Abstract
Objectives:
A systematic review of the literature and meta-analysis was conducted to assess the nature and quality of the evidence for the use of hearing instruments in adults with a unilateral severe to profound sensorineural hearing loss.
Design:
The PubMed, EMBASE, MEDLINE, Cochrane, CINAHL, and DARE databases were searched with no restrictions on language. The search included articles from the start of each database until February 11, 2015. Studies were included that (a) assessed the impact of any form of hearing instrument, including devices that reroute signals between the ears or restore aspects of hearing to a deaf ear, in adults with a sensorineural severe to profound loss in one ear and normal or near-normal hearing in the other ear; (b) compared different devices or compared a device with placebo or the unaided condition; (c) measured outcomes in terms of speech perception, spatial listening, or quality of life; (d) were prospective controlled or observational studies. Studies that met prospectively defined criteria were subjected to random effects meta-analyses.
Results:
Twenty-seven studies reported in 30 articles were included. The evidence was graded as low-to-moderate quality having been obtained primarily from observational before-after comparisons. The meta-analysis identified statistically significant benefits to speech perception in noise for devices that rerouted the speech signals of interest from the worse ear to the better ear using either air or bone conduction (mean benefit, 2.5 dB). However, these devices also degraded speech understanding significantly and to a similar extent (mean deficit, 3.1 dB) when noise was rerouted to the better ear. Data on the effects of cochlear implantation on speech perception could not be pooled as the prospectively defined criteria for meta-analysis were not met. Inconsistency in the assessment of outcomes relating to sound localization also precluded the synthesis of evidence across studies. Evidence for the relative efficacy of different devices was sparse but a statistically significant advantage was observed for rerouting speech signals using abutment-mounted bone conduction devices when compared with outcomes after preoperative trials of air conduction devices when speech and noise were colocated (mean benefit, 1.5 dB). Patients reported significant improvements in hearing-related quality of life with both rerouting devices and following cochlear implantation. Only two studies measured health-related quality of life and findings were inconclusive.
Conclusions:
Devices that reroute sounds from an ear with a severe to profound hearing loss to an ear with minimal hearing loss may improve speech perception in noise when signals of interest are located toward the impaired ear. However, the same device may also degrade speech perception as all signals are rerouted indiscriminately, including noise. Although the restoration of functional hearing in both ears through cochlear implantation could be expected to provide benefits to speech perception, the inability to synthesize evidence across existing studies means that such a conclusion cannot yet be made. For the same reason, it remains unclear whether cochlear implantation can improve the ability to localize sounds despite restoring bilateral input. Prospective controlled studies that measure outcomes consistently and control for selection and observation biases are required to improve the quality of the evidence for the provision of hearing instruments to patients with unilateral deafness and to support any future recommendations for the clinical management of these patients.
Keywords: Air conduction, Bone conduction, Cochlear implantation, Contralateral routing of signals, Localization, Meta-analysis, Quality of life, Re-routing devices, Restorative devices, Single-sided deafness, Speech perception, Systematic review, Unilateral deafness, Unilateral hearing loss
Adults with a unilateral severe-to-profound sensorineural hearing loss report difficulties with listening in many everyday situations. Current hearing instruments attempt to alleviate these difficulties either by rerouting sounds from the impaired ear to the non-impaired ear or by restoring hearing in the impaired ear. A systematic review and meta-analysis evaluated the evidence for their use. The evidence was of low-to-moderate quality. Meta-analyses found benefits to speech perception in noise and reductions in listening difficulty. Controlled trials are needed to provide higher-quality evidence for hearing instrument use in unilateral deafness and to support recommendations for the clinical management of these patients.
Supplemental Digital Content is available in the text.
Introduction
The onset of unilateral deafness in adulthood is often sudden and idiopathic (Baguley et al. 2006). Even a small asymmetry between the ears has the potential to impose an audiological handicap, particularly in situations with multiple people speaking at the same time (Noble & Gatehouse 2004). Consequently, the near or total loss of hearing in one ear gives rise to substantial difficulties with listening in most everyday situations (Dwyer et al. 2014). Unilateral deafness impairs the ability to understand speech in noise and to localize sounds and also limits awareness of sounds that are located on the side of the impaired ear (IE; McLeod et al. 2008). These difficulties and their consequences for social and vocational activities can lead to feelings of annoyance, embarrassment, and helplessness (Giolas & Wark 1967).
One approach to improve the awareness of sounds on the side of the IE is to reroute signals to the contralateral, nonimpaired ear. This contralateral routing of signals was first achieved by connecting a hearing aid microphone on the side of the IE to a hearing aid on the non-IE (Harford & Barry 1965; Harford & Dodds 1966). A similar result is now achieved via wireless communication between two behind-the-ear devices (Valente 1995). Due to limitations in the frequency response of early rerouting devices, an alternative approach was to fit a high-powered in-the-ear-canal hearing aid in the IE to stimulate the nonimpaired cochlea via conduction through the cranial bones (Valente et al. 1995). Candidacy for bone-anchored hearing devices that were originally developed for conductive or mixed losses has also been extended to include unilateral sensorineural deafness (Niparko et al. 2003). Recently, cochlear implantation (CI) has been considered for unilateral deafness, initially for suppressing tinnitus (Van de Heyning et al. 2008) but subsequently for the restoration of binaural hearing (Vermeire & Van de Heyning 2009).
Related Systematic Reviews
Baguley et al. (2006) compared outcomes with bone- and air conduction rerouting devices (BCD and ACD, respectively) in adults with a unilateral sensorineural deafness. A meta-analysis of four studies suggested that BCD may provide additional benefits over ACD by improving speech perception in noise and reducing self-reported listening difficulties. Neither device improved the ability to localize sounds. Peters et al. (2015) also compared the evidence for ACD and BCD. Six studies provided evidence for a reduction in self-reported difficulties from the use of rerouting devices. A clear advantage for either conduction modality was not observed. Neither review included studies evaluating the effectiveness of devices in one conduction modality only compared with the unaided condition. No systematic review was identified whose stated purpose was to review the evidence for rerouting devices compared with the unaided condition. Both van Zon et al. (2015) and Blasco and Redleaf (2014) reviewed the evidence for CI in adults with unilateral deafness. Both reviews identified some benefits to sound localization, listening difficulties, and quality of life after CI.
Comparing the evidence for the different interventions (ACD, BCD, and CI) across these reviews is restricted by differences in their inclusion criteria, limitations on the language of included articles, quality assessment procedures, and methodologies for synthesizing evidence. No previous review has permitted the inclusion of studies that assessed any forms of hearing instruments other than ACD, BCD, and CI.
Purpose
A systematic review of the literature was conducted to assess the evidence for whether hearing instruments, including but not limited to rerouting devices and any device that restores input to the IE (“restorative devices”), are effective in improving listening skills impaired in unilateral deafness (speech perception and sound localization), reducing associated listening difficulty (hearing-related quality of life), and improving overall health and well-being (health-related quality of life). The review also sought to compare restorative and rerouting devices, and to compare air- and bone conduction rerouting devices to the unaided condition. All previous reviews have noted a lack of randomized controlled trials and therefore the review included both prospective controlled and observational studies.
The difficulties with understanding speech reported by unilaterally deaf adults result in part from the attenuating effect of the head’s acoustic shadow on talkers located toward the IE (Harford et al. 1966). It was, therefore, anticipated that rerouting and restorative devices would improve speech perception in noise when speech signals were presented toward the IE, as both overcome the head shadow effect. As a result of these effects, both categories of device were also expected to lead to reductions in at least some forms of difficulty with listening in everyday situations. Difficulties with the spatial aspects of hearing reported by these individuals result from impaired access to the binaural cues of interaural level and time (Noble & Gatehouse 2004). It was, therefore, hypothesized that restorative devices would improve the ability to localize sounds by restoring functional hearing in both ears, an ability that would not be restored by rerouting devices as they do not provide input bilaterally.
Materials and Methods
A systematic review of the literature was carried out in accordance with recommendations for undertaking (Centre for Reviews and Dissemination 2009; Higgins & Green 2009) and reporting (Stroup et al. 2000; Moher et al. 2009) reviews of healthcare interventions. Before commencing the review, an unpublished internal protocol was developed that specified the search strategy, selection criteria, data extraction procedures, method of quality assessment, and terms under which study data would be pooled for meta-analysis.
Search Strategy
A literature search was conducted by the investigators of the following electronic databases: MEDLINE, EMBASE, PubMed, CINAHL, DARE, and the Cochrane Databases. The MEDLINE and EMBASE databases were searched using the OvidSP software (Ovid Technologies Ltd.). Other databases were searched using their public-facing websites. The search was conducted with no restrictions on language from 1946 or the start date of the database, whichever was earlier. The search results were last updated on February 11, 2015 (see Table, Supplemental Digital Content 1 (http://links.lww.com/EANDH/A264), which lists a representative example of the search strategy adapted for the MEDLINE and EMBASE databases).
Selection Criteria
The criteria for inclusion in the review were defined in terms of participants, intervention(s), comparators, outcomes, and study designs (PICOS) as follows: (P) adults with a pure-tone average audiometric threshold ≤30 dB HL in one ear (averaged across 0.5, 1, 2, and 4 kHz) and >70 dB HL in the other ear. The description of the poorer ear should indicate that the loss was sensorineural in origin or that the sensorineural component was severe to profound. Where the description does not rule out well-preserved cochlear function and bone conduction thresholds are reported, the air bone gap should be ≤10 dB from 1 to 4 kHz; (I) any hearing instrument; (C) hearing instruments and placebo devices, no intervention; (O) speech perception in quiet and in noise, sound localization, hearing- and health-related quality of life, complications and adverse events; (S) controlled trials and prospective observational studies. Studies that included other populations had to report data from the eligible population separately. The minimum duration of follow-up was 1 week for rerouting devices and 3 months for restorative devices to ensure there was sufficient time for acclimatization. All studies were reviewed independently by PTK and SNS. Initial screening was conducted based on titles and abstracts. Full text articles were retrieved for screening where any uncertainty existed about a study’s eligibility. Authors were contacted if other methods of obtaining study information were unsuccessful. Foreign language articles were translated. Disagreements between reviewers were resolved by consensus. Reference lists of articles that met the inclusion criteria were also searched for potentially eligible articles. Published abstracts, articles published in nonpeer reviewed publications (“gray literature”), and unpublished studies were not included in this review.
Data Extraction
Information was extracted from each study under the following headings: number of patients, type of intervention device, type of comparator device, control group, outcomes, duration of follow-up, and level of evidence. Levels of evidence were determined based on study design and other quality factors (Centre for Evidence-Based Medicine 2009). The form used for data extraction was piloted on a small number of studies before use. Data extraction was performed independently by SNS and LL. Where data were extracted from the text of articles, disagreements were resolved by a third author (PTK). Disagreements about numerical data extracted from figures were resolved by averaging.
Quality Assessment
A quality assessment was conducted based on selected factors that may increase the risk of bias (Downs & Black 1998). The quality factors were randomized allocation, blinding, ethical approval, statement of eligibility criteria, power calculation, appropriate control group, confounds identified and controlled for, missing data accounted for, and funding source(s) declared. Quality assessments were conducted at the study level and were performed independently by SNS and LL with disagreements resolved by consensus. The results of the quality assessment were used to assess the risk that the observed results may have been biased due to the manner in which the study was conducted.
Meta-Analysis and Data Synthesis
The quality assessment was not used to select studies for meta-analysis due to the lack of a validated set of quality criteria upon which to base selection (Jüni et al. 1999). Instead, data were pooled and subjected to meta-analysis if a standardized self-report instrument or assessment methodology was used and there was no substantial difference in design between the studies that were pooled into a single meta-analysis. Where these criteria were met, data were subjected to a random effects meta-analysis with a restricted maximum-likelihood estimator using the “metafor” package for the R statistical software (Viechtbauer 2010). The use of a random effects approach to the meta-analysis was based on an assumption that effect sizes would vary across studies not only due to the fact that they used different samples of participants (as assumed in a fixed effect approach) but also due to differences in the way the studies were conducted (Borenstein et al. 2010).
Effect sizes were calculated as standardized mean differences (SMDs) in which the mean difference between treatments was divided by the pooled standard deviation (between group) or by the SD of the differences (within group). Heterogeneity was described by expressing the total heterogeneity as a percentage of the total variability (I2) and its significance was tested using a χ2 test. This approach to quantifying heterogeneity provides an intuitive value from 0 to 100% where a higher value indicates greater inconsistency in effect sizes across studies. I2 values less than 40% and for which the associated χ2 test was also nonsignificant (p > 0.10) were considered as indicative of low heterogeneity (Higgins & Green 2009). Publication bias was assessed using the trim and fill method (Duval & Tweedie 2000). An estimate of the number of missing studies was derived using the R0 estimator to test the null hypothesis that the number of missing studies was zero (Duval 2005). If the null hypothesis was rejected, the sensitivity of the results to publication bias was assessed by calculating an adjusted summary estimate. The direction and statistical significance of the observed effects in each study were also tabulated.
Results
A total of 778 articles were identified and subjected to a three-stage screening process (Fig. 1). The full texts of 298 articles that passed the initial title and abstract screen were retrieved without requiring contact with authors. A total of 249 articles were judged to not have met the inclusion criteria and were excluded (see Table, Supplemental Digital Content 2 (http://links.lww.com/EANDH/A265), which provides additional details about the reasons for exclusions after full-text review). Of the remaining 49 articles that did meet the criteria, 19 were excluded either because the duration of follow-up was insufficient or outcomes for the eligible population were not reported separately (Table 1). Therefore, 30 articles reporting 27 separate studies were included in the review (Table 2).
Fig. 1.
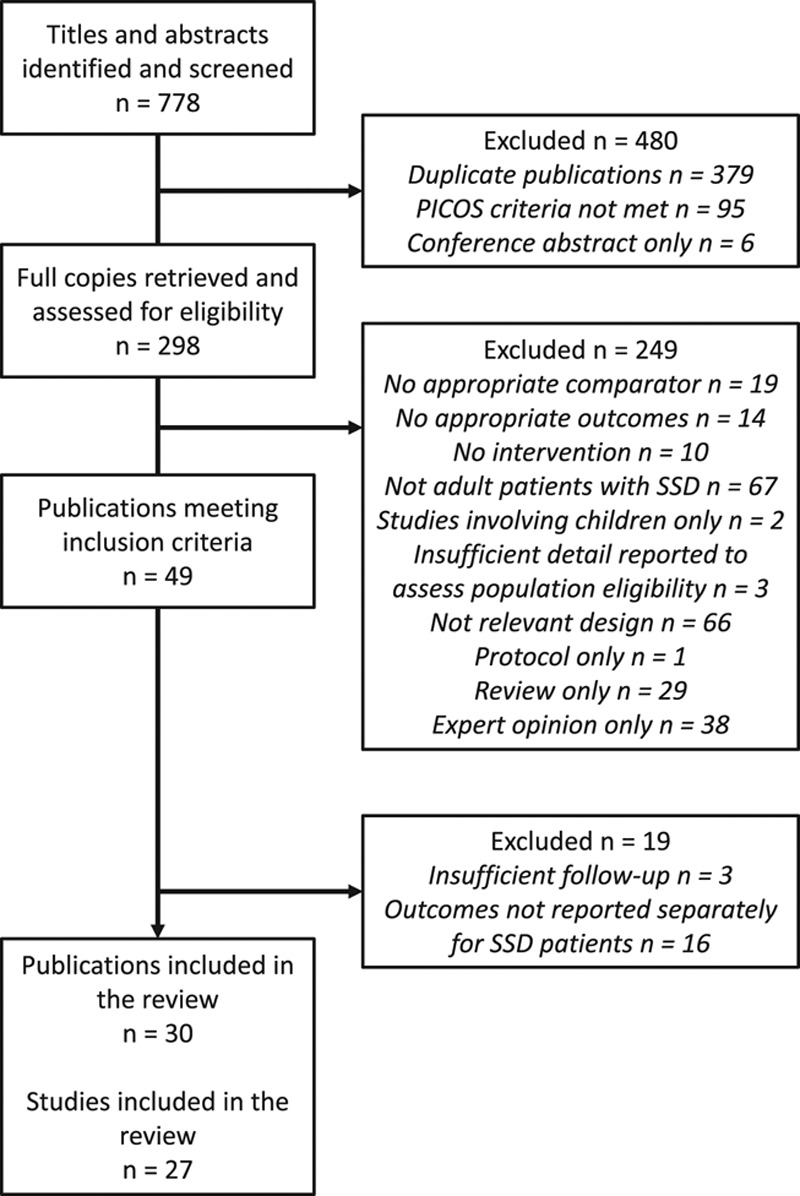
Flow chart of the process for selecting studies for inclusion in the review.
Table 1.
Articles that met the inclusion criteria but were excluded from the review with justification (see Table, Supplemental Digital Content 2 (http://links.lww.com/EANDH/A265), for further information on the reasons for excluding articles that did not meet the inclusion criteria)
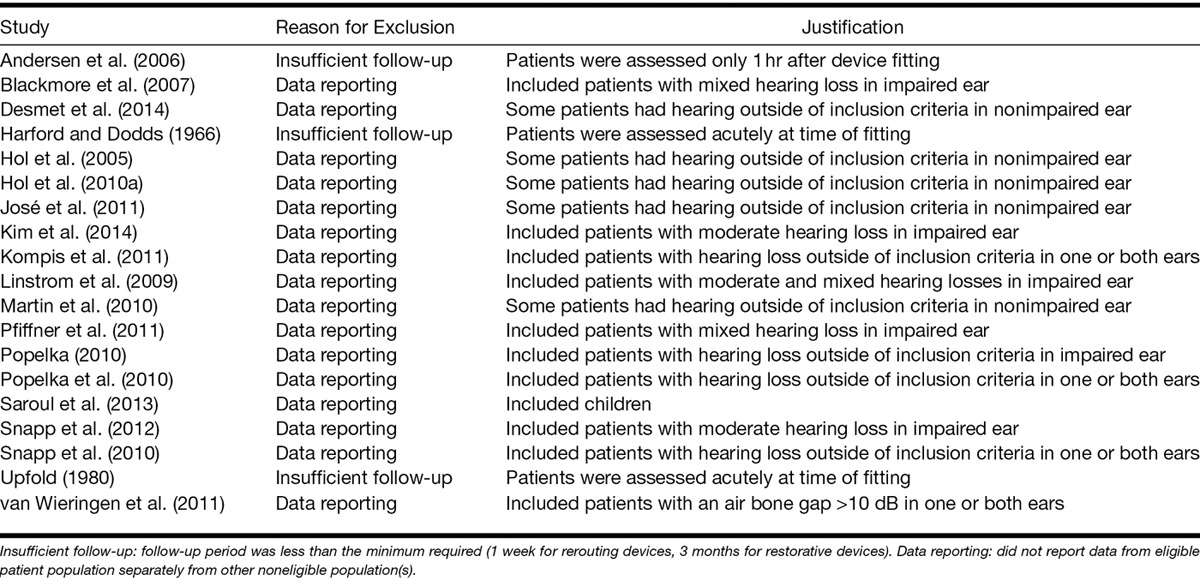
Table 2.
Characteristics of studies included in the review
The studies evaluated hearing instruments that fell into the broad categories of rerouting devices and restorative devices. The rerouting devices included those based on ACD and BCD. The restorative devices were limited to CIs. No other form of hearing instrument was identified in the screening process. The comparators for both categories of device included the unaided condition and other rerouting devices. All studies that assessed ACDs compared them with BCDs, with two studies randomizing the order of the interventions (Hol et al. 2010b; Arndt et al. 2011a, b) and four studies evaluating ACD use before BCD use (Bosman et al. 2003; Niparko et al. 2003; Wazen et al. 2003; Lin et al. 2006). Bone conduction rerouting devices included those mounted on a head-band, on a surgically inserted abutment, on an oral prosthesis, and those inserted into the ear canal. As there were no a priori expectations about differences between these varying approaches to bone conduction, all BCD devices were grouped together for all analyses.
The majority of studies were before-after comparisons in which patients acted as their own control. Three studies included matched control groups that did not receive a hearing instrument, two of which were case–control designs (Gluth et al. 2010; House et al. 2010) and one was a cohort study (Punte et al. 2013). The minimum duration of follow-up varied considerably and was dependent on the type of intervention. All studies assessing outcomes after CI followed patients for at least 6 months, whereas follow-up periods were far shorter for studies of rerouting devices, with one study following patients for only 18 days (Desmet et al. 2012). This variation reflects the fact that rerouting devices present additional information to an already-functioning ear, whereas CIs require a longer period of adjustment to the novel form of stimulation in the deaf ear.
The studies were judged to be of low-to-moderate quality. None reported conducting a power calculation. Seven studies (26%) included a control group but four of those used nonmatched controls (individuals with normal hearing or conductive hearing losses). None reported identifying or controlling for confounding factors. Four studies (15%) randomized the order of interventions but did not provide any information about randomization methodology or concealment according to best-practice guidelines for the reporting of randomized controlled trials (Schulz et al. 2010). None of these four studies reported outcomes based on participants’ initial allocation and used randomization to control for order effects when providing multiple interventions to the same participants rather than to control for biases. Missing data were controlled for in only two studies (7%) and by excluding participants with incomplete data, an approach that can introduce bias if those who failed to return for follow-up appointments did so because of lack of benefit (Nüesch et al. 2009). Ten studies (37%) did not state clear eligibility criteria, 18 studies (67%) did not declare funding sources, and 11 studies (41%) did not state whether ethical approval had been obtained.
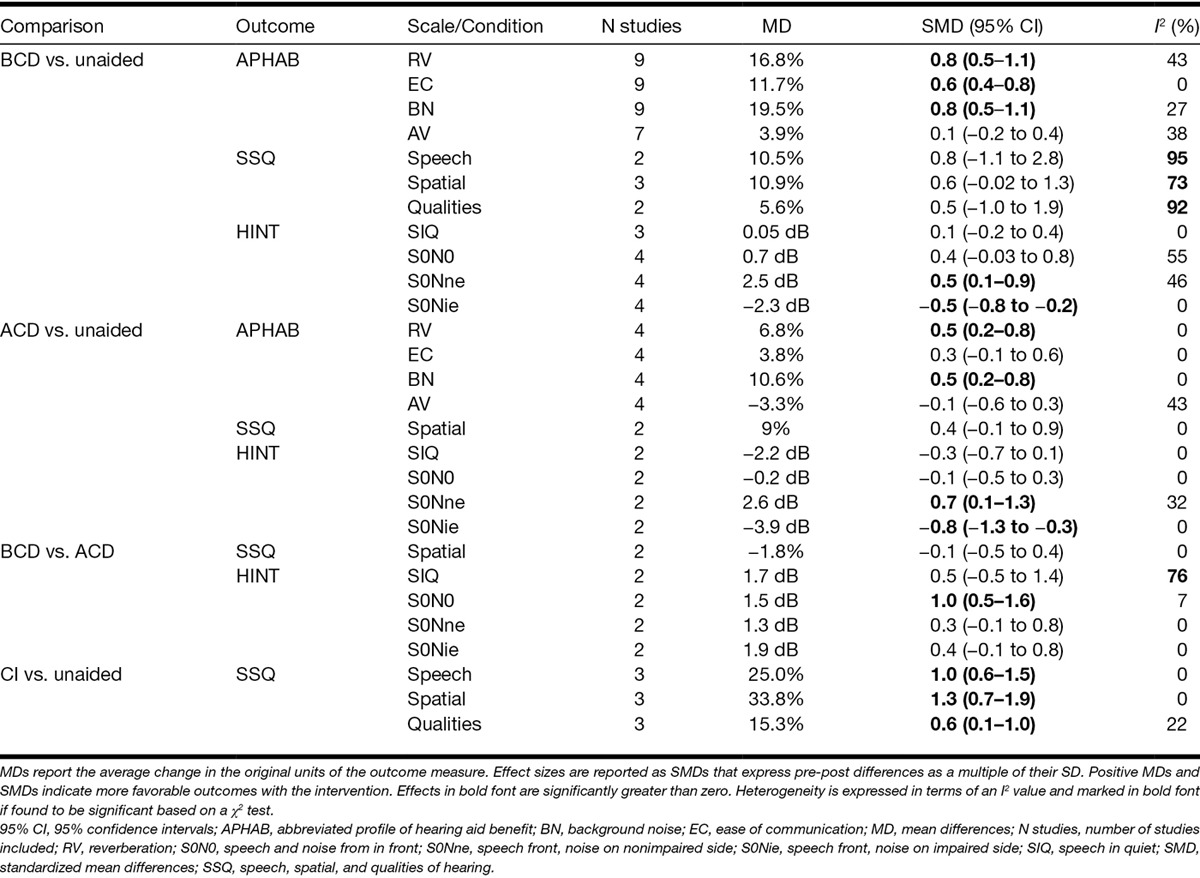
Due to heterogeneity in the design and outcomes of those studies that included control groups, summary effect sizes based on a random effects meta-analysis were computed for within-group effects only. No evidence of publication bias was found using the trim and fill method and therefore adjusted summary effect sizes were not calculated. Effect sizes were categorized as small, medium, or large if their value exceeded 0.3, 0.7, or 1.2 based on an average pre-post correlation of 0.53 (Barcikowski & Robey 1985). The statistical significance and direction of effects within studies (Tables 3 and 4; see Tables, Supplemental Digital Contents 3 and 4, for annotated version of these tables) and the results of the meta-analysis of effects across studies (Figs. 2 and 3, Table 5) are discussed for each outcome measure separately in the following sections.
Table 3.
Statistical significance and direction of effects for each category of outcome measure from studies that compared BCDs to the unaided condition (see Table, Supplemental Digital Content 3 (http://links.lww.com/EANDH/A266), for an annotated version of this table)
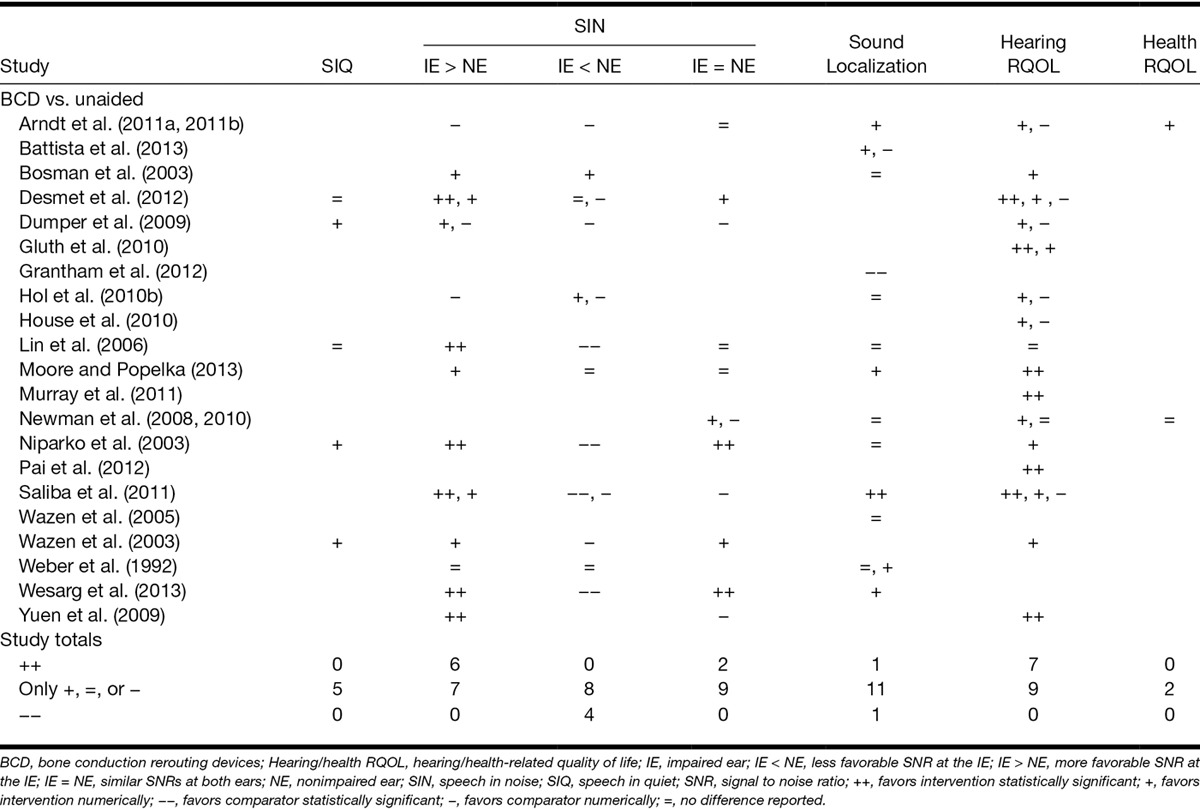
Table 4.
Statistical significance and direction of effects for each category of outcome measure in studies that compared ACDs and CI with BCDs and to the unaided condition (see Table, Supplemental Digital Content 4, for an annotated version of this table)
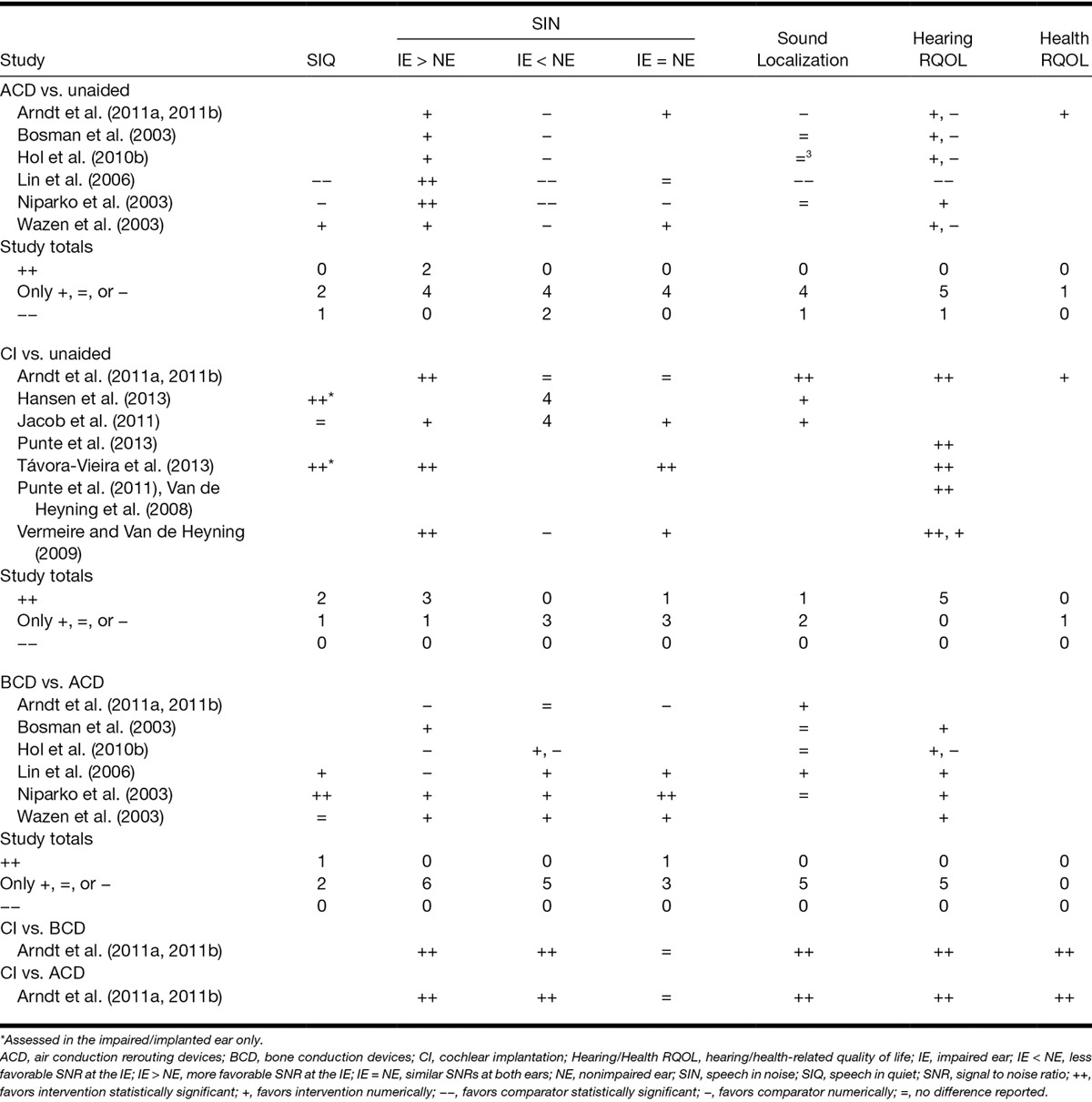
Fig. 2.
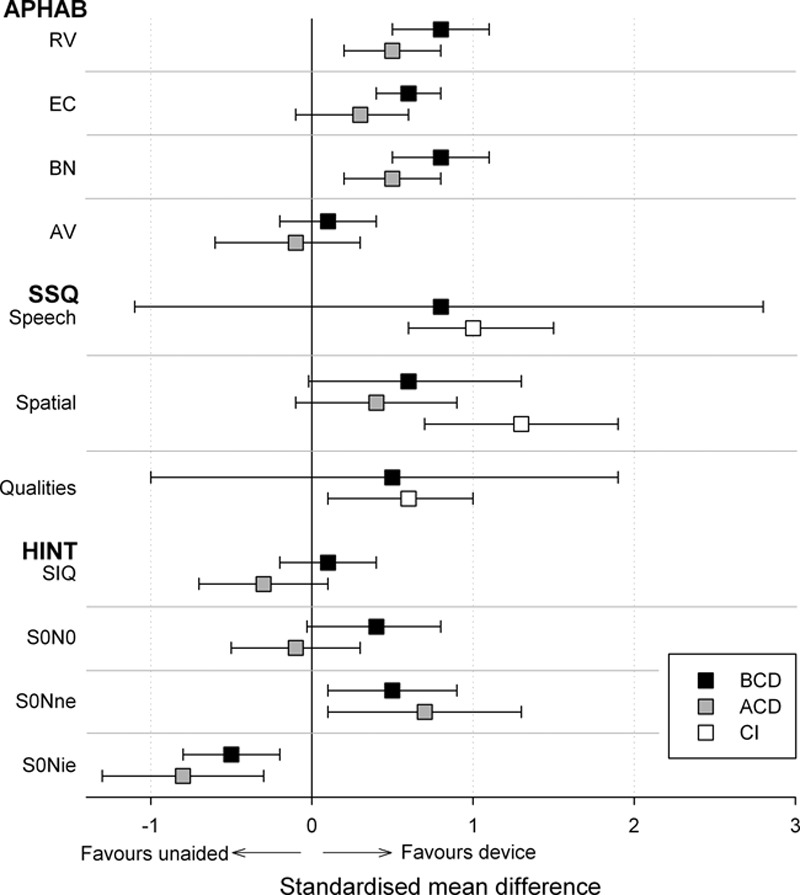
A summary of the random effects meta-analysis results comparing air- and bone conduction rerouting (ACD and BCD, respectively) and CI to the unaided condition. Symbols indicate the summary effect size for each subscale of two self-reported outcome measures (APHAB and SSQ) and for each condition of a behavioural measure of speech perception in noise (HINT). Error bars report 95% confidence intervals for the summary effects. ACD indicates air conduction rerouting devices; APHAB, abbreviated profile of hearing aid benefit; BCD, bone conduction rerouting devices; CI, cochlear implantation; SSQ, speech, spatial, and qualities of hearing.
Fig. 3.
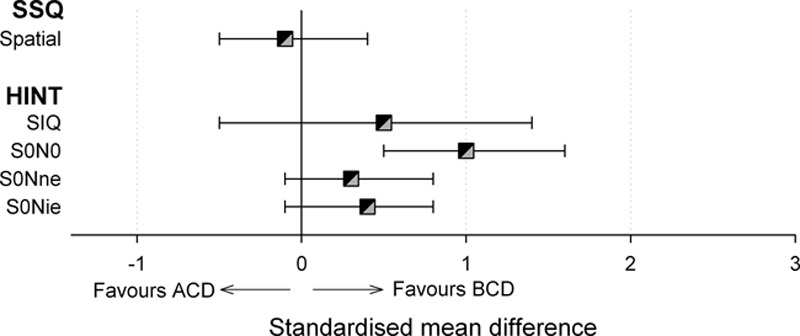
A summary of the random effects meta-analysis results comparing BCDs with ACDs. Symbols indicate the summary effect size for each subscale of a self-reported outcome measure (SSQ) and for each condition of a behavioral measure of speech perception in noise (HINT). Error bars report 95% confidence intervals for the summary effects. ACD indicates air conduction rerouting devices; BCD, bone conducting rerouting devices; SSQ, speech, spatial, and qualities of hearing.
Table 5.
Random effects meta-analyses of speech perception (HINT Sentence test) and hearing-related quality of life (APHAB and SSQ) data
Speech Perception in Quiet
Rerouting Versus Unaided • Five studies examined the effect of using a rerouting device on speech perception in quiet (Tables 3 and 4, “SIQ”). Speech perception was evaluated using either the HINT sentence test (Nilsson et al. 1994) or the Leuven Intelligibility Sentence test (Van Wieringen & Wouters 2008). In all five studies, speech was presented directly in front of the patient. The only statistically significant effect in any individual study was a decrease in speech perception accuracy when participants used an ACD (Lin et al. 2006). Meta-analyses of two studies evaluating ACD effects (Niparko et al. 2003; Wazen et al. 2003) and three studies evaluating BCD effects (Niparko et al. 2003; Wazen et al. 2003; Dumper et al. 2009) identified no significant change in speech-reception thresholds (SRT) following use of either type of device (see Figure, Supplemental Digital Content 5 and 6 (http://links.lww.com/EANDH/A268 and http://links.lww.com/EANDH/A269), which list the individual study estimates for ACD and BCD, respectively). No significant heterogeneity across the studies was observed (Table 5, “HINT-SIQ”).
BCD Versus ACD • Of those studies that compared ACD and BCD effects on speech perception in quiet, only one study found significantly greater benefits from bone conduction (Table 4). However, no statistically significant effect was observed when data were pooled across the two studies that reported SRTs in quiet (Niparko et al. 2003; Wazen et al. 2003). Significant heterogeneity in their effect sizes was also observed (Table 5, “HINT-SIQ”; see Figure, Supplemental Digital Content 7 (http://links.lww.com/EANDH/A270), which lists the individual study estimates for the difference between BCD and ACD).
CI Versus Unaided • Two studies reported a statistically significant improvement in speech perception in quiet after CI (Table 4, “SIQ”). However, in both cases, speech perception was assessed when participants listened using only their implanted ear. Neither study reported equivalent outcomes when participants also had the use of their nonimplanted ear.
CI Versus Rerouting • No study compared speech perception in quiet with a CI and with any form of rerouting device.
Interim Summary • There is a lack of evidence to suggest that rerouting devices or CI can provide benefits to speech perception in quiet compared with the unaided condition, or that one category of hearing instrument may be more beneficial than another.
Speech Perception in Noise
Studies varied the spatial location of the speech and noise stimuli relative to the IE or non-IE (NE). Spatial configurations were classified as either creating a similar signal to noise ratio (SNR) at the two ears (IE = NE), a more favorable SNR at the IE (IE > NE), or a less favorable SNR at the IE (IE < NE). Consistent assessment methodologies were only observed among studies that used the HINT sentence test and meta-analyses were conducted only where identical speech and noise locations were used across studies.
Rerouting Versus Unaided • Thirteen studies reported changes in speech perception in noise after use of a rerouting device (Tables 3 and 4, “SIN”). Individual studies found significant benefits for both ACD and BCD when the IE had a more favorable SNR than the NE (IE > NE). Conversely, statistically significant deficits were found for both rerouting modalities when the IE had a less favorable SNR (IE < NE). When both ears had a similar SNR (IE = NE), significant benefits were only found for bone conduction. The number of studies reporting nonsignificant effects for all configurations of speech and noise was similar to or greater than the number reporting significant effects.
Meta-analyses of data from the HINT sentence test identified significant benefits from both BCD and ACD when speech was presented from in front and noise was presented toward the NE (Fig. 2; Table 5, “HINT-S0Nne”). SRTs were also impaired significantly by both device types when noise was presented toward the IE (“HINT-S0Nie”). No significant effect of rerouting was identified when speech and noise were colocated (“HINT-S0N0”; see Figure, Supplemental Digital Content 5 and 6 (http://links.lww.com/EANDH/A268 and http://links.lww.com/EANDH/A269), which list the individual study estimates for ACD and BCD, respectively).
BCD Versus ACD • Only one study reported significantly better speech perception in noise with BCD compared to ACD (Niparko et al. 2003). A meta-analysis of HINT data from two studies (Niparko et al. 2003; Wazen et al. 2003) found that outcomes were more favorable with BCD but only when both ears had a similar SNR (Fig. 3; Table 5, “HINT-S0N0”; see Figure, Supplemental Digital Content 7 (http://links.lww.com/EANDH/A270), which lists the individual study estimates for the difference between BCD and ACD).
CI Versus Unaided • Three of the four studies reporting speech perception in noise outcomes before and after CI found significant benefits when the implanted ear had a more favorable SNR (IE > NE; Table 4, “SIN”). Only one study found significant benefits when both ears had a similar SNR (IE = NE; Távora-Vieira et al. 2013). Results for the spatial configuration that created a more favorable SNR at the NE were inconclusive with no study reporting a significant benefit or deficit after implantation. Heterogeneity in the assessment methodologies meant that data could not be pooled across studies for meta-analysis.
CI Versus Rerouting • One study compared outcomes after CI with outcomes after the use of either ACD or BCD devices (Arndt et al. 2011a, 2011b). Speech perception was reported to be significantly better after CI compared with the preoperative use of both an ACD and BCD when either ear had a more favorable SNR.
Interim Summary • The available evidence suggests that rerouting devices provide benefits to speech perception in noise when the SNR is more favorable at the IE but degrade speech perception when the SNR is less favorable at the IE. There is an absence of evidence for any effect of rerouting signals on speech perception when the SNR is similar at both ears. There is also a lack of evidence for the effects of cochlear implant use on speech perception in noise due to variations in testing methodologies across studies. The evidence for additional benefits from one device type over another is limited and inconclusive.
Sound Lateralization and Localization
Sound localization was assessed in terms of a patient’s ability to determine the location of a sound from two or more possible locations. Methodologies for assessing localization were heterogeneous and precluded any meta-analyses from being conducted.
Rerouting Devices Versus Unaided • Only one study reported a significant benefit to sound localization from the use of a rerouting device (Tables 3 and 4, “LOC”). Saliba et al. (2011) reported an improvement in the ability to localize a sound to one of four spatial quadrants (front left/right, rear left/right) with BCD use. Two studies reported significant deficits to localization performance after use of a rerouting device, one for BCD (Grantham et al. 2012) and one for ACD (Lin et al. 2006).
BCD Versus ACD • All five studies that compared the effect of ACD and BCD on sound localization found no difference with performance at chance levels regardless of device modality (Table 4, “LOC”).
CI Versus Unaided • Only one of the three studies that reported localization outcomes after CI found a statistically significant improvement compared with the unaided condition (Arndt et al. 2011a, b).
CI Versus Rerouting • Only one study compared localization accuracy after CI with rerouting devices and observed that localization was significantly more accurate after CI compared with both ACD and BCD (Arndt et al. 2011a, 2011b).
Interim Summary • The evidence suggests that rerouting signals to the NE does not improve the ability to determine the location of a sound. There is currently a lack of evidence to indicate whether CI can restore the ability to localize sounds and meta-analysis of the available evidence is limited by the use of inconsistent testing methodologies.
Hearing- and Health-Related Quality of Life
The most frequent measures of hearing-related quality of life included the abbreviated profile of hearing aid benefit (APHAB, 14 studies) and the speech, spatial, and qualities of hearing scale (SSQ, 7 studies). Measures of health-related quality of life were limited to two studies, with one using the SF-36 (Newman et al. 2008) and the other using the Health Utilities Index Mark 3 (HUI3; Arndt et al. 2011a, 2011b).
Rerouting Devices Versus Unaided • Significant benefits for BCD were reported in seven studies (Tables 3 and 4, hearing/health-related quality of life [“hearing RQOL”]). A majority of these studies measured outcomes using the APHAB and significant benefits were found on the ease of communication, background noise, and reverberation subscales. No study reported a significant benefit to hearing-related quality of life with ACD but one study did report a significant decrease in quality of life relating to an increased aversion to loud sounds (Lin et al. 2006). Two studies measured the impact of rerouting devices on health-related quality of life and reported no significant benefits (Newman et al. 2008; Arndt et al. 2011a, 2011b; Tables 3 and 4, “health RQOL”).
Meta-analyses were conducted using data from studies that compared APHAB before and after use of a rerouting device separately for BCD and ACD devices (Fig. 2). Significant benefits from BCD use were found for all subscales except for aversion to loud sounds (AV) and significant benefits for ACD were found only for background noise and reverberation (Table 5, “APHAB”; see Figure, Supplemental Digital Content 8 and 9 (http://links.lww.com/EANDH/A271 and http://links.lww.com/EANDH/A272), which lists the individual study estimates for BCD and ACD, respectively). Additional meta-analyses were conducted on SSQ data but no significant effects were identified for either bone or air conduction (Table 5, “SSQ”; see Figure, Supplemental Digital Content 10 and 11 (http://links.lww.com/EANDH/A273 and http://links.lww.com/EANDH/A274), which list the individual study estimates for ACD and BCD, respectively).
BCD Versus ACD • None of the five studies that compared rerouting modalities found statistically significant differences in hearing-related quality of life between BCD and ACD (Table 4, “hearing RQOL”). Only SSQ data could be pooled across studies and only for the spatial dimension (Fig. 3). No significant effect of conduction modality was found (Table 5, “SSQ”; see Figure, Supplemental Digital Content 12 (http://links.lww.com/EANDH/A275), which lists the individual study estimates for the difference between BCD and ACD).
CI Versus Unaided • All studies that compared hearing-related quality of life before and after CI found significant benefits (Table 4, “hearing RQOL”). Three studies reported a significant decrease in self-reported difficulties with listening using the SSQ (Vermeire et al. 2009; Arndt et al. 2011a, 2011b; Távora-Vieira et al. 2013). A meta-analysis of this SSQ data found significant decreases in listening difficulty on the speech, spatial, and qualities subscales (Fig. 2, Table 5 “SSQ”; see Figure, Supplemental Digital Content 13 (http://links.lww.com/EANDH/A276), which lists the individual study estimates for CI).
CI Versus Rerouting • Only one study compared CI with rerouting devices (Arndt et al. 2011a, 2011b). The study reported significant benefits on measures of hearing-related (SSQ) and health-related (HUI3) quality of life after implantation compared with 3-week trials of both an ACD and a headband-mounted BCD (Table 4).
Interim Summary • Compared with the unaided condition, both rerouting devices and CI appear to have beneficial effects on hearing-related quality of life by reducing the level of listening difficulty experienced in everyday situations. There is currently an absence of evidence that either conduction modality for rerouting signals between the ears reduces listening difficulties more than the other. No conclusion can yet be drawn about whether CI provides additional reductions to listening difficulty compared with rerouting devices. There is a lack of evidence for the effects of any intervention on health-related quality of life.
Complications and Adverse Events
Three studies reported information about complications related to BCD use. Gluth et al. (2010) reported eight cases of skin reactions around the site of an abutment. Soft-tissue surgery was performed in 2 cases and 1 case required relocation of the abutment due to infection. One patient reported pain that led to discontinued use of the device. Wazen et al. (2003) reported one case of skin ulceration around an abutment that healed and did not affect device use. Murray et al. (2011) reported 4 cases with minor soft-tissue changes and 1 case of minor irritation after a trial of a dental-mounted BCD. None of the 27 studies discussed the reporting of adverse events.
Discussion
The scientific literature was searched using a systematic approach to identify studies that evaluated benefits of hearing instruments in adults with a unilateral sensorineural severe to profound hearing loss. Studies primarily examined changes in self-reported difficulties with listening and behavioral measures of speech perception before and after providing patients with a device that either rerouted sounds from the impaired to the NE or restored aspects of hearing to the IE.
Rerouting Devices
The evidence suggests that rerouting devices can both improve and degrade speech perception in noise but can also reduce self-reported difficulty with listening. Statistically significant benefits to speech perception were found only when the SNR was more favorable at the impaired than at the NE. In this situation, a rerouting device increases the SNR at the NE by overcoming the head-shadow effect (Pumford 2005). Some evidence of heterogeneity in the effect sizes was also found for both air and bone conduction devices (ACD, I2 = 32%; BCD, I2 = 46%). For example, the size of the benefit from BCD devices ranged from an improvement in SNR of just 0.4 dB (Saliba et al. 2011) to a more substantial 4.4 dB (Niparko et al. 2003). Thus, uncertainty remains about the size of the benefit that patients may receive even under listening conditions that favor the use of a rerouting device and whether the magnitude of the benefit would be clinically meaningful.
When the SNR was less favorable at the IE, rerouting actually hindered performance. The meta-analysis revealed that the average size of the degradation in performance on the HINT sentence test (increase in SNR: ACD: 3.9 dB, BCD: 2.3 dB) was similar to or larger than the size of the benefit observed when the SNR was more favorable at the IE (decrease in SNR: ACD: 2.6 dB, BCD: 2.5 dB). Patients may, therefore, require counseling to form appropriate expectations about the situations in which benefit may be obtained and those in which the use of a rerouting device may be counterproductive to listening. The fact that the effect of a rerouting device can be highly dependent on the listening situation in which it is used may also explain why the meta-analysis found significant effects on one self-reported outcome (APHAB) but not another (SSQ), and also why moderate-to-high levels of heterogeneity (I2 >4 0%) were observed on many of their subscales.
The evidence also suggests that rerouting devices do not aid the listener to localize sounds. This conclusion is compatible with the fact that localization abilities are largely dependent on binaural cues (Akeroyd 2006) and rerouting devices do not restore two-eared hearing. Some have speculated that these devices may provide cues that enable a listener to distinguish sounds on the left from sounds on the right (lateralization) by distorting the spectral content of sounds transmitted through the device (Harford & Barry 1965; Vaneecloo et al. 2001). The ability of the current review to synthesize evidence for the effects of rerouting devices on spatial listening was hindered by heterogeneity in the assessment methodologies used across studies. However, even when multiple studies assessed spatial listening using the same self-report questionnaire (SSQ), the meta-analysis identified that there were only small and nonsignificant effects for both ACD (effect size, 0.4) and BCD (effect size, 0.6) with significant heterogeneity in effect sizes across studies for the latter (BCD, I2 = 79%). Therefore, the current review identified a lack of evidence to suggest that devices that reroute sounds to the NE provide reliable cues to support spatial hearing.
Restorative Devices
This review identified limited evidence for the effects of CI on speech perception in noise. Although significant benefits were reported individual by three studies when the SNR was more favorable at the IE, the evidence could not be synthesized and subjected to a meta-analysis in this and other configurations of speech and noise due to fact that the assessment methodologies were not consistent across studies. The meta-analysis did identify effects relating to reductions in self-reported difficulties with listening to speech that were medium in size (SSQ speech: mean reduction 25%, SMD = 1.0) and consistent across studies (I2 = 0%). Although this evidence may suggest that the impact of any benefits to speech perception after implantation may extend to situations in everyday life, further evidence for the effects of CI on speech perception under controlled conditions is required to establish the bases of these reductions in listening difficulties.
CI also has the capacity to improve localization for individuals with residual hearing in the contralateral ear (Seeber et al. 2004). However, only one out of four studies that assessed sound localization before and after CI identified a statistically significant improvement (Arndt et al. 2011a, 2011b). The lack of a single power calculation raises doubts over whether the studies were capable of detecting such an effect. Although implantation undoubtedly restores some form of bilateral input, it is still unclear whether it can restore spatial hearing in the unilaterally deaf and over what timescale. The consistent (I2 = 0%) and large (SMD > 1.2) reduction in self-reported spatial listening difficulties after implantation (SSQ spatial: mean reduction 34%) could suggest that the assessment methods were insensitive to the particular benefits experienced by these individuals. Alternatively, implantation may have improved the ability to lateralize sounds, which these studies did not assess, or may have created a perception of spatial listening by improving the awareness of sounds on the impaired side. As with the self-reported reductions in difficulties with listening to speech, elucidating the bases of these perceived benefits to spatial listening will require further assessments of sound localization abilities under controlled conditions and the use of consistent testing methodologies across studies.
Choice of Intervention
Evidence for the relative effectiveness of the various interventions identified in the review was sparse. A meta-analysis of two studies (Niparko et al. 2003; Wazen et al. 2003) suggested that BCD conferred additional benefits to speech perception compared with ACD but only when speech and noise were colocated. The effect was of medium size (SMD = 1.0) and consistent across the two studies (I2 = 7%). However, the nature of the improvement was that listeners could tolerate an additional decrease in SNR of only 1.5 dB on average and it is unclear whether the size of this effect would be considered clinically meaningful.
The two studies that were included in that meta-analysis were from a subset of four studies that compared BCD and ACD using nonrandomised designs (Bosman et al. 2003; Niparko et al. 2003; Wazen et al. 2003; Lin et al. 2006; Table 4, BCD versus ACD). As all these studies evaluated BCDs mounted on a surgically implanted abutment, it is possible that those who had committed to surgery in these nonrandomised studies were primarily interested in receiving a BCD and may have been less willing to report benefit from ACD use during a preoperative trial. Although two studies did randomized the order of ACD and BCD trials by using head-band mounted BCD models (Hol et al. 2010b; Arndt et al. 2011a, 2011b), they did not assess speech perception consistently and therefore it was not possible to pool their data for meta-analysis. Thus, the statistically significant benefit observed for BCD over ACD in one particular spatial configuration of speech and noise should be interpreted with caution.
There are physical differences between BCD and ACD devices that could plausibly give rise to preferences for one over the other including whether the patient has to wear two devices (ACD) or only one (BCD) and whether the NE is occluded (ACD) or not (BCD). Differences in the intensity of rehabilitation, which was likely to be higher after abutment-mounted BCD provision compared with ACD provision, may also produce differences in outcome that are not a direct function of the conduction modality. However, no study was identified that compared the relative benefits of an abutment-mounted BCD and an ACD where these potential confounding factors were controlled for, and only one study compared CI to either ACD or BCD (Arndt et al. 2011a, 2011b). There is, therefore, a lack of evidence for the relative benefits of different rerouting modalities and for the additional benefits, if any, of CI over the rerouting of signals to the NE.
Recommendations
The lack of prospective controlled trials and the exclusion of patients who provided incomplete data raise concerns about the potential for selection bias to influence the observed effects. As a result, no recommendations for the management of unilaterally deaf adults can be based on the current evidence. The following recommendations, therefore, aim to ensure that future studies are of sufficient quality to strengthen the evidence base for hearing instruments for unilateral deafness. The primary recommendation is that randomized controlled trials should be conducted to compare ACD and BCD devices (whether mounted on a head-band or abutment) to the unaided condition and to each other, and also to compare CI with rerouting devices. If further cohort or case–control studies are undertaken, they should be well designed, appropriately powered, planned prospectively with detailed inclusion criteria, and should recruit from multiple sites. The effect sizes provided by the current meta-analysis (Table 5) could be used to inform the sample size calculations for future studies.
It is also recommended that those conducting further studies of the effectiveness of hearing instruments for unilateral severe to profound sensorineural hearing loss consider supporting future efforts to synthesize new and existing evidence when selecting outcome measures and when reporting the results of any further studies. Where studies seek to measure the benefits to speech perception in noise, it is recommended that outcomes should include SRTs for IE < NE, IE > NE, and IE = NE conditions as the current evidence suggests they may be useful in demonstrating both the benefits and potential drawbacks of different hearing instruments. Where studies aim to assess benefits to spatial listening abilities, it is recommended that the methodology should include the presentation of sounds to the left and right of straight ahead to permit the reporting of performance in terms of percent correct sound lateralization. Few studies have assessed lateralization explicitly and uncertainty remains as to whether certain hearing instruments may only aid gross spatial judgments rather than finer judgments of spatial location. Where studies wish to assess these finer judgments using localization tasks, it is recommended that the reporting of results includes the mean unsigned localization error. The current literature has employed a range of localization performance metrics that cannot be compared directly and mean unsigned error represents a summary statistic that is straightforward to compute and to interpret.
In the absence of a patient-reported outcome instrument that has been validated specifically for use in unilaterally deaf adults and to detect the effects of rerouting and restorative devices, it is recommended that future studies should assess hearing-related quality of life using at least one of the two most commonly used instruments, the APHAB and the SSQ. These instruments appear to be sensitive to the impact of device use and may indicate whether improvements on laboratory-based tests generalize to everyday listening situations. On a similar basis, it is recommended that all future studies include a generic preference-based instrument for measuring health-related quality of life that is sensitive to interventions for hearing, such as the HUI3, as only a single study was found to collect and report data on health-related quality of life. The use of generic instruments to provide preference-based valuations of the health states associated with the use of different hearing instruments (“utility” values) is necessary to inform the health-economic evaluations that are increasingly underpinning commissioning decisions in publicly funded healthcare systems.
Acknowledgments
The authors thank Dr. Susan Arndt for providing access to individual-level data for two studies (Arndt et al. 2011a, 2011b) to facilitate their inclusion in the meta-analyses.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com).
This study was supported by infrastructure funding from the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
P. T. K. and S. N. S. screened the articles for inclusion in the review. S. N. S. and L. L. extracted the data from included articles. S. N. S. and L. L. performed the quality assessment of the included studies. P. T. K. conducted the analyses and drafted the manuscript. All authors contributed to and approved the final version.
P. T. K. is coordinating a trial of cochlear implantation in adults with unilateral deafness that is part-funded by a manufacturer of cochlear implants, Cochlear Europe Ltd, and supported through the provision of study devices by a manufacturer of CROS hearing aids, Phonak UK.
References
- Akeroyd M. A.The psychoacoustics of binaural hearing. Int J Audiol(2006)45Suppl 1S25–S33. [DOI] [PubMed] [Google Scholar]
- Andersen H. T., Schrøder S. A., Bonding P.Unilateral deafness after acoustic neuroma surgery: Subjective hearing handicap and the effect of the bone-anchored hearing aid. Otol Neurotol(2006)27809–814. [DOI] [PubMed] [Google Scholar]
- Arndt S., Aschendorff A., Laszig R., et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011a;32:39–47. doi: 10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- Arndt S., Laszig R., Aschendorff A., et al. Unilateral deafness and cochlear implantation: Audiological diagnostic evaluation and outcomes. HNO. 2011b;59:437–446. doi: 10.1007/s00106-011-2318-8. [DOI] [PubMed] [Google Scholar]
- Baguley D. M., Bird J., Humphriss R. L., et al. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol(2006)316–14. [DOI] [PubMed] [Google Scholar]
- Barcikowski R. S., Robey R. R.Sample size selection in the single group repeated measures analysis(1985)Chicago, IL, USA: Paper presented at the Annual Convention of the American Educational Research Association [Google Scholar]
- Battista R. A., Mullins K., Wiet R. M., et al. Sound localization in unilateral deafness with the Baha or TransEar device. JAMA Otolaryngol Head Neck Surg(2013)13964–70. [DOI] [PubMed] [Google Scholar]
- Blackmore K. J., Kernohan M. D., Davison T., et al. Bone-anchored hearing aid modified with directional microphone: Do patients benefit? J Laryngol Otol(2007)121822–825. [DOI] [PubMed] [Google Scholar]
- Blasco M. A., Redleaf M. I.Cochlear implantation in unilateral sudden deafness improves tinnitus and speech comprehension: Meta-analysis and systematic review. Otol Neurotol(2014)351426–1432. [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P., et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods(2010)197–111. [DOI] [PubMed] [Google Scholar]
- Bosman A. J., Hol M. K., Snik A. F., et al. Bone-anchored hearing aids in unilateral inner ear deafness. Acta Otolaryngol(2003)123258–260. [DOI] [PubMed] [Google Scholar]
- Centre for Evidence-Based Medicine Centre for Evidence-Based Medicine Levels of Evidence(2009)Oxford: CEBM [Google Scholar]
- Centre for Reviews and Dissemination. Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care(2009)York: University of York [Google Scholar]
- Desmet J. B., Wouters K., De Bodt M., et al. Comparison of 2 implantable bone conduction devices in patients with single-sided deafness using a daily alternating method. Otol Neurotol(2012)331018–1026. [DOI] [PubMed] [Google Scholar]
- Desmet J., Wouters K., De Bodt M., et al. Long-term subjective benefit with a bone conduction implant sound processor in 44 patients with single-sided deafness. Otol Neurotol(2014)351017–1025. [DOI] [PubMed] [Google Scholar]
- Downs S. H., Black N.The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health(1998)52377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumper J., Hodgetts B., Liu R., et al. Indications for bone-anchored hearing AIDS: A functional outcomes study. J Otolaryngol Head Neck Surg(2009)3896–105. [PubMed] [Google Scholar]
- Duval S. J.H. R. Rothstein, A. J. Sutton, M. Borenstein.The trim and fill method. In Publication Bias in Meta-analysis: Prevention, Assessment, and Adjustments(2005)Chichester, England: Wiley; pp. 127–144. [Google Scholar]
- Duval S., Tweedie R.Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics(2000)56455–463. [DOI] [PubMed] [Google Scholar]
- Dwyer N. Y., Firszt J. B., Reeder R. M.Effects of unilateral input and mode of hearing in the better ear: Self-reported performance using the speech, spatial and qualities of hearing scale. Ear Hear(2014)35126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giolas T. G., Wark D. J.Communication problems associated with unilateral hearing loss. J Speech Hear Disord(1967)32336–343. [DOI] [PubMed] [Google Scholar]
- Gluth M. B., Eager K. M., Eikelboom R. H., et al. Long-term benefit perception, complications, and device malfunction rate of bone-anchored hearing aid implantation for profound unilateral sensorineural hearing loss. Otol Neurotol(2010)311427–1434. [DOI] [PubMed] [Google Scholar]
- Grantham D. W., Ashmead D. H., Haynes D. S., et al. Horizontal plane localization in single-sided deaf adults fitted with a bone-anchored hearing aid (Baha). Ear Hear(2012)33595–603. [DOI] [PubMed] [Google Scholar]
- Hansen M. R., Gantz B. J., Dunn C.Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Ménière’s disease. Otol Neurotol(2013)341681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford E., Barry J.A rehabilitative approach to the problem of unilateral hearing impairment: The contralateral routing of signals CROS. J Speech Hear Disord(1965)30121–138. [DOI] [PubMed] [Google Scholar]
- Harford E., Dodds E.The clinical application of CROS. A hearing aid for unilateral deafness. Arch Otolaryngol(1966)83455–464. [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S.Cochrane Handbook for Systematic Reviews of Interventions(2009)The Cochrane Collaboration; West Sussex, England: Wiley & Sons. Available from www.cochrane-handbook.org. [Google Scholar]
- Hol M. K., Bosman A. J., Snik A. F., et al. Bone-anchored hearing aids in unilateral inner ear deafness: An evaluation of audiometric and patient outcome measurements. Otol Neurotol(2005)26999–1006. [DOI] [PubMed] [Google Scholar]
- Hol M. K., Kunst S. J., Snik A. F., et al. Bone-anchored hearing aids in patients with acquired and congenital unilateral inner ear deafness (Baha CROS): Clinical evaluation of 56 cases. Ann Otol Rhinol Laryngol. 2010a;119:447–454. doi: 10.1177/000348941011900704. [DOI] [PubMed] [Google Scholar]
- Hol M. K., Kunst S. J., Snik A. F., et al. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. Eur Arch Otorhinolaryngol. 2010b;267:889–896. doi: 10.1007/s00405-009-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J. W., Kutz J. W., Jr, Chung J., et al. Bone-anchored hearing aid subjective benefit for unilateral deafness. Laryngoscope(2010)120601–607. [DOI] [PubMed] [Google Scholar]
- Jacob R., Stelzig Y., Nopp P., et al. Audiological results with cochlear implants for single-sided deafness. HNO(2011)59453–460. [DOI] [PubMed] [Google Scholar]
- José M. R., Campos P. D., Mondelli M. F.Unilateral hearing loss: Benefits and satisfaction from the use of hearing aids. Braz J Otorhinolaryngol(2011)77221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüni P., Witschi A., Bloch R., et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA(1999)2821054–1060. [DOI] [PubMed] [Google Scholar]
- Kim D. Y., Kim T. S., Shim B. S., et al. Hearing gain with a BAHA test-band in patients with single-sided deafness. Am J Otolaryngol(2014)3537–41. [DOI] [PubMed] [Google Scholar]
- Kompis M., Pfiffner F., Krebs M., et al. Factors influencing the decision for Baha in unilateral deafness: The Bern benefit in single-sided deafness questionnaire. Adv Otorhinolaryngol(2011)71103–111. [DOI] [PubMed] [Google Scholar]
- Lin L. M., Bowditch S., Anderson M. J., et al. Amplification in the rehabilitation of unilateral deafness: Speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol(2006)27172–182. [DOI] [PubMed] [Google Scholar]
- Linstrom C. J., Silverman C. A., Yu G. P.Efficacy of the bone-anchored hearing aid for single-sided deafness. Laryngoscope(2009)119713–720. [DOI] [PubMed] [Google Scholar]
- Martin T. P., Lowther R., Cooper H., et al. The bone-anchored hearing aid in the rehabilitation of single-sided deafness: Experience with 58 patients. Clin Otolaryngol(2010)35284–290. [DOI] [PubMed] [Google Scholar]
- McLeod B., Upfold L., Taylor A.Self reported hearing difficulties following excision of vestibular schwannoma. Int J Audiol(2008)47420–430. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ(2009)339b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. C., Popelka G. R.Preliminary comparison of bone-anchored hearing instruments and a dental device as treatments for unilateral hearing loss. Int J Audiol(2013)52678–686. [DOI] [PubMed] [Google Scholar]
- Murray M., Miller R., Hujoel P., et al. Long-term safety and benefit of a new intraoral device for single-sided deafness. Otol Neurotol(2011)321262–1269. [DOI] [PubMed] [Google Scholar]
- Newman C. W., Sandridge S. A., Wodzisz L. M.Longitudinal benefit from and satisfaction with the Baha system for patients with acquired unilateral sensorineural hearing loss. Otol Neurotol(2008)291123–1131. [DOI] [PubMed] [Google Scholar]
- Newman C. W., Sandridge S. A., Oswald L. M.Relationship between expectations and satisfaction for Baha implant system in patients with single-sided deafness. Semin Hear(2010)3115–27. [Google Scholar]
- Nilsson M., Soli S. D., Sullivan J. A.Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am(1994)951085–1099. [DOI] [PubMed] [Google Scholar]
- Niparko J. K., Cox K. M., Lustig L. R.Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol(2003)2473–78. [DOI] [PubMed] [Google Scholar]
- Noble W., Gatehouse S.Interaural asymmetry of hearing loss, speech, spatial and qualities of hearing scale (SSQ) disabilities, and handicap. Int J Audiol(2004)43100–114. [DOI] [PubMed] [Google Scholar]
- Nüesch E., Trelle S., Reichenbach S., et al. The effects of excluding patients from the analysis in randomised controlled trials: Meta-epidemiological study. BMJ(2009)339b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai I., Kelleher C., Nunn T., et al. Outcome of bone-anchored hearing aids for single-sided deafness: A prospective study. Acta Otolaryngol(2012)132751–755. [DOI] [PubMed] [Google Scholar]
- Peters J. P., Smit A. L., Stegeman I., et al. Review: Bone conduction devices and contralateral routing of sound systems in single-sided deafness. Laryngoscope(2015)125218–226. [DOI] [PubMed] [Google Scholar]
- Pfiffner F., Kompis M., Flynn M., et al. Benefits of low-frequency attenuation of baha® in single-sided sensorineural deafness. Ear Hear(2011)3240–45. [DOI] [PubMed] [Google Scholar]
- Popelka G. R.SoundBite hearing system by sonitus medical: A new approach to single-sided deafness. Semin Hear(2010)31393–409. [Google Scholar]
- Popelka G. R., Derebery J., Blevins N. H., et al. Preliminary evaluation of a novel bone-conduction device for single-sided deafness. Otol Neurotol(2010)31492–497. [DOI] [PubMed] [Google Scholar]
- Pumford J.Benefits of probe-mic measures with CROS/BiCROS fittings. Hear J(2005)5034–40. [Google Scholar]
- Punte A. K., De Ridder D., Van de Heyning P.On the necessity of full length electrical cochlear stimulation to suppress severe tinnitus in single-sided deafness. Hear Res(2013)29524–29. [DOI] [PubMed] [Google Scholar]
- Punte A. K., Vermeire K., Hofkens A., et al. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int(2011)12Suppl 1S26–S29. [DOI] [PubMed] [Google Scholar]
- Saliba I., Nader M. E., El Fata F., et al. Bone anchored hearing aid in single sided deafness: Outcome in right-handed patients. Auris Nasus Larynx(2011)38570–576. [DOI] [PubMed] [Google Scholar]
- Saroul N., Nicolas S., Akkari M., et al. Long-term benefit and sound localization in patients with single-sided deafness rehabilitated with an osseointegrated bone-conduction device. Otol Neurotol(2013)34111–114. [DOI] [PubMed] [Google Scholar]
- Schulz K. F., Altman D. G., Moher D.CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ(2010)340c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber B. U., Baumann U., Fastl H.Localization ability with bimodal hearing aids and bilateral cochlear implants. J Acoust Soc Am(2004)1161698–1709. [DOI] [PubMed] [Google Scholar]
- Snapp H. A., Fabry D. A., Telischi F. F., et al. A clinical protocol for predicting outcomes with an implantable prosthetic device (Baha) in patients with single-sided deafness. J Am Acad Audiol(2010)21654–662. [DOI] [PubMed] [Google Scholar]
- Snapp H., Angeli S., Telischi F. F., et al. Postoperative validation of bone-anchored implants in the single-sided deafness population. Otol Neurotol(2012)33291–296. [DOI] [PubMed] [Google Scholar]
- Stroup D. F., Berlin J. A., Morton S. C., et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA(2000)2832008–2012. [DOI] [PubMed] [Google Scholar]
- Távora-Vieira D., Boisvert I., McMahon C. M., et al. Successful outcomes of cochlear implantation in long-term unilateral deafness: Brain plasticity? Neuroreport(2013)24724–729. [DOI] [PubMed] [Google Scholar]
- Upfold L. J.The evaluation of CROS aids with the unilateral listener. Scand Audiol(1980)985–88. [DOI] [PubMed] [Google Scholar]
- Valente M.Fitting options for unilateral hearing loss. Hear J(1995)4810–48. [Google Scholar]
- Valente M., Potts L. G., Valente M., et al. Wireless CROS versus transcranial CROS for unilateral hearing loss. Am J Audiol(1995)452–59. [Google Scholar]
- Van de Heyning P., Vermeire K., Diebl M., et al. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol(2008)117645–652. [DOI] [PubMed] [Google Scholar]
- van Wieringen A., Wouters J.LIST and LINT: Sentences and numbers for quantifying speech understanding in severely impaired listeners for Flanders and the Netherlands. Int J Audiol(2008)47348–355. [DOI] [PubMed] [Google Scholar]
- van Wieringen A., De Voecht K., Bosman A. J., et al. Functional benefit of the bone-anchored hearing aid with different auditory profiles: Objective and subjective measures. Clin Otolaryngol(2011)36114–120. [DOI] [PubMed] [Google Scholar]
- van Zon A., Peters J. P., Stegeman I., et al. Cochlear implantation for patients with single-sided deafness or asymmetrical hearing loss: A systematic review of the evidence. Otol Neurotol(2015)36209–219. [DOI] [PubMed] [Google Scholar]
- Vaneecloo F. M., Ruzza I., Hanson J. N., et al. The monaural pseudo-stereophonic hearing aid (BAHA) in unilateral total deafness: A study of 29 patients. Rev Laryngol Otol Rhinol (Bord)(2001)122343–350. [PubMed] [Google Scholar]
- Vermeire K., Van de Heyning P.Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol(2009)14163–171. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W.Conducting meta-analyses in R with the metafor Package. J Stat Soft(2010)361–48. [Google Scholar]
- Wazen J. J., Spitzer J. B., Ghossaini S. N., et al. Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngol Head Neck Surg(2003)129248–254. [DOI] [PubMed] [Google Scholar]
- Wazen J. J., Ghossaini S. N., Spitzer J. B., et al. Localization by unilateral BAHA users. Otolaryngol Head Neck Surg(2005)132928–932. [DOI] [PubMed] [Google Scholar]
- Weber B. A., Roush J., McElveen J. T., JrApplication of an implantable bone conduction hearing device to patients with unilateral sensorineural hearing loss. Laryngoscope(1992)102538–542. [DOI] [PubMed] [Google Scholar]
- Wesarg T., Aschendorff A., Laszig R., et al. Comparison of speech discrimination in noise and directional hearing with 2 different sound processors of a bone-anchored hearing system in adults with unilateral severe or profound sensorineural hearing loss. Otol Neurotol(2013)341064–1070. [DOI] [PubMed] [Google Scholar]
- Yuen H. W., Bodmer D., Smilsky K., et al. Management of single-sided deafness with the bone-anchored hearing aid. Otolaryngol Head Neck Surg(2009)14116–23. [DOI] [PubMed] [Google Scholar]


