Abstract
The aim of the present study was to investigate the PAQR3 gene expression and its methylation level in colorectal cancer tissues, as well as the association with colorectal cancer clinical data. In total, 54 cases of colorectal cancer tissue samples and normal adjacent tissue samples were collected between June, 2013 and July, 2014. RT-PCR and western blot analysis were used to detect the mRNA and protein levels of PAQR3 in colorectal samples, respectively. MSP was used to detect the methylation level of PAQR3 gene in colorectal samples, which was compared with colorectal data. The results showed that a decreased expression level of PAQR3 mRNA in colorectal cancer tissues and the expression reduction rate was 57.4% (31/54). Similarly, the expression level of PAQR3 protein was reduced in cancer tissues, and the reduction rate was 46.3% (25/54), while the protein expression reduction rate in cancer adjacent tissue was 5.6% (3/54), and the difference was statistically significant (P<0.05). Furthermore, the methylation rates of PAQR3 in cancer tissues and cancer adjacent tissues were 33.3% (18/54) and 5.6% (3/54), respectively. In addition, PAQR3 mRNA and protein levels in colorectal cancer tissues were associated with the differentiation degree, lymphatic metastasis and tumor infiltration depth. The methylation level of PAQR3 was associated with age, differentiated degree, lymphatic metastasis and tumor infiltration depth. In conclusion, the expression of PAQR3 mRNA and protein in colorectal cancer was reduced and methylation of PAQR3 occurred. Although the PAQR3 mRNA and protein levels were not associated with gender, age or the location of tumor, there was an association with differentiation degree, lymphatic metastasis and tumor infiltration depth. In addition, the methylation level of PAQR3 was not correlated with gender or tumor location, but was correlated with age, differentiation degree, lymphatic metastasis and tumor infiltration depth.
Keywords: colorectal cancer, PAQR3, mRNA, western blot analysis, methylation
Introduction
In recent years, the mortality rate for colorectal cancer is over 600,000 patients annually (1). Early discovery, diagnosis and treatment are imperative for patients with colorectal cancer. The occurrence of colorectal cancer is the result of combined actions of multiple genes, factors and steps. Epigenetic change is a type of abnormal gene expression without the occurrence of gene sequence changes, including histone markers and gene methylation. Gene methylation is an early tumor event, and the process is reversible, which provides theoretical evidence for methylation-relevant gene application and clinical early diagnosis and treatment (2,3).
PAQR3 is a cancer suppressor gene and is involved in various signal channels that can influence biological processes such as energy metabolism, cell proliferation, differentiation and the maturity of reproductive cells, and can negatively regulate the Ras/Raf/MEK/ERK signal channel (4). In experiments in nude mice with colorectal cancer, it was verified that PAQR3 plays a role in cancer inhibition in colorectal cancer tissues, and the deficiency of PAQR3 expression reduced the survival time of gene-knockout nude mice (4). In addition, a low expression of PAQR3 in tumor tissues of gastric cancer, breast cancer, melanoma and liver cancer has been demonstrated. PAQR3 was able to enhance the sensitivity of breast cancer SK-BR-3 cells to epirubicin (5–13). However, studies should be performed on cancer suppressor gene PAQR3 mRNA and protein levels of colorectal cancer to assist in the diagnosis and treatment of colorectal cancer.
PAQR3 is a member of the progesterone and adiponectin receptor (PAQR) family, which plays a role in cancer inhibition in colorectal cancer cells, and is involved in biological processes such as signal transduction, energy metabolism, cell proliferation, differentiation and the maturity of reproductive cells. The purpose of adjusting physiological processes such as proliferation, differentiation, apoptosis, malignant transformation of cells and the occurrence and development of cancer is reached through the negative regulation of the Ras/Raf/MEK/ERK signal channel (5–9). In addition, the AKT signal channel is negatively regulated by PAQR3 through two mechanisms. After knockout of PAQR3, the expression of p110α in the cytoplast in combination with the dictyosome is decreased, PAQR3 combined and changed the subcellular localization of p110α of PI3K of compounds in the cytoplast, and prevented the combination with the p85 control site, resulting in the inactivation of PI3K and the phosphorylation of AKT, a process that influences the signal channel of insulin. By contrast, PAQR3 is able to block the Gβ locus of GPCR, and inhibits the function of AKT in GRCR-activated Gβ/γ locus. In gastric cancer tissues, PAQR3 negatively regulates the phosphorylation of ERK and AKT, which demonstrates that PAQR3 plays a role in the process of negatively regulating EMT through inhibition of the ERK and AKT signal channel (10–13). As the cancer suppressor gene in colorectal cancer, the silencing mechanism of PAQR3 remains to be determined.
The aim of the present study was to investigate PAQR3 gene expression and its methylation level in colorectal cancer tissues, as well as the association with colorectal cancer clinical data. The results of the present study provide theoretical evidence for the diagnosis and treatment of colorectal cancer.
Patients and methods
Tissue extraction
In total, 54 pairs of colorectal cancer and normal tissues, respectively, were collected from the Department of General Surgery of the Affiliated Hospital of Hebei University, between June, 2013 and July, 2014. Inclusion criteria for the study were that, the patients did not receive radiotherapy, chemotherapy or immunotherapy prior to surgery. Any tumor tissues that had already necrosis on collection of cancer tissue samples, were excluded. Normal tissues that were >10 cm from the edge of cancer tissue were collected as the cancer adjacent normal tissues. Full-thickness of the tissue was selected, and the diagnostic results were confirmed by the postoperative pathological results. There were 32 male and 22 female patients, 19 patients with rectal cancer and 35 patients with colon cancer, with an age range of 36–82 years and an average age of 59.69 years. The samples were collected during surgery, placed in liquid nitrogen, and preserved at −80°C for ≤6 months.
Reagents
The Omega E.Z.N.A.™ overall RNA extraction kit, Vazyme HiScript First Strand cDNA synthesis kit, RT-qPCR fluorescence quantitation kit, Qiagen EpiTect Fast DNA Bisulfite kit, proteinase K, RNaseA, RNA enzyme EP tubes, PAQR3 and β-actin antibodies were all purchased from Shijiazhuang Huiyou Biological Technology Co., Ltd. (Shijiazhuang, China). β-actin, PAQR3, PAQR3 methylation and non-methylation primers were synthesized according to the design of Primer Premier 5.0 software, their specificities were detected through PubMed Blast, and all the primers were produced by Sangon Biotech Co. Ltd. (Shanghai, China). STE lysate, SDS, agarose, chloroform, isoamyl, absolute ethanol, 75% absolute ethanol, Tris-phenol, phenol/chloroform/isoamylol (25:24:1), chloroform/isoamylol (24:1). TE buffer solution and anhydrous sodium acetate were used in the process of DNA extraction and nucleic acid electrophoresis. Protein lysis buffer, PMSF, 1X PBS, and 5X loading buffer were used for protein isolation. Gel electrophoresis, separation gel and running buffer, methanol, and 1X transfer membrane buffer were used for western blot analysis. Coomassie blue staining solution, and Coomassie brilliant blue bleaching liquid were used for quantification of the isolated proteins. PVDF membrane was used for immunoblotting. The membrane was incubated in primary antibody, and the secondary antibody was added in 5% skim milk in TBST. Materials for western blot analysis were provided by the Central Laboratory of the Affiliated Hospital of Hebei University.
Quantitative RT-PCR
Tissue RNA extraction
Tissue (30 mg) was collected and RNA was extracted according to the instructions of the Omega E.Z.N.A.™ RNA extraction kit. Product (2 µl) was collected for separation on 1% sepharose gel for electrophoresis. Reverse transcription was carried out after the ELISA test, and the content (2,000 ng/µl) and ratio (OD: 1.9–2.0) were determined.
Reverse transcription
RNA content was adjusted according to RNA quantitative results. RNA (1 µg) was collected, and reverse transcription was carried out according to the instructions of the Vazyme HiScript First Strand cDNA synthesis kit. The reaction conditions were denaturation at 25°C for 5 min, annealing at 42°C for 30 min, and elongation at 85°C for 5 min.
PCR
PCR primers were designed using Primer Premier 5.0. The reference gene was β-actin primer: 5′-GTGGACATCCGCAAAGAC-3′ (F), 5′-AAAGGGTGTAACGCAACTAA-3′ (R), and the length of the product was 302 bp. The PDCD4 primer was 5′-TGGGAGTGACGCCCTTAGAA-3′ (F), 5′-TCCACCTCCTCCACATCATACA-3′ (R), and the length of the product was 171 bp. The PAQR3 primer was 5′-TCTGTATGCTTTGCTCTGTGGG-3′ (F), 5′-TTTGCCATTGCTGCGTGAG-3′ (R), and the length of the product was 252 bp. The reaction conditions were 95°C for 5 min, 95°C for 10 sec, 60°C for 32 sec, for 40 cycles; and 95°C for 15 sec, 60°C for 60 sec, 95°C for 15 sec. The results were normalized to β-actin.
Western blot analysis
Protein expression levels in tissues were analyzed using western blot analysis as previously described (14). The membranes were incubated with primary goat antibody in a dilution of 1:500 (anti-PAQR3; cat. no. sc-161992; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. The membrane was washed with TBST and incubated with a peroxidase-conjugated secondary polyclonal rabbit-anti-goat antibody (dilution: 1:1,000; cat. no. sc-2768; Santa Cruz Biotechnology) for 1 h. Western blot film was scanned and the membrane was stripped and reprobed with antibody against β-actin (dilution: 1:1,500) to confirm equal sample loading.
Methylation-specific PCR (MSP)
The phenol/chloroform extraction method was used to extract tissue DNA. Hydrosulphite modification was then performed according to the instructions on the EpiTect Fast DNA Bisulfite kit. PAQR3 methylation and non-methylation primers were designed and produced using MethPrimer software (Sangon Biotech Co., Ltd.).
The PAQR3 methylation primer was: (M) 5′-TTGTTGAAGAGCGCGTATTATATC-3′ (F), 5′-TAAAAACCCGAAAATCTACTCGTA-3′ (R), and non-methylation primer was: (U) 5′-TTGTTGAAGAGTGTGTATTATATTGA-3′ (F), 5′-TAAAAAACCCAAAAATCTACTCATA-3′ (R). The PAQR4 methylation primer was: (M) 5′-TTTAGTTTCGGTTTCGTCGTTAC-3′ (F), 5′-GAAAAATCTCTAACCCTTCTCGC-3′ (R), and non-methylation primer was: (U) 5′-TTTAGTTTTGGTTTTGTTGTTATGA-3′ (F), 5′-CAAAAAATCTCTAACCCTTCTCACT-3′ (R). The reference gene β-actin primer was: 5′-GTGGACATCCGCAAAGAC-3′ (F), 5′-AAAGGGTGTAACGCAACTAA-3′ (R).
Statistical analysis
Data were analysed using statistical software SPSS 16.0 (Chicago, IL, USA). The χ2 test and Spearman relevant analysis, and two-tailed test were used for comparisons between groups. P<0.05 was considered statistically significant.
Results
PAQR3 mRNA level in colorectal cancer tissues
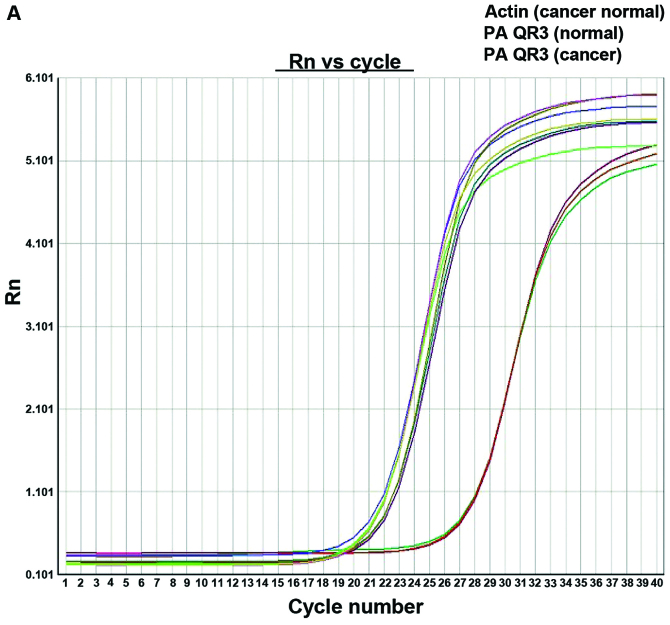
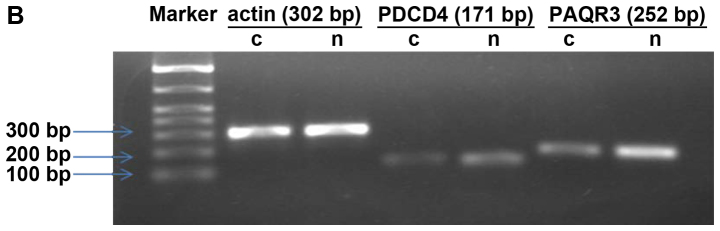
The PAQR3 mRNA expression level in colorectal cancer tissues was markedly reduced and the expression reduction rate was 57.4% (31/54), with PAQR3 mRNA in cancer tissues being 9.321.97, while that of normal adjacent tissues was 8.442.42. The difference was of statistical significance (P=0.001) (Fig. 1).
Figure 1.
RT-qPCR for PAQR3 mRNA expression level for normal and cancer cells. (A) Trend for Rn (fluorescence signal) vs. cycle number for colorectal cancer and normal adjacent tissues. (B) Agarose gel electrophoresis for actin (302 bp), PCD4 (171 bp), and PAQR3 (252 bp) PCR product. C, colorectal cancer tissues; N, normal cancer adjacent tissues.
PAQR3 protein level in colorectal cancer tissues
The expression level of PAQR3 protein was significantly low, the protein expression reduction rate in cancer tissue was 46.3% (25/54), while the protein expression reduction rate in cancer adjacent tissue was 5.6% (3/54). The difference was of statistical significance (P<0.05) (Fig. 2).
Figure 2.
PAQR3 protein expression condition.
PAQR3 gene methylation level in colorectal cancer tissues
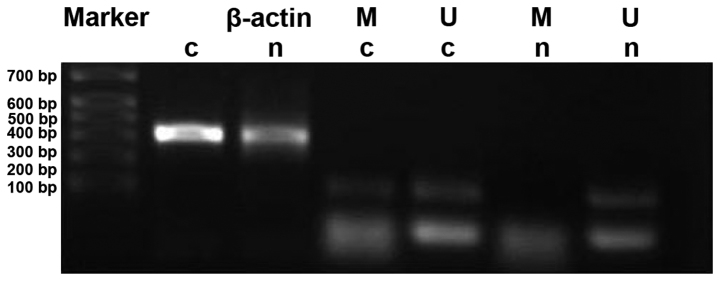
Methylation rates of PAQR3 in cancer tissues and cancer adjacent tissues were 33.3% (18/54) and 5.6% (3/54), respectively. The methylation rate of PAQR3 in cancer tissues was significantly higher than that in the corresponding normal cancer adjacent tissues (P<0.05) (Fig. 3).
Figure 3.
Agarose gel electrophoresis for the amplified products of PAQR3 MSP in colorectal cancer tissues. Marker, marker β-actin as internal reference; C, colorectal cancer tissues; N, normal cancer adjacent tissues; M, methylation primer; N, non-methylation primer; MSP, methylation-specific PCR.
Association of the PAQR3 mRNA and protein levels and colorectal cancer clinical parameters
Statistical software was detected using the Fisher's exact test probability method. The PAQR3 mRNA and protein levels were not correlated with gender, age or tumor location, but were statistically significant for differentiation degree, lymphatic metastasis and tumor infiltration depth (Table I).
Table I.
Association of the PAQR3 mRNA and protein levels and colorectal cancer clinical parameters.
| Colorectal cancer tissues | |||||||
|---|---|---|---|---|---|---|---|
| Clinical parameters | N=54 | Cases of PAQR3 reduced protein (31) | PAQR3 protein reduction rate (%) | P-value | PAQR3 mRNA cases, 31 | PAQR3 mRNA rate (%) | P-value |
| Gender | |||||||
| Male | 32 | 18 | 56.2 | 0.836 | 15 | 46.9 | 0.059 |
| 9 | 9 | ||||||
| Female | 22 | 13 | 59.1 | 16 | |||
| 4 | 4 | 72.7 | |||||
| Age (years) | |||||||
| <60 | 29 | 17 | 68.0 | 0.144 | 19 | 65.5 | 0.194 |
| 8 | |||||||
| ≥60 | 25 | 14 | 48.3 | 12 | 48.0 | ||
| 5 | 5 | ||||||
| Location | |||||||
| Colon | 35 | 19 | 54.3 | 0.529 | 23 | 65.7 | 0.094 |
| 6 | 6 | ||||||
| Rectum | 19 | 12 | 63.2 | 8 | 42.1 | ||
| 7 | 7 | ||||||
| Differentiated degree | |||||||
| Level I–II | 30 | 12 | 40.0 | 0.004a | 11 | 36.7 | 0.001a |
| 9 | 9 | ||||||
| Level III | 24 | 19 | 79.2 | ||||
| 4 | 20 | 83.3 | |||||
| Lymphatic metastasis | |||||||
| No | 28 | 11 | 39.3 | 0.005a | 12 | 42.9 | 0.025a |
| 9 | 9 | ||||||
| Yes | 26 | 20 | 76.9 | 19 | 73.1 | ||
| 4 | |||||||
| Depth of infiltration | |||||||
| Muscularis mucosae | 31 | 14 | 45.2 | 0.035a | 13 | 41.9 | 0.008a |
| Around serosa | 23 | 17 | 73.9 | 18 | 78.3 | ||
Significant differences.
Association between PDCD4 gene methylation and colorectal cancer clinical data
The methylation level of PAQR3 gene in colorectal cancer tissues was not correlated with gender or tumor location, but was associated with age, differentiation degree, lymphatic metastasis and tumor infiltration depth. The higher the age of the patient, the lower the differentiation degree observed, and the deeper the lymphatic metastasis and tumor infiltration the higher the methylation rate found, and vice versa (Table II).
Table II.
Association between PDCD4 gene methylation level and colorectal cancer clinical data.
| Colorectal cancer tissues | ||||
|---|---|---|---|---|
| Clinical data | n | Cases of PAQR3 methylation n | PAQR3 methylation rate (%) | P-value |
| Gender | ||||
| Male | 32 | 11 | 34.4 | 0.845 |
| Female | 22 | 7 | 31.8 | |
| Age (years) | ||||
| <60 | 29 | 6 | 20.7 | 0.034a |
| ≥60 | 25 | 12 | 48.0 | |
| Location | ||||
| Colon | 35 | 10 | 28.6 | 0.314 |
| Rectum | 19 | 8 | 42.1 | |
| Differentiated degree | ||||
| Level I–II | 30 | 15 | 50.0 | 0.004b |
| Level III | 24 | 3 | 12.5 | |
| Lymphatic metastasis | ||||
| No | 28 | 4 | 14.3 | 0.036a |
| Yes | 26 | 14 | 53.8 | |
| Depth of infiltration | ||||
| Muscularis mucosae | 31 | 14 | 45.2 | 0.032a |
| Around serosa | 23 | 4 | 17.4 | |
Represents differences
represents significant differences.
Discussion
The function of the PAQR3 gene and it inhibitory role in colorectal cancer was identified by Chen and Xie in 2009 (5). The gene is involved in various signal channels of cells, which can influence biological processes such as energy metabolism, cell proliferation, differentiation and the mature reproductive cells. It is also known as PKTG, which can encode 7-fold transmembrane protein of 37 kDa, N-end towards cytoplasm, C-end towards organelle, and is situated at the Golgi membrane, which can negatively regulate the Ras/Raf/MEK/ERK signal channel. This signal channel is one of the typical routes of the crystal-induced mitogen-activated protein kinase cascade signal. The PAQR3 protein competitively binds to RAF, and disturbs the combination between RAF and the downstream signal molecule substrate, which is used to identify extracellular signals and transfer extracellular stimulating signals into cells. This can achieve the purpose of physiological processes such as regulating cell proliferation, differentiation, apoptosis and malignant transformation of cells, as well as the occurrence and development of cancer (5–9). Wang et al also verified that PAQR3 plays a role in cancer inhibition in colorectal cancer tissues, and the deficiency of PAQR3 expression reduces the survival time of gene-knockout nude mice (15). In the study on human melanoma A375, the overexpression of PAQR3 clearly inhibited the amplification and malignant transformation of cells, and in the nude mouse transplantation tumor experiment, the re-expression of PAQR3 through siRNA clearly inhibited the effects of subcutaneous tumors, and the stimulation of abnormally activated ERK downstream was inhibited (16). In addition, the expression of PAQR3 in tumor tissues, such as breast cancer, was reduced, and PAQR3 enhanced the sensitivity of breast cancer SK-BR-3 cells to epirubicin (17).
The silencing mechanism of PAQR3 is not clear, thus, the gene promoter mRNA and protein levels in colorectal cancer tissues were detected to determine their association. In the present study, through the detection of tissue samples of 54 pairs of patients with colorectal cancer, it was verified that the expression of PAQR3 in colorectal cancer is reduced, and this shows that PAQR3 mRNA and protein levels in colorectal cancer tissues was not associated with gender, age or tumor location, but were correlated with differentiation degree, lymphatic metastasis and tumor infiltration depth. Previous studies only mentioned that the expression level of PAQR3 protein was reduced; however, the association between the reduction rate of protein expression and clinical data in colorectal cancer tissues was not clear (12,15). Due to the limitation of the number of samples, this result was not completely consistent.
Wang et al detected that the reduction rate of PAQR3 mRNA expression in colorectal cancer was 82.3% (51/62), which is different from our results of 57.4% (31/54) (18). However, compared with normal cancer adjacent tissues, statistical significance was observed. Authors of that study mentioned that the reduction rate of PAQR3 mRNA expression was higher in male compared to female patients, which is consistent with the epidemiological analysis results that male patients have higher morbidity in colorectal cancer compared to female patients (19). In the present study, there were more male samples collected than female samples. However, the results showed that, the reduction of PAQR3 expression was not associated with gender, which was a result based on sample and regional difference. In addition, Wang et al found that the PAQR3 mRNA level in colorectal cancer tissues was not associated with tumor location or age, which is consistent with the results of the present study. However, its relationship with tumor differentiation degree was not analyzed. In the present study, the PAQR3 mRNA level was relevant to lymphatic metastasis, tumor infiltration depth and tumor differentiation degree, and this was considered to be relevant to the number of samples collected. These indexes can be used as prognosis monitoring indicators of patients and remain to be confirmed by clinical follow-up research.
Through the detection of the methylation level of PAQR3 gene in colorectal cancer tissues, the present study evaluated methylation in colorectal cancer, and its association with clinical data. Gene methylation is an early event of tumorigenesis that can be used as diagnostic evidence of colorectal cancer. The methylation rate of PAQR3 was 33.3% (18/54), which was of statistical significance, compared to the methylation level of the normal cancer adjacent tissues. To the best of our knowledge, there are currently no studies on PAQR3 gene methylation, and the results of our study verified that PAQR3 gene methylation is involved in the formation of colorectal cancer, and can be used as the tumor marker thereof. At the same time we found that PAQR3 methylation level was relevant to age; thus, the older the patient was, the higher the PAQR3 gene methylation rate. The analysis of the relevance between gene methylation and age shows that, with the increase of age, methylation of the organism was higher. The age-relevant methylation level changing mechanism remains to be clarified, and this relationship has received controversion results from different research groups. First of all, with increasing age, enzymatic activity in the human body is altered, promoting gene methylation. This may be influenced by geographical environmental elements, random selection and sample size, although this difference was not statistically significant (20). The high methylation level of PAQR3 gene is associated with differentiated degree, lymphatic metastasis and tumor infiltration depth. Concerning gene methylation is the early event of tumorigenesis (21), the lower the differentiated degree the higher the gene methylation level was, and at a low gene expression level the ability of cancer inhibition was minimal. With the increase of differentiation degree, the possibility of methylation occurrence was less. Therefore, the lower the differentiation degree was in tumor tissues, the higher the possibility of gene methylation. PAQR3 methylation was relevant to tumor infiltration depth of colorectal cancer and lymphatic metastasis, and considering the consequence of PAQR3 gene silencing, PAQR3 can be used as the monitoring prognostic indicator of colorectal cancer. The present results verified the role that PAQR3 gene methylation plays in colorectal cancer, which provides effective theoretical evidences for further in vitro experiments, reverse gene methylation and re-expression of cancer suppressor genes.
References
- 1.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 2.Carmona FJ, Azuara D, Berenguer-Llergo A, Fernández AF, Biondo S, de Oca J, Rodriguez-Moranta F, Salazar R, Villanueva A, Fraga MF, et al. DNA methylation biomarkers for noninvasive diagnosis of colorectal cancer. Cancer Prev Res (Phila) 2013;6:656–665. doi: 10.1158/1940-6207.CAPR-12-0501. [DOI] [PubMed] [Google Scholar]
- 3.Tapp HS, Commane DM, Bradburn DM, Arasaradnam R, Mathers JC, Johnson IT, Belshaw NJ. Nutritional factors and gender influence age-related DNA methylation in the human rectal mucosa. Aging Cell. 2013;12:148–155. doi: 10.1111/acel.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HG, Zhang WJ, Ding Q, Peng G, Zou ZW, Liu T, Cao RB, Fei SJ, Li PC, Yang KY, Hu JL, Dai XF, Wu G, Li PD. Identification of PAQR3 as a new candidate tumor suppressor in hepatocellular carcinoma. Oncol Rep. 2014;32:2687–2695. doi: 10.3892/or.2014.3532. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Xie XD. The functional research of RKTG gene. J Cell Biol. 2009;31:9–14. [Google Scholar]
- 6.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 7.Feng L, Xie X, Ding Q, Luo X, He J, Fan F, Liu W, Wang Z, Chen Y. Spatial regulation of Raf kinase signaling by RKTG. Proc Natl Acad Sci USA. 2007;104:14348–14353. doi: 10.1073/pnas.0701298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/S0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 9.Fan F, Feng L, He J, Wang X, Jiang X, Zhang Y, Wang Z, Chen Y. RKTG sequesters B-Raf to the Golgi apparatus and inhibits the proliferation and tumorigenicity of human malignant melanoma cells. Carcinogenesis. 2008;29:1157–1163. doi: 10.1093/carcin/bgn119. [DOI] [PubMed] [Google Scholar]
- 10.Xie X, Zhang Y, Jiang Y, Liu W, Ma H, Wang Z, Chen Y. Suppressive function of RKTG on chemical carcinogen-induced skin carcinogenesis in mouse. Carcinogenesis. 2008;29:1632–1638. doi: 10.1093/carcin/bgn139. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu M, Bai O, Liu W, Xie X, Wang Z, et al. RKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinoma. Oncogene. 2010;29:5404–5415. doi: 10.1038/onc.2010.270. [DOI] [PubMed] [Google Scholar]
- 12.Ling ZQ, Guo W, Lu XX, Zhu X, Hong LL, Wang Z, Wang Z, Chen Y. A Golgi-specific protein PAQR3 is closely associated with the progression, metastasis and prognosis of human gastric cancers. Ann Oncol. 2014;25:1363–1372. doi: 10.1093/annonc/mdu168. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Xie X, Zhang Y, Luo X, Wang X, Fan F, Zheng D, Wang Z, Chen Y. Regulation of G-protein signaling by RKTG via sequestration of the G betagamma subunit to the Golgi apparatus. Mol Cell Biol. 2010;30:78–90. doi: 10.1128/MCB.01038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padda RS, Gkouvatsos K, Guido M, Mui J, Vali H, Pantopoulos K. A high-fat diet modulates iron metabolism but does not promote liver fibrosis in hemochromatotic Hjv−/− mice. Am J Physiol Gastrointest Liver Physiol. 2015;308:G251–G261. doi: 10.1152/ajpgi.00137.2014. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li X, Fan F, Jiao S, Wang L, Zhu L, Pan Y, Wu G, Ling ZQ, Fang J, et al. PAQR3 plays a suppressive role in the tumorigenesis of colorectal cancers. Carcinogenesis. 2012;33:2228–2235. doi: 10.1093/carcin/bgs245. [DOI] [PubMed] [Google Scholar]
- 16.Ding Q, Huo L, Yang JY, Xia W, Wei Y, Liao Y, Chang CJ, Yang Y, Lai CC, Lee DF, et al. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Cancer Res. 2008;68:6109–6117. doi: 10.1158/0008-5472.CAN-08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang JB, Luo XR, Kong LQ, Xiang TX, Ren GS. The influence of sensitivity of PAQR3 to Epirubicin of breast cancer cells SK-br-3. J Third Military Medical University. 2013;35:1658–1662. (In Chinese) [Google Scholar]
- 18.Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both β-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene. 2008;27:1527–1535. doi: 10.1038/sj.onc.1210793. [DOI] [PubMed] [Google Scholar]
- 19.Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res. 2005;65:11282–11286. doi: 10.1158/0008-5472.CAN-05-3469. [DOI] [PubMed] [Google Scholar]
- 20.Lillycrop KA, Hoile SP, Grenfell L, Burdge GC. DNA methylation, ageing and the influence of early life nutrition. Proc Nutr Soc. 2014;73:413–421. doi: 10.1017/S0029665114000081. [DOI] [PubMed] [Google Scholar]
- 21.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]