and 17 (94.44%) patients of a total of 18 patients who underwent the procedure were treated with ACEIs/ARBs (P = 0.025). Of the 8 patients who were treated with HCQ but were not on ACEIs/ARBs, 1 patient required a rebiopsy. All 8 patients treated with ACEIs/ARBs but not treated with HCQ required a rebiopsy. Of 21 patients who were on both treatments, 9 required rebiopsy. There was no difference between the 2 groups regarding treatment with corticosteroids or ISs. Comparative data for these groups are shown on Table 3.
TABLE 3.
Comparison Between Patients Who Did Not Have a Rebiopsy and Those Who Had a Rebiopsy
Response to Treatment
Response to treatment was evaluated at 6 months, 1 year, 2 years, and 5 years after the first renal biopsy. A hundred percent of the patients were treated with corticosteroids (range, 0.5–1 mg/kg per day) after their first biopsy. Fifteen patients were started on ISs (4 patients were treated with azathioprine, 6 with cyclophosphamide/azathioprine, 2 with cyclophosphamide, 2 with cyclophosphamide/cyclosporine, 1 with azathioprine/mycophenolate) prescribed for extrarenal causes: serositis (n = 3), immune thrombocytopenic purpura (n = 2), neuropsychiatric manifestation (n = 3), hemolytic anemia (n = 2), pericarditis (n = 1), vasculitis (n = 3), and severe myositis (n = 1). After the first biopsy, 20 (83.33%) of 24 patients from the responder group and 5 (50%) of 10 patients from the nonresponder group (P = 0.03428) were being treated with HCQ, whereas 17 (70.83%) of 24 patients from the responder group and 10 (100%) of 10 patients from the nonresponder group (P = 0.03217) were being treated with ACEIs/ARBs.
A patient who presented deterioration of renal function as a form of presentation before the first renal biopsy required dialysis as from the first year of follow-up.
Six months after the first biopsy, there are data available from 37 patients: 9 (24.32%) CR, 19 (51.35%) PR, and 9 (24.32%) NR; at 1 year, of 34 evaluated patients, 10 patients (29.41%) were rated as CR, 14 (41.17%) as PR, and 10 (29.41%) as NR; after 2 years of the first biopsy, of 34 patients, 14 patients (41.17%) were CR, 11 (26.47%) were PR, and 9 (41.17%) were NR; after 5 years of the first biopsy, of 33 patients evaluated, 12 patients (36.36%) were rated as CR, 7 (21.21%) as PR, and 14 (42.42%) as NR. The reason for nonresponse was HT in 1 (10%) of 10 patients after 1 year of follow-up, 6 (66.66%) of 9 patients after 2 years, and 11 (78.57%) of 14 patients after 5 years.
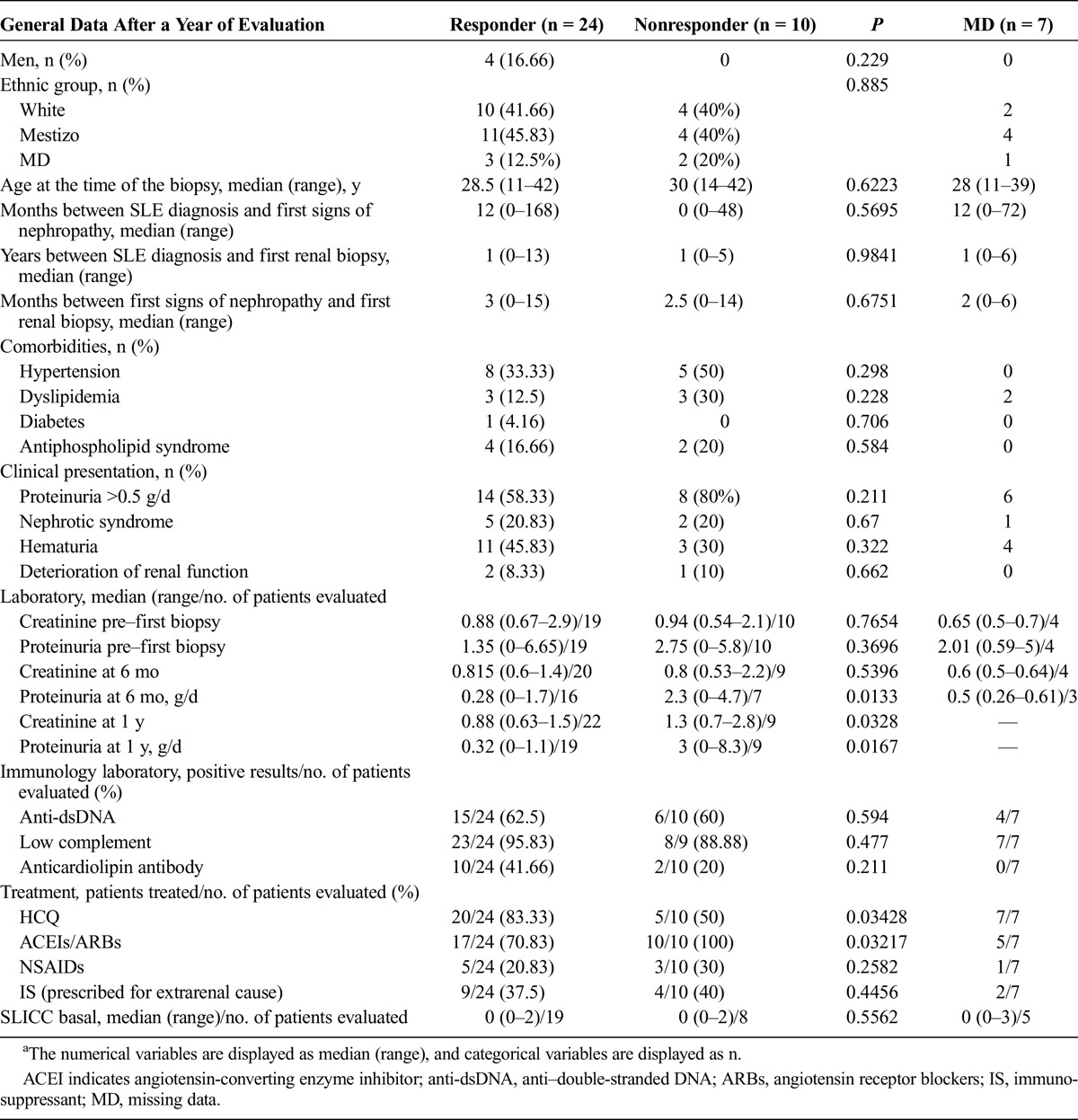
Table 4 shows comparative results of groups defined according to response to treatment at 1 year after the first biopsy. When comparing nonresponder group versus responder group after 1 year of follow-up, it was observed that already at a follow-up time of 6 months proteinuria levels were higher in the nonresponder group.
TABLE 4.
General Data of Patients With LN Class II Over 1 Year of Follow-up From the First Biopsy (Comparison Between Responders and Nonresponders to Treatment)
At 5 years after the first biopsy, there were data available from 33 patients; the outcome was favorable for 19 patients and unfavorable for 14 patients. No patients died.
Although there were few patients with complete data at 5 years from the first biopsy, upon comparison, responder and nonresponder groups showed similar values except for proteinuria values. It was possible to have 5 years of follow-up after the first biopsy of 14 nonresponder patients and 19 responder patients. Already at 6 months after the first biopsy, the median levels of proteinuria were higher in nonresponders (8 patients) versus responders (12 patients) (13 missing data) with a 24-hour proteinuria median level of 2.4 g/d (range, 0–4.7 g/d) versus 0.35 g/d (range, 0–1.8 g/d) (P = 0.01), respectively. One year after the first biopsy, the median levels of proteinuria were also higher in nonresponders (11 patients) versus responders (14 patients) (8 missing data) with a 24-hour proteinuria median level of 0.87 g/d (range, 0.17–8.3 g/d) versus 0.26 g/d (range, 0–3.4 g/d) (P = 0.01), respectively.
The same comparison carried out 2 years after the first biopsy (data not shown) rendered results similar to those obtained 1 and 5 years after the first biopsy.
DISCUSSION
There are a limited number of publications on the long-term progress of patients with LN class II.2,8–10 This series of Argentine patients is, to the best of our knowledge, the broadest case series published to date.
Although mesangial nephropathy is considered a benign variant of LN,5–7 this study shows that a rebiopsy was advised for 51.21% of patients with LN class II histology, and of the 18 patients who had a second biopsy, 94.44% showed HT, this being the most frequent cause of an unfavorable outcome at 5 years.
Systemic lupus erythematosus is the paradigm of autoimmune disease mediated by immune complexes; for this reason, histological progression of LN class II into more severe classes could be accounted for the basis of Koffler and colleagues’18 hypothesis, which proposed that LN is secondary to the deposition of immune complexes and complement, first in the mesangium and, when the phagocytic capacity of mesangial cells is exhausted, in the subendothelium along the glomerular basement membrane.
The HT frequency reported by other authors varies between 14.81% and 47.4%,2,8–10 and the median time at rebiopsy from 33 to 58 months.2,8,10 These data are consistent with those published for this series of cases. Because most subsequent biopsies were performed close to 3 years from the first biopsy, HT is considered a late event. This could be explained if HT is understood as a consequence of different stages along a continuum of increasingly advanced renal disease that begins in the mesangium and progresses to more severe classes of nephritis, as stated by Ginzler et al.19
Consistently with Tam et al.,2 who reported 52.6% of unfavorable outcome in a series of Chinese patients after 4.8 years of follow-up, in this series of cases it was possible to determine 42.42% of unfavorable outcome at 5 years. It is difficult to account for the determinants of a favorable or an unfavorable outcome in patients’ progress in the long term, something also noted by other authors.2,7 In patients with mild renal manifestations, Cruchaud20 observed that biopsies showed the existence of scarce histological lesions but significant deposits of immune complexes and proposed then that in these cases renal damage results from complex pathogenic mechanisms with possible interaction of factors related to the potential nephrotoxic capacity of immune complexes and factors protecting the host.
It is well known that the ethnic group has an influence on the prognosis of renal involvement due to lupus21–23 with a higher risk to develop LN and with a worse outcome in the mestizo ethnic group than in the white ethnic group.24,25 Thirty-nine percent of the patients in this study were mestizo including 7 of the 17 patients who presented HT. No significant differences were found regarding response or HT between mestizo and white ethnic groups, although the conclusion that this is due to the size of the sample cannot be dismissed.
Consistently with other published articles, where it was observed that clinical presentation of this histological class can be other than mild,2,9,26 in this series of 41 cases, the manifestation at first biopsy was proteinuria greater than 0.5 g/d in 28 patients and deterioration of renal function in 3 patients. Because the clinical presentation of mesangial nephritis can be identical to the more aggressive classes, it is important to carry out the biopsy because it is the only parameter that can differentiate one from the others.27,28
Nephrotic syndrome is frequently associated with proliferative and membranous classes, but not with class II; however, several clinical cases have been reported of patients with mesangial LN with this syndrome.7,26,29 Possible explanations for the occurrence of the nephrotic syndrome as clinical manifestation at first biopsy in this series would be the following: treatment with nonsteroidal antiinflammatory drugs, which is associated to the minimal change disease,30 the association between mesangial nephropathy and minimal change nephropathy,26,31 or the production by the mesangial cells of inflammatory mediators, which could increase the glomerular basal membrane permeability, as suggested by Stankeviciute et al.26 It is not possible to assert which was the pathogenic mechanism for the nephrotic syndrome in this series because only 1 patient was being treated with nonsteroidal antiinflammatory drugs at the time of the first biopsy, and because of the lack of electron microscopy, it is not possible to rule out minimal change disease.
All patients were treated with corticosteroids at a dosage of 0.5 to 1 mg/kg per day after the first biopsy, and after a year, 70% of the patients were responders. At 5 years of follow-up after the first biopsy, 42.42% of patients were nonresponders, and of the 14 nonresponder patients, 11 showed HT. When comparing the nonresponder group versus the responder group at 5 years’ follow-up, it was observed that the median proteinuria levels at 6 months were significantly higher in the nonresponder group. This suggests that proteinuria levels at 6 months could be a predictor of unfavorable outcome in the long term. This is consistent with what has been put forward in 2 articles about predictors of long-term renal outcome for patients with proliferative lupus glomerulonephritis, in the Euro-Lupus Nephritis Cohort; in these articles, it was shown that the best predictor of a good long-term renal outcome is the decrease in proteinuria levels to less than 1 g/d after 6 months32 and to less than 0.8 g/d after 12 months,33 since the beginning of a treatment with low-dosage cyclophosphamide. Besides this, the study published by Yang et al.,3 which includes 1814 patients, of whom 127 were of class II, showed that the persistence of proteinuria with values greater than 0.5 g/d is a risk factor for evolution into end-stage renal disease.
There is not much evidence as regards the treatment that LN class II patients must receive. According to the American College Rheumatology 2012 guidelines,34 this histological class does not generally require immunosuppressive treatment (evidence level C). Patients were treated with corticosteroids at a dosage higher than the recommended one by the European League Against Rheumatism group,35 and they showed good response in the short term, but this treatment could not avoid the long-term unfavorable outcome in almost half of the patients. Then, for nonresponder patients with proteinuria levels higher than 1 g/d at 6 months, it should be advisable to consider a combined treatment with corticosteroids and azathioprine, as was suggested by the European League Against Rheumatism group.35
The use of HCQ is recommended for all patients who received a diagnosis of SLE.36,37 All the patients who did not undergo a rebiopsy were treated with HCQ after the first renal biopsy, whereas only 55.55% of the 18 patients who had a rebiopsy were treated with this drug, which corroborates the nephroprotective effect of this treatment demonstrated in previous publications.38,39
This study presents limitations inherent to its retrospective design, such as the unavailability of data, the impossibility of carrying out complementary studies such as electron microscopy techniques, and the size of the sample. It is necessary to carry out prospective studies and with a higher number of patients to strengthen the findings of this study.
CONCLUSIONS
In this study, it is possible to observe a high rate of HT in the long-term follow-up. Proteinuria levels at 6 months made it possible to identify patients who will have a long-term unfavorable outcome and who, because of that, will benefit from a more aggressive treatment. The results suggest that HCQ presented a nephroprotective effect.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–424. [DOI] [PubMed] [Google Scholar]
- 2.Tam LS, Li EK, Lai FM, et al. Mesangial lupus nephritis in Chinese is associated with a high rate of transformation to higher grade nephritis. Lupus. 2003;12:665–671. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Liang D, Zhang H, et al. Long-term renal outcomes in a cohort of 1814 Chinese patients with biopsy-proven lupus nephritis. Lupus. 2015;24:1468–1478. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama H, Okuyama H, Yamaya H. Clinicopathological insights into lupus glomerulonephritis in Japanese and Asians. Clin Exp Nephrol. 2011;15:321–330. [DOI] [PubMed] [Google Scholar]
- 5.Houng DL, Papo T, Beaufils H, et al. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine (Baltimore). 1999;78:148–166. [DOI] [PubMed] [Google Scholar]
- 6.Appel GB, Cohen DJ, Pirani CL, et al. Long-term follow-up of patients with lupus nephritis. A study based on the classification of the World Health Organization. Am J Med. 1987;83:877–885. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin DS, Gluck MC, Lowenstein J, et al. Lupus nephritis. clinical course as related to morphologic forms and their transitions. Am J Med. 1977;62:12–30. [DOI] [PubMed] [Google Scholar]
- 8.Lee SG, Cho YM, So MW, et al. ISN/RPS 2003 class II mesangial proliferative lupus nephritis: a comparison between cases that progressed to class III or IV and cases that did not. Rheumatol Int. 2012;32:2459–2464. [DOI] [PubMed] [Google Scholar]
- 9.Arévalo-Martínez FG, Andrade-Ortega L, Irazoque-Palazuelos F, et al. Presentación atípica y evolución clínica de la nefropatía lúpica mesangial. Estudio de 20 pacientes. Reumatol Clin. 2006;2:4–9. [DOI] [PubMed] [Google Scholar]
- 10.Rubio-Rivas M, Gómez-Junyent J, Simonetti A, et al. Proliferative mesangial lupus nephritis: description of a cohort of 27 patients. Med Clin (Barc). 2012;139:341–345. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 12.Churg J, Sobin LH. Renal Disease: Classification and Atlas of Glomerular Disease. Igaku-Shoin: Tokyo, Japan; 1982. [Google Scholar]
- 13.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. [DOI] [PubMed] [Google Scholar]
- 14.Pons-Estel B, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore). 2004;83:1–17. [DOI] [PubMed] [Google Scholar]
- 15.Gladman DD, Ginzler E, Goldsmith CH, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. [DOI] [PubMed] [Google Scholar]
- 16.Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–813. [DOI] [PubMed] [Google Scholar]
- 17.Gordon C, Jayne D, Pusey C, et al. European consensus statement on the terminology used in the management of lupus glomerulonephritis. Lupus. 2009;18:257–263. [DOI] [PubMed] [Google Scholar]
- 18.Koffler D, Agnello V, Thoburn R, et al. Systemic lupus erythematosus: prototype of immune complex nephritis in man. J Exp Med. 1971;134:169–179. [PMC free article] [PubMed] [Google Scholar]
- 19.Ginzler EM, Nicastri AD, Chen CK, et al. Progression of mesangial and focal to diffuse lupus nephritis. N Engl J Med. 1974;291:693–696. [DOI] [PubMed] [Google Scholar]
- 20.Cruchaud A, Chenais F, Fournié GJ. Immune complex deposits in systemic lupus erythematosus kidney without histological or functional alterations. Eur J Clin Invest. 1975;5:297–309. [DOI] [PubMed] [Google Scholar]
- 21.Bastian HM, Roseman JM, McGwin G., Jr Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–160. [DOI] [PubMed] [Google Scholar]
- 22.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney International. 2006;69:1846–1851. [DOI] [PubMed] [Google Scholar]
- 23.Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons- Estel GJ, Alarcón GS, Hachuel S, et al. Anti-malarials exert a protective effect while Mestizo patients are at increased risk of developing SLE renal disease: data from a Latin-American cohort. Rheumatology (Oxford). 2012;51:1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pons-Estel GJ, Catoggio LJ, Cardiel MH, et al. Lupus in Latin-American patients: lessons from the GLADEL cohort. Lupus. 2015;24:536–545. [DOI] [PubMed] [Google Scholar]
- 26.Stankeviciute N, Wellington J, Bakir A, et al. Mesangial lupus nephritis with associated nephrotic syndrome. J Am Soc Nephrol. 1997;8:1199–1204. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh YP, Wen YK, Chen ML. The value of early renal biopsy in systemic lupus erythematosus patients presenting with renal involvement. Clin Nephrol. 2012;77:18–24. [DOI] [PubMed] [Google Scholar]
- 28.Rovin BH, Parikh S, Alvarado A. The kidney biopsy in lupus nephritis: is it still relevant? Rheum Dis Clin North Am. 2014;40:537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe T. Nephrotic syndrome in mesangial proliferative lupus nephritis. Pediatr Int. 2007;49:1009–1011. [DOI] [PubMed] [Google Scholar]
- 30.Ravnskov U. Glomerular, tubular and interstitial nephritis associated with non-steroidal anti-inflammatory drugs. Evidence of a common mechanism. Br J Clin Pharmacol. 1999;47:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deji N, Sugimoto T, Kanasaki M, et al. Emerging minimal-change nephrotic syndrome in a patient with chronic mesangial proliferative lupus nephritis. Intern Med. 2007;46:991–995. [DOI] [PubMed] [Google Scholar]
- 32.Housseau F, Vasconcelos C, D’Cruz D, et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum. 2004;50:3934–3940. [DOI] [PubMed] [Google Scholar]
- 33.Dall’Era M, Cisternas M, Smilek D, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis Cohort. Arthritis Rheum. 2015;67:1305–1313. [DOI] [PubMed] [Google Scholar]
- 34.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res(Hoboken). 2012;64:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertsias GK, Tektonidou M, Amoura Z, et al. Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and pediatric lupus nephritis. Ann Rheum Dis. 2012;71:1772–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bykerk V, Sampalis J, Esdaili JM, et al. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med. 1991;324:150–154. [DOI] [PubMed] [Google Scholar]
- 37.Shinjo SK, Bonfá E, Wojdyla D, et al. Antimalarial treatment may have a time-dependent effect on lupus survival. Arthritis Rheum. 2010;62:855–862. [DOI] [PubMed] [Google Scholar]
- 38.Sisó A, Ramos-Casals M, Bové A, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus. 2008;17:281–288. [DOI] [PubMed] [Google Scholar]
- 39.Pons-Estel GJ, Alarcon GS, Burgos PI, et al. Mestizos with systemic lupus erythematosus develop renal disease early while antimalarials retard its appearance: data from a Latin American cohort. Lupus. 2013;22:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]


