Abstract
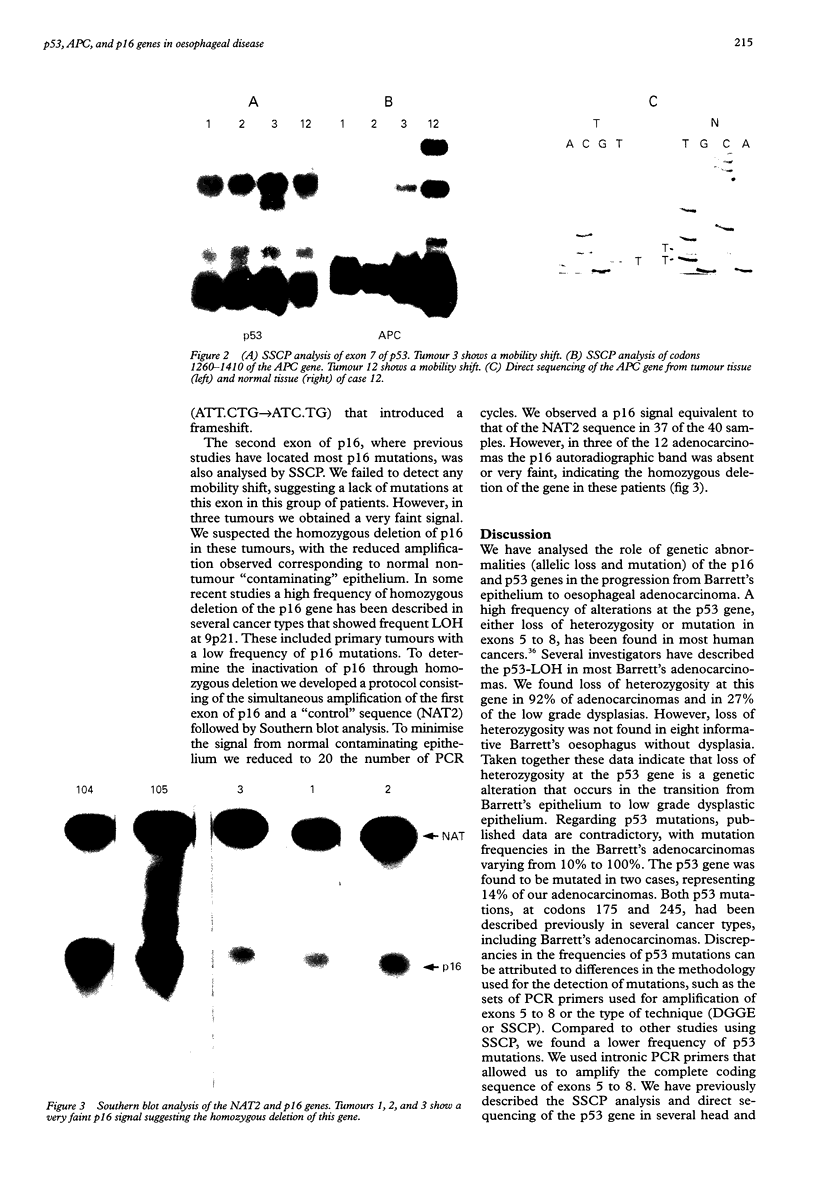
AIMS: To study the loss of heterozygosity and the presence of mutations at the p53, p16/CDKN2, and APC genes in Barrett's oesophagus, low grade dysplastic oesophageal epithelium, and adenocarcinoma of the oesophagus; to relate the presence of alterations at these genes with the progression from Barrett's oesophagus to adenocarcinoma. METHODS: DNA was extracted from paraffin blocks containing tissue from Barrett's oesophagus (12 samples), low grade dysplasia (15 cases), and adenocarcinoma (14 cases). Loss of heterozygosity (LOH) at the p53, p16, and APC genes was determined by comparing the autoradiographic patterns of several microsatellite markers between the normal tissue and the malignant tissue counterpart. SSCP was used to determine the presence of mutations at p53 (exons 5 to 8), p16 (exon 2), and APC. Homozygous deletion of the p16 gene was defined through polymerase chain reaction followed by Southern blot. RESULTS: LOH at the p53, p16, and APC genes was not observed in Barrett's oesophagus without dysplasia, and increased to 90% (p53), 89% (p16), and 60% (APC) in the adenocarcinomas. The p53 gene was mutated in only two adenocarcinomas (codons 175 and 245). In one case a mutation at the APC gene (codon 1297) was found. No patient had mutation at the second exon of p16. However, this gene was homozygously deleted in three of the 12 adenocarcinomas. CONCLUSIONS: The tumour suppressor genes p53, p16, and APC are often deleted in adenocarcinomas derived from Barrett's oesophagus. Mutations at these genes are also found in the adenocarcinomas, including the homozygous deletion of the p16 gene. However, the absence of genetic alterations in the Barrett's oesophagus and the low grade dysplastic epithelia suggest that mutations at these genes develop later in the progression from Barrett's oesophagus to adenocarcinoma.
Full text
PDF

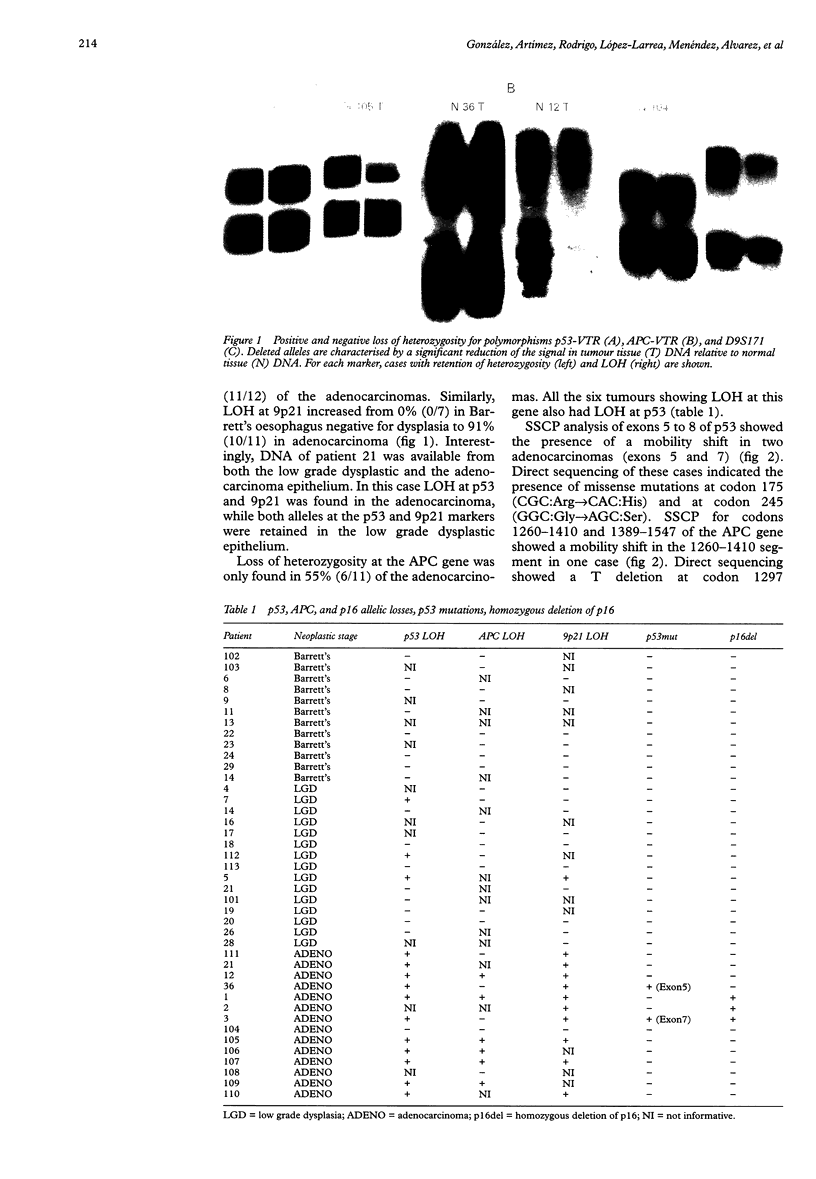


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blount P. L., Galipeau P. C., Sanchez C. A., Neshat K., Levine D. S., Yin J., Suzuki H., Abraham J. M., Meltzer S. J., Reid B. J. 17p allelic losses in diploid cells of patients with Barrett's esophagus who develop aneuploidy. Cancer Res. 1994 May 1;54(9):2292–2295. [PubMed] [Google Scholar]
- Blount P. L., Meltzer S. J., Yin J., Huang Y., Krasna M. J., Reid B. J. Clonal ordering of 17p and 5q allelic losses in Barrett dysplasia and adenocarcinoma. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3221–3225. doi: 10.1073/pnas.90.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P. L., Ramel S., Raskind W. H., Haggitt R. C., Sanchez C. A., Dean P. J., Rabinovitch P. S., Reid B. J. 17p allelic deletions and p53 protein overexpression in Barrett's adenocarcinoma. Cancer Res. 1991 Oct 15;51(20):5482–5486. [PubMed] [Google Scholar]
- Cairns P., Polascik T. J., Eby Y., Tokino K., Califano J., Merlo A., Mao L., Herath J., Jenkins R., Westra W. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995 Oct;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Casson A. G., Mukhopadhyay T., Cleary K. R., Ro J. Y., Levin B., Roth J. A. p53 gene mutations in Barrett's epithelium and esophageal cancer. Cancer Res. 1991 Aug 15;51(16):4495–4499. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- González M. V., Pello M. F., López-Larrea C., Suárez C., Menéndez M. J., Coto E. Loss of heterozygosity and mutation analysis of the p16 (9p21) and p53 (17p13) genes in squamous cell carcinoma of the head and neck. Clin Cancer Res. 1995 Sep;1(9):1043–1049. [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Fléjou J. F., Muzeau F., Potet F., Laurent-Puig P., Fékété F., Thomas G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett's esophagus. Gastroenterology. 1994 Oct;107(4):1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Huang Y., Boynton R. F., Blount P. L., Silverstein R. J., Yin J., Tong Y., McDaniel T. K., Newkirk C., Resau J. H., Sridhara R. Loss of heterozygosity involves multiple tumor suppressor genes in human esophageal cancers. Cancer Res. 1992 Dec 1;52(23):6525–6530. [PubMed] [Google Scholar]
- Huang Y., Meltzer S. J., Yin J., Tong Y., Chang E. H., Srivastava S., McDaniel T., Boynton R. F., Zou Z. Q. Altered messenger RNA and unique mutational profiles of p53 and Rb in human esophageal carcinomas. Cancer Res. 1993 Apr 15;53(8):1889–1894. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Levine D. S., Haggitt R. C., Blount P. L., Rabinovitch P. S., Rusch V. W., Reid B. J. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993 Jul;105(1):40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- Marchetti A., Buttitta F., Pellegrini S., Campani D., Diella F., Cecchetti D., Callahan R., Bistocchi M. p53 mutations and histological type of invasive breast carcinoma. Cancer Res. 1993 Oct 1;53(19):4665–4669. [PubMed] [Google Scholar]
- McKinley M. J., Budman D. R., Grueneberg D., Bronzo R. L., Weissman G. S., Kahn E. DNA content in Barrett's esophagus and esophageal malignancy. Am J Gastroenterol. 1987 Oct;82(10):1012–1015. [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Ando H., Nagase H., Nishisho I., Horii A., Miki Y., Mori T., Utsunomiya J., Baba S., Petersen G. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Miura K., Aoki T., Nishihira T., Mori S., Nakamura Y. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer Res. 1994 Jul 1;54(13):3396–3397. [PubMed] [Google Scholar]
- Neshat K., Sanchez C. A., Galipeau P. C., Blount P. L., Levine D. S., Joslyn G., Reid B. J. p53 mutations in Barrett's adenocarcinoma and high-grade dysplasia. Gastroenterology. 1994 Jun;106(6):1589–1595. doi: 10.1016/0016-5085(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Neshat K., Sanchez C. A., Galipeau P. C., Cowan D. S., Ramel S., Levine D. S., Reid B. J. Barrett's esophagus: a model of human neoplastic progression. Cold Spring Harb Symp Quant Biol. 1994;59:577–583. doi: 10.1101/sqb.1994.059.01.065. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Phillips R. W., Wong R. K. Barrett's esophagus. Natural history, incidence, etiology, and complications. Gastroenterol Clin North Am. 1991 Dec;20(4):791–816. [PubMed] [Google Scholar]
- Rabinovitch P. S., Reid B. J., Haggitt R. C., Norwood T. H., Rubin C. E. Progression to cancer in Barrett's esophagus is associated with genomic instability. Lab Invest. 1989 Jan;60(1):65–71. [PubMed] [Google Scholar]
- Reid B. J., Blount P. L., Rubin C. E., Levine D. S., Haggitt R. C., Rabinovitch P. S. Flow-cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992 Apr;102(4 Pt 1):1212–1219. [PubMed] [Google Scholar]
- Reid B. J., Weinstein W. M., Lewin K. J., Haggitt R. C., VanDeventer G., DenBesten L., Rubin C. E. Endoscopic biopsy can detect high-grade dysplasia or early adenocarcinoma in Barrett's esophagus without grossly recognizable neoplastic lesions. Gastroenterology. 1988 Jan;94(1):81–90. doi: 10.1016/0016-5085(88)90613-0. [DOI] [PubMed] [Google Scholar]
- Rusch V. W., Levine D. S., Haggitt R., Reid B. J. The management of high grade dysplasia and early cancer in Barrett's esophagus. A multidisciplinary problem. Cancer. 1994 Aug 15;74(4):1225–1229. doi: 10.1002/1097-0142(19940815)74:4<1225::aid-cncr2820740408>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Zhou X., Yin J., Lei J., Jiang H. Y., Suzuki Y., Chan T., Hannon G. J., Mergner W. J., Abraham J. M. Intragenic mutations of CDKN2B and CDKN2A in primary human esophageal cancers. Hum Mol Genet. 1995 Oct;4(10):1883–1887. doi: 10.1093/hmg/4.10.1883. [DOI] [PubMed] [Google Scholar]
- Tamura G., Maesawa C., Suzuki Y., Tamada H., Satoh M., Ogasawara S., Kashiwaba M., Satodate R. Mutations of the APC gene occur during early stages of gastric adenoma development. Cancer Res. 1994 Mar 1;54(5):1149–1151. [PubMed] [Google Scholar]
- Tarmin L., Yin J., Zhou X., Suzuki H., Jiang H. Y., Rhyu M. G., Abraham J. M., Krasna M. J., Cottrell J., Meltzer S. J. Frequent loss of heterozygosity on chromosome 9 in adenocarcinoma and squamous cell carcinoma of the esophagus. Cancer Res. 1994 Dec 1;54(23):6094–6096. [PubMed] [Google Scholar]
- Zhou X., Suzuki H., Shimada Y., Imamura M., Yin J., Jiang H. Y., Tarmin L., Abraham J. M., Meltzer S. J. Genomic DNA and messenger RNA expression alterations of the CDKN2B and CDKN2 genes in esophageal squamous carcinoma cell lines. Genes Chromosomes Cancer. 1995 Aug;13(4):285–290. doi: 10.1002/gcc.2870130409. [DOI] [PubMed] [Google Scholar]