Abstract
Angiogenesis is required for the growth and metastasis of solid tumors. The anti-malarial agent dihydroartemisinin (DHA) demonstrates potent anti-angiogenic activity, but the underlying molecular mechanisms are not yet fully understood. During the process of angiogenesis, endothelial cells migrating from existing capillaries may undergo programmed cell death after detaching from the extracellular matrix, a process that is defined as anchorage-dependent apoptosis or anoikis. In the present study, DHA-induced cell death was compared in human umbilical vein endothelial cells (HUVECs) cultured in suspension and attached to culture plates. In suspended HUVECs, the cell viability was decreased and apoptosis was increased with the treatment of 50 µM DHA for 5 h, while the same treatment did not affect the attached HUVECs. In addition, 50 µM DHA increased the phosphorylation of c-Jun N-terminal kinase (JNK) in suspended HUVECs, but not in attached HUVECs, for up to 5 h of treatment. The JNK inhibitor, SP600125, reversed DHA-induced cell death in suspended HUVECs, suggesting that the JNK pathway may mediate DHA-induced endothelial cell anoikis. The data from the present study indicates a novel mechanism for understanding the anti-angiogenic effects of DHA, which may be used as a component for chemotherapy.
Keywords: dihydroartemisinin, c-Jun N-terminal kinase signaling, angiogenesis, endothelial cell, anoikis
Introduction
Angiogenesis is an important physiological process, through which novel blood vessels develop from existing vessels (1). Persistent and uncontrolled angiogenesis is involved in the pathogenesis of rheumatoid arthritis, atherosclerosis, diabetes, ocular retinopathy and tumors (2,3). In particular, tumor angiogenesis is crucial for solid tumor growth, invasion and metastasis (4). During tumor growth, the production of angiogenic factors, including members of the vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) families, from tumor cells results in the induction of capillary spouting and the subsequent growth of novel vessels in tumors from surrounding host vessels (3,5). As the vessels in the tumor are tortuous, with sluggish flow and a lack of surrounding pericytes, tumor cells readily invade into novel vessels and form tumor emboli (3). In addition, tumor cells seed in distance organs, where they undergo secondary angiogenesis. Therefore, the disruption of angiogenesis has been considered as an effective therapeutic strategy in the treatment of solid tumors (6).
Angiogenesis is a multi-step process involving endothelial cell activation, proliferation, migration, differentiation, maturation and tube formation (3,7). Endothelial cells are central for angiogenesis. Previous studies have demonstrated that the inhibition of endothelial cell death is an essential prerequisite to maintain vascular remodeling and angiogenesis (8). There are several types of morphological distinct cell death exhibited by endothelial cells (9). Adhesion to the extracellular matrix (ECM) is a crucial for survival of endothelial cells (7). During tumor angiogenesis, the vascular basement membrane is degraded after endothelial cell activation, and then the endothelial cells migrate into the subendothelial space without attachment to the ECM (8). Similarly to other anchorage-dependent cells, endothelial cells undergo cell death after detachment from the underlying ECM, which is defined as anoikis (10).
Anti-malarial agent dihydroartemisinin (DHA) is a semi-synthetic derivative of artemisinin that is extracted from the herbaceous plant, Artemisia annua (11). DHA exhibits potent antitumor and anti-angiogenesis effects and has therefore emerged as a potential component for cancer chemotherapies (12). DHA inhibits endothelial cell proliferation and migration via the downregulation of the nuclear factor-κB and extracellular signal-regulated kinase (ERK) signaling pathways (13–15). Several studies have suggested that the anti-angiogenic effects of DHA may be partly associated with its role in promoting the apoptosis of endothelial cells (12,16). However, the effects of DHA on endothelial cell anoikis have not yet been studied.
In the present study, human umbilical vein endothelial cells (HUVECs) in suspension were used as a model for anoikis. The cells in suspension or attached to culture plates were treated with DHA. The cell death of HUVECs in these two models was determined. Notably, 5 h treatment of 50 µM DHA significantly increased the cell death of HUVECs in suspension, but not for HUVECs attached to the plates. In addition, DHA specifically activated the c-Jun N-terminal kinase (JNK) pathway in suspended HUVECs, and the inhibition of the JNK pathway reversed the cell death of HUVECs in suspension. These results suggest that DHA promotes endothelial cell anoikis via the activation of the JNK pathway.
Materials and methods
Cell culture
HUVECs were purchased from Lonza Group Ltd. (Basel, Switzerland) and were cultured in endothelial basal cell medium-2 supplemented with EGM-2-MV bullet kit (Lonza Group Ltd.) and antibiotics (100 international units/ml penicillin and 100 µg/ml streptomycin). The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. The HUVECs grown on culture plates were used as attached cells. A group of confluent cells were trypsinized for 2 min and detached to form single cell suspension. These cells were cultured in suspension by slow rotation in culture flasks and collected at 5 h as suspended HUVECs. DHA (Sigma-Aldrich, St. Louis, MO, USA) and the JNK inhibitor, SP600125 (Cell Signaling Technology, Inc., Danvers, MA, USA), were dissolved in dimethyl sulfoxide (DMSO).
Trypan blue exclusion assay
Cell viability was assessed at 5 h after 50-µM DHA treatment. The single cell suspensions were prepared and diluted 1:1 with 0.4% trypan blue (w/v in 0.9% NaCl; Santa Cruz Biotechnology, Dallas, TX, USA). The dye-free cells were calculated under a light microscope.
Flow cytometry
The cell death of HUVECs induced by DHA was detected by using Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining (NeoBioscience, Shenzhen, China), according to the manufacturer's instructions. Briefly, single cell suspensions from attached or suspended HUVECs were prepared, washed with phosphate-buffered saline (PBS) and resuspended in binding buffer containing Annexin V-FITC (0.25%) and PI (1 µg/ml). An aliquot of 1×105 cells were examined using a FACSAria II flow cytometer (BD Biosciences, San Jose, CA, USA). The percentages of positive cells were analyzed using the FACSDiva version 6.0 acquisition and analysis software (BD Biosciences).
Western blotting
HUVECs were collected and washed with cold PBS, then lysed in radioimmunoprecipitation assay buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 50 mM NaF, 1% NP40, 0.1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethane sulfonyl fluoride and 1 mg/ml leupeptin]. Protein concentrations were determined using bicinchoninic acid assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts of protein were separated by 8% SDS-polyacrylamide gel electrophoresis and transferred to the polyvinylidene fluoride (PVDF) membrane. After being blocked with 5% skimmed milk overnight, the PVDF membranes were incubated with primary antibodies in PBS-Tween at 4°C. The primary antibodies used were rabbit monoclonal anti-phosphorylated- (p-)JNK (1:500; 4668; Cell Signaling Technology, Inc.), rabbit polyclonal anti-JNK (1:500; 9252; Cell Signaling Technology, Inc.) and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000; SAB2100894, Sigma-Aldrich). Immunoreactivity was visualized by using a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:2,000; 7074; Cell Signaling Technology, Inc.) and a chemiluminescence kit (Pierce ECL; Thermo Fisher Scientific, Inc., Waltham, MA USA). The densitometry analyses were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). GAPDH levels were used as controls for protein loading.
Statistical analyses
Each experiment was performed at least 3 times. Statistical analyses were performed using SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA). The results are presented as the mean ± standard deviation. A Student's t-test was used for statistical comparisons between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
DHA inhibits the cell viability of suspended endothelial cells
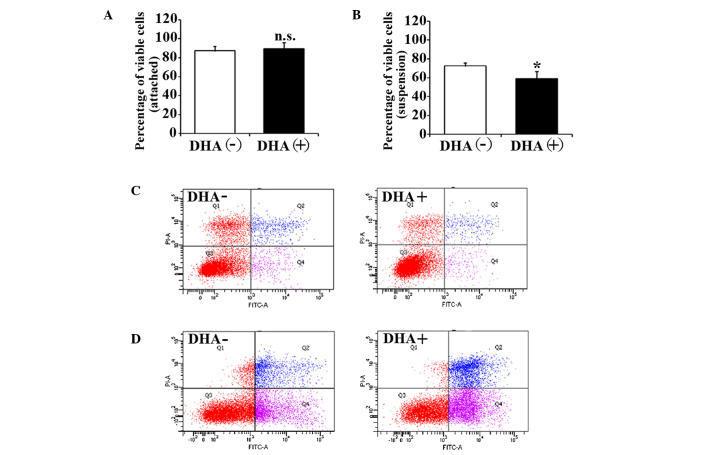
The effects of DHA on the cell viability of HUVECs were evaluated by trypan blue exclusion assay. After 5 h in suspension, the cell viability of HUVECs in the non-treatment group was significantly decreased compared with the attached HUVECs (88.3±4.6% vs. 73.6±3.1%; P=0.03). The percentage of viable cells in attached HUVECs was not affected by 50 µM DHA after 5 h incubation (88.3±4.6% vs. 90.7±6.1%; P=0.23) (Fig. 1A). However, the percentage of viable cells in suspended HUVECs was significantly decreased after the same DHA treatment (73.6±3.1% vs. 58.7±8.1%; P=0.02) (Fig. 1B). Therefore, DHA is likely to inhibit the cell viability of suspended endothelial cells but not attached endothelial cells.
Figure 1.
Viability and apoptosis of HUVECs treated with DHA. Percentages of viable cells from (A) attached or (B) suspended HUVECs treated with 50 µM DHA for 5 h; n=4. *P<0.05 vs. DHA (−). Representative images of (C) attached or (D) suspended HUVECs treated with 50 µM DHA for 5 h by flow cytometry analysis with Annexin V/PI-staining. DHA, dihydroartemisinin; HUVEC, human umbilical vein endothelial cell; n.s., non-significant; PI, propidium iodide; FITC, fluorescein isothiocyanate.
DHA induces apoptosis in suspended endothelial cells
To investigate the apoptotic status of the unviable cells, HUVECs with DHA treatment were analyzed by flow cytometry with Annexin V and PI staining. Consistent with viability assays, increased apoptosis was observed in suspended HUVECs compared with attached HUVECs in the non-treatment group (15.9±2.1% vs. 27.1±3.5%; P=0.04), suggesting that the suspended endothelial cells undergo anoikis. DHA did not alter apoptosis in attached HUVECs (15.9±2.1% vs. 16.3±1.7%; P=0.31) (Fig. 1C), but induced a significant increase of the apoptosis in suspended HUVECs (27.1±3.5% vs. 39.3±4.4%; P=0.02) (Fig. 1D). This indicates that DHA specifically enhances anoikis in endothelial cells.
DHA activates the JNK pathway in suspended endothelial cells
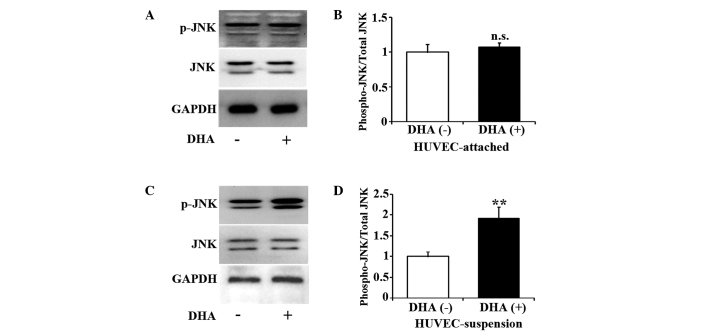
JNK is one of the major components of the mitogen-activated protein kinase cascade, and participates in cell death signaling pathways (17). Studies have suggested that the JNK pathway may mediate anoikis in epithelial cells (18,19). Therefore, the present study examined the activation of JNK in DHA treated HUVECs by western blotting. As shown in Fig. 2A and B, for up to 5 h incubation with 50 µM DHA, the level of p-JNK remained unchanged in attached HUVECs (P=0.13). However, the same treatment of DHA significantly increased p-JNK in suspended HUVECs (P=0.01) (Fig. 2C and D), suggesting that DHA activates JNK pathway in suspended endothelial cells but not in attached endothelial cells.
Figure 2.
DHA activates JNK signaling pathway in suspended HUVECs. (A) Representative immunoblots and (B) densitometry analysis of p-JNK and JNK in attached HUVECs treated with 50 µM DHA for 5 h; n=3. (C) Representative immunoblots and (D) densitometry analysis of p-JNK and JNK in suspended HUVECs treated with 50 µM DHA for 5 h; n=3. **P<0.01 vs. DHA (−). DHA, dihydroartemisinin; JNK, c-Jun N-terminal kinase; p-JNK, phosphorylated-JNK; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HUVEC, human umbilical vein endothelial cell; n.s., non-significant.
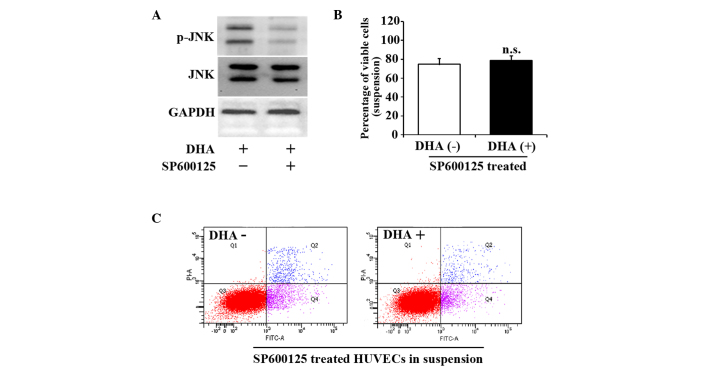
JNK inhibitor SP600125 reverses HUVEC anoikis induced by DHA
SP600125 is a cell-permeable and selective inhibitor of the JNK pathway (20). To further verify the role of the JNK signaling pathway in the cell death of suspended HUVECs, 10 µM SP600125 was applied to suspended HUVECs for 1 h prior to DHA treatment. Fig. 3A indicates that SP600125 successfully prevented the increase of p-JNK. SP600125 treatment abrogated the decrease of viable cells in suspended HUVECs treated with DHA (74.6±4.5% vs. 78.9±6.6%; P=0.19) (Fig. 3B). SP600125 also attenuated DHA-induced apoptosis in suspended HUVECs (28.6±2.5% vs. 30.3±4.9%; P=0.12) (Fig. 3C). These results suggest that JNK signaling pathway mediates DHA induced anoikis in endothelial cells.
Figure 3.
JNK inhibitor, SP600125, reverses the viability and apoptosis of suspended HUVECs induced by DHA. (A) Representative immunoblots of p-JNK and JNK in suspended HUVECs treated with DHA and SP600125. (B) Percentage of viable cells from suspended HUVECs treated with DHA and SP600125; n=4. (C) Representative images of flow cytometry analyses of Annexin V/PI-staining in suspended HUVECs treated with DHA and SP600125. DHA, dihydroartemisinin; JNK, c-Jun N-terminal kinase; p-JNK, phosphorylated-JNK; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HUVEC, human umbilical vein endothelial cell; n.s., non-significant; PI, propidium iodide; FITC, fluorescein isothiocyanate.
Discussion
DHA possesses strong anti-angiogenic activities, but the molecular mechanisms are not yet fully understood (13,14,21). The present study examined the effects of DHA on endothelial cell death in attached and suspended HUVECs. After 5 h incubation, 50 µM DHA induced cell death in suspended endothelial cells, but not in attached endothelial cells. In addition, DHA increased the expression of p-JNK, and blocking the JNK pathway abrogated DHA-induced cell death in suspended endothelial cells. The present study indicates that DHA induces endothelial cell anoikis via activation of the JNK pathway.
Anoikis is induced by lack of correct cell or ECM attachment, and anoikis resistance is important for tumor metastasis (10). During tumor angiogenesis, a group of endothelial cells migrate across the basement membrane, leading to insufficient cell-matrix interactions and subsequent anoikis (1). Consistent with the findings of other reports, the present study revealed the additional occurrence of cell death in untreated endothelial cells in suspension, a model in which endothelial cells are completely detached from the ECM. Endothelial cell anoikis is crucial for tumor angiogenesis and presents a target for anti-angiogenic therapy. In the current study, treatment with a low concentration of DHA (50 µM) for a short-term exposure time (5 h) was observed to induce cell death in suspended HUVECs. The same treatment did not affect cell survival of attached HUVECs. This suggests that DHA inhibits angiogenesis, which is at least partially due to the induction of endothelial cell anoikis.
The JNK pathway, which appears to be activated by detachment from the ECM, is critical for tumor cell apoptosis (22). However, its role in anoikis has been controversial. Several studies have reported that the activation of the JNK signaling pathway mediates anoikis in cancer cell lines (19,23,24). In addition, B cell lymphoma-2 has been found to suppress the suspension-induced activation of JNK signaling, which requires the proteolytic function associated with interleukin-1-β-converting enzyme (18). Conversely, the study by Khwaja et al (25) reported that the JNK pathway is not associated with anoikis in epithelial cells. In the present study, DHA was demonstrated to promote anoikis in suspended HUVECs through activation of the JNK signaling pathway. In endothelial cells, detachment results in a rapid rise in the level of reactive oxygen species (ROS), which modulate the activity of the JNK signaling pathway (26). DHA increases the level of ROS in several cancerous cell lines (27,28). Another artemisinin derivative, artesunate, significantly inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells (29). Therefore, DHA may induce an increase in ROS and then activate then JNK signaling pathway in suspended HUVECs.
The JNK pathway may also interact with focal adhesion kinase (FAK) signaling in mediating anoikis. FAK is a key component of cell-substratum adhesions, and disruption of FAK signaling results in a loss of substrate adhesion and anoikis in endothelial cells (30). Glucocorticoids induce osteocyte anoikis by blocking FAK signaling and activating JNK. In addition, FAK blocks the ras-related C3 botulinum toxin substrate 1/JNK pathway in vascular smooth muscle cells (31). However, in lung adenocarcinoma cells, FAK regulates anoikis independently of the JNK pathway (32). DHA directly decreases the level of p-FAK in ovarian cancer cells (33). Thus, the role of FAK signaling in DHA induced anoikis requires further investigation.
In summary, the present study demonstrates that DHA induces endothelial cell anoikis, which is mediated by the activation of the JNK pathway. DHA may be considered as a promising angiogenesis inhibitor for clinical application. The findings of the present study will aid the current understanding of the molecular mechanisms underlying the anti-angiogenic effects of DHA.
Acknowledgements
The present study was supported by the Medical Science and Technology Development Plan of Shandong Province (Jinan, China; grant no. 2013WS0137). The authors are grateful for the support provided to Professor Ju Liu from the Shandong Taishan Scholarship (Jinan, China).
References
- 1.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Deutsch U, Jeong J, Lobe CG. Constitutive notch signaling in adult transgenic mice inhibits bFGF-induced angiogenesis and blocks ovarian follicle development. Genesis. 2014;52:809–816. doi: 10.1002/dvg.22790. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 8.Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: A role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a006080. pii: a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Zhou HJ, Wu XH. Dihydroartemisinin downregulates vascular endothelial growth factor expression and induces apoptosis in chronic myeloid leukemia K562 cells. Cancer Chemother Pharmacol. 2006;57:213–220. doi: 10.1007/s00280-005-0002-y. [DOI] [PubMed] [Google Scholar]
- 12.Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142:126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Dong F, Zhou X, Li C, Yan S, Deng X, Cao Z, Li L, Tang B, Allen TD, Liu J. Dihydroartemisinin targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis. Cancer Biol Ther. 2014;15:1479–1488. doi: 10.4161/15384047.2014.955728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong F, Tian H, Yan S, Li L, Dong X, Wang F, Li J, Li C, Cao Z, Liu X, Liu J. Dihydroartemisinin inhibits endothelial cell proliferation through the suppression of the ERK signaling pathway. Int J Mol Med. 2015;35:1381–1387. doi: 10.3892/ijmm.2015.2140. [DOI] [PubMed] [Google Scholar]
- 15.Guo L, Dong F, Hou Y, Cai W, Zhou X, Huang AL, Yang M, Allen TD, Liu J. Dihydroartemisinin inhibits vascular endothelial growth factor-induced endothelial cell migration by a p38 mitogen-activated protein kinase-independent pathway. Exp Ther Med. 2014;8:1707–1712. doi: 10.3892/etm.2014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HH, Zhou HJ, Wang WQ, Wu GD. Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother Pharmacol. 2004;53:423–432. doi: 10.1007/s00280-003-0751-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Kapron CM. Differential induction of MAP kinase signalling pathways by cadmium in primary cultures of mouse embryo limb bud cells. Reprod Toxicol. 2010;29:286–291. doi: 10.1016/j.reprotox.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun-N-terminal kinase in anoikis; suppression by bcl-2 and crmA. J Cell Biol. 1996;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardone MH, Salvesen GS, Widmann C, Johnson G, Frisch SM. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/S0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 20.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S, Jeong IH, Shin WS, Lee S. Growth inhibition activity of thioacetal artemisinin derivatives against human umbilical vein endothelial cells. Bioorg Med Chem Lett. 2003;13:3665–3668. doi: 10.1016/j.bmcl.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Rivera Rosado LA, Moon SY, Zhang B. Silencing of D4-GDI inhibits growth and invasive behavior in MDA-MB-231 cells by activation of Rac-dependent p38 and JNK signaling. J Biol Chem. 2009;284:12956–12965. doi: 10.1074/jbc.M807845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krestow JK, Rak J, Filmus J, Kerbel RS. Functional dissociation of anoikis-like cell death and activity of stress activated protein kinase. Biochem Biophys Res Commun. 1999;260:48–53. doi: 10.1006/bbrc.1999.0863. [DOI] [PubMed] [Google Scholar]
- 25.Khwaja A, Downward J. Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J Cell Biol. 1997;139:1017–1023. doi: 10.1083/jcb.139.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li AE, Ito H, Rovira II, Kim KS, Takeda K, Yu ZY, Ferrans VJ, Finkel T. A role for reactive oxygen species in endothelial cell anoikis. Circ Res. 1999;85:304–310. doi: 10.1161/01.RES.85.4.304. [DOI] [PubMed] [Google Scholar]
- 27.Kong R, Jia G, Cheng ZX, Wang YW, Mu M, Wang SJ, Pan SH, Gao Y, Jiang HC, Dong DL, Sun B. Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5. PloS One. 2012;7:e37222. doi: 10.1371/journal.pone.0037222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SJ, Kim MS, Lee JW, Lee CH, Yoo H, Shin SH, Park MJ, Lee SH. Dihydroartemisinin enhances radiosensitivity of human glioma cells in vitro. J Cancer Res Clin Oncol. 2006;132:129–135. doi: 10.1007/s00432-005-0052-x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng R, Li C, Wei L, Li L, Zhang Y, Yao Y, Gu X, Cai W, Yang Z, Ma J, et al. The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Invest Ophthalmol Vis Sci. 2013;54:3400–3409. doi: 10.1167/iovs.12-11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Q, Rounds S. Focal adhesion kinase and endothelial cell apoptosis. Microvasc Res. 2012;83:56–63. doi: 10.1016/j.mvr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundberg LJ, Galante LM, Bill HM, Mack CP, Taylor JM. An endogenous inhibitor of focal adhesion kinase blocks Rac1/JNK but not Ras/ERK-dependent signaling in vascular smooth muscle cells. J Biol Chem. 2003;278:29783–29791. doi: 10.1074/jbc.M303771200. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Meng X, Jin Y, Bai J, Zhao Y, Cui X, Chen F, Fu S. Inhibitory role of focal adhesion kinase on anoikis in the lung cancer cell A549. Cell Biol Int. 2008;32:663–670. doi: 10.1016/j.cellbi.2008.01.292. [DOI] [PubMed] [Google Scholar]
- 33.Wu B, Hu K, Li S, Zhu J, Gu L, Shen H, Hambly BD, Bao S, Di W. Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Rep. 2012;27:101–108. doi: 10.3892/or.2011.1505. [DOI] [PubMed] [Google Scholar]