Abstract
The effect of β2-adrenergic receptor (AR) overexpression on interleukin (IL)-10 content secreted by cardiomyocytes of heart failure (HF) rats was investigated. A rat model of chronic HF was established by partially banding abdominal aorta and the cardiomyocytes were isolated with collagenase II. The cardiomyocytes were then transfected with adenovirus type 5-ADRβ2-enhanced green fluorescent protein (EGFP) for 48 h to observe the changes of β2-AR protein expression using western blot analysis. The IL-10 level was detected by ELISA. The experiment was divided into seven groups: Control, HF, HF+EGFP, HF+β2, sham, sham+EGFP and sham+β2 groups. Compared with the sham-operated group, left ventricular diastolic dimension, and left ventricular systolic dimension were increased (P<0.05), whereas ejection fraction and fractional shortening were decreased (P<0.05) in the HF group. Compared with the sham group, the cardiomyocyte survival rate of the HF group was significantly reduced (P<0.05). Compared with the control or sham group, the β2-AR protein level of the HF group showed no significant differences (P>0.05). Compared with the HF and HF+EGFP groups, the expression of β2-AR protein of cardiomyocytes was increased in the HF+β2 group (P<0.05). Compared with the sham group, IL-10 content secreted by cardiomyocytes in the HF group was increased (P<0.05). Compared with the HF and HF+EGFP groups, IL-10 content in the HF+β2 group was increased significantly (P<0.05). In conclusion, the concentration of IL-10 secreted by cardiomyocytes of HF rats was increased. The overexpression of β2-AR in the cardiomyocytes of HF rats was able to enhance the secretion of IL-10.
Keywords: heart failure, β2-adrenergic receptor, gene transfection, interleukin-10
Introduction
Chronic heart failure (CHF) is the last stage for the majority of cardiovascular diseases, and is a leading cause of mortality (1). Its prognosis is worse than many malignant tumors. Excessive enhancement of sympathetic nerve excitability is the important pathological and physiological ground for the development and progression of CHF (1). β-adrenergic receptor (AR) is a major receptor of the sympathetic nerve system, and βl-AR, β2-AR and β3-AR of β-AR exist on myocardial cells, with βl-AR and β2-AR being the most frequent. In HF, due to the selective reduction of βl-AR, the physiological effects of β2-AR were significantly improved (2).
Experimental results showed that in ischemic myocardial cells of domestic rabbits, the enhancement of β2-AR gene expression can improve the myocardial contractility and cell survival rate (3). By contrast, an increasing number of experimental studies demonstrated that immune activation and inflammatory reaction, with the increase of inflammatory factors as their symbols, also play an important role in the process of CHF. Interleukin (IL)-10 is one of the most important anti-inflammatory cytokines that has been identified, and which plays a role in lowering the levels of pro-inflammatory factors such as tumor necrosis factor α (TNF-α), IL-1 and IL-6, and has protective effects on the heart (4). It has been reported that the application of immunoregulation to increase IL-10 can improve the symptoms of HF (5). Previous findings showed that agonist β2-AR is capable of inhibiting the generation of multiple pro-inflammatory factors. However, currently there are no literature reports on the overexpression of β2-AR to clarify whether gene transfection in HF can influence the release of anti-inflammatory cytokine IL-10 of myocardial cells (6,7).
The present study examined the effect of β2-adrenergic receptor (AR) overexpression on interleukin (IL)-10 content secreted by cardiomyocytes of heart failure (HF) rats. The results showed that the concentration of IL-10 secreted by cardiomyocytes of HF rats was increased. The overexpression of β2-AR in the cardiomyocytes of HF rats was able to enhance the secretion of IL-10.
Materials and methods
Experimental animals
Healthy male Sprague-Dawley (SD) rats (n=100), weighing 180–220 g, were provided by the Experimental Animal Center of Xuzhou Medical College (Jiangsu, China). Animal experiments were approved by the ethics committee of Xuzhou Medical College
Experimental reagents
Recombinant adenovirus with target gene β2-AR [adenovirus type 5 (Ad5)-ADRβ2-enhanced green fluorescent protein (EGFP)] and recombinant adenovirus only with EGFP (Ad5-EGFP) were structured by Vector Gene Technology Co., Ltd. (Beijing, China). Collagenase type II was purchased from Worthington Biochemical Co. (Freehold, NJ, USA). M199 culture medium, carnitine, taurine and double antibody were purchased from Sigma (St. Louis, MO, USA), and bovine serum albumin (BSA) was purchased from Sunshine Biotechnology Co., Ltd. (Shanghai, China). Anti-β2-AR (H-20): sc-569 was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), and anti-β-actin was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The NBT/BCIP kit was purchased from Promega Corp. (Madison, WI, USA), and the rats IL-10 ELISA kit was obtained from eBioscience, Inc. (San Diego, CA, USA).
Methods
Establishment of a rat model with CHF
Abdominal aortic banding was used for the rat model of CHF, and abdominal aortas were isolated without banding for rats in the sham-operated group. Color Doppler imaging (GE Healthcare, Piscataway, NJ, USA; Vivid7, provided by the Xuzhou Central Hospital) and 10S probe (probe frequency: 11.0 MHz) were used to detect cardiac structure and function 12 weeks after the operation. Left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), LVE shortening fraction (FS) and ejection fraction (EF) were measured, respectively. Five cardiac cycles were selected to calculate the mean value of each ultrasound measurement value. The screening criteria included: rats with poor appetite, no gain in weight, low spirit, low activities, fluffy and lackluster coat of fur, and tachypnea, and cardiac color Doppler ultrasound index EF <60%. Rats conforming to the above criteria were included into the HF group. Rats in the HF group were randomly divided into the HF control group (HF), EGFP-transfected group (HF+EGFP) and β2-AR-EGFP-transfected group (HF+β2). Rats in the sham-operated group were also randomly divided into the control group (sham), EGFP-transfected group (sham+EGFP) and β2-AR-EGFP-transfected group (sham+β2).
Isolation and culture of myocardial cells of rats
Collagenase type II dissociation method was used for the isolation of myocardial cells of rats. Abdomens of rats were injected with heparin sodium (1,500 U/150 g) 30 min prior to anesthesia. Thoracotomy was immediately employed to obtain hearts after anesthesia, the hearts were placed in cold 1 mmol/l calciferous KH solution to cease beating, and were immediately hung on the Langendorff perfusion apparatus (ADInstrument, Colorado Springs, CO, USA). After 5 min retrograde perfusion through aorta with 1 mmol/l calciferous KH solution, the calciferous KH solution was replaced by low-calcium KH solution for another 5 min perfusion, and then converting enzyme solution circulatory perfusion was used for 10–15 min. The temperature of the whole perfusion process was kept at 37°C, and the flow rate was kept at 8–10 ml/min. Once the textures of hearts softened, the myocardium was dissected and cultured in the culture dish. Myocardium was cut into pieces, oscillated and incubated in enzyme solution for 5 min. A nylon net (300 µm) was used for filtration, the filter solution was centrifuged at 16 × g (400 rpm) for 1 min, the supernatant was discarded and naturally settled in 1 mmol/l calciferous KH solution after re-suspension and repeated three times. The visual counting method was utilized to calculate the density of myocardial cells, and cells were cultured using M199 culture solution with 0.2% BSA in the living cell density of 1×105/ml.
Gene transfection of myocardial cells of rats
Recombinant adenovirus with β2-AR or EGFP genes were used to transfect myocardial cells of rats, at a multiplicity of infection of 100. At 48 h after transfection, the myocardial cells were removed from the incubator, observed and photographed under a fluorescence microscope (AF6000, Leica (Wetzlar, Germany). Five views were randomly selected and transfection efficiencies, as the number/total number of stab cells with the expression of green fluorescence under the same view, were calculated, respectively.
Detection of β2-AR protein expression by western blotting
At 48 h after transfection, adenovirus-transfected myocardial cells were collected, cleaned in culture medium twice, centrifuged 16 × g (400 rpm) for 1 min and the supernatant was discarded, and homogenate with protease inhibitor cocktail was added. The cells were broken by ultrasound, after measuring its protein concentration by the Lowry method, 4 × Laemmli SDS-polyacrylamide gel (PAGE) loading buffer of the same volume was added to the sample with the same protein content, and was placed in a boiling water bath for 5 min. Denatured protein sample (100 µg) of the same volume was loaded on SDS-PAGE for ionophoretic separation. Subsequently, separated proteins were transferred to a nitrocellulose (NC) membrane using the semi-dry transferring method. The NC membrane was incubated for 3 h at room temperature with primary mouse monoclonal anti-β2 AR antibody at a dilution of 1:1000 (Sigma-Aldrich, St. Louis, MO, USA, catalog no.: SAB4504272) and then transferred to 4°C for overnight incubation. TBST was used for washing the membrane three times, followed by incubation with polyclonal goat-anti-mouse secondary antibody (Sigma-Aldrich, catalog no.: A6715) for 2 h at room temperature. TBST buffer was used to wash the membrane for 5 min, three times. For the chemiluminescent reaction, the NBT/BCIP kit was used for coloration in newly prepared AP coloring solution. Images were captured and data analysed using software such as Image J, Sigmastat and Sigmaplot (7). Optical density of the control group was set at 1, and other groups were represented by its multiple.
Detection of IL-10 content by ELISA
After 48 h of incubation, myocardial cells in each group were centrifuged at 389 × g for 20 min. The culture supernatants were collected, and preserved at −80°C. ELISA was used to detect the content of IL-10 released by myocardial cells, and the procedures were in accordance with the instructions of the kit (eBioscience, San Diego, CA, USA).
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. Measurement data were presented as mean ± standard deviation. Double independent sample t-test was employed for the treatment of results of the ultrasound cardiac function measuring of rats. After homogeneity test of variances, western blot data and ELISA detection results were statistically analyzed by one-way analysis of variance. As for variance comparison among groups, the LSD test was used for homogeneity, while Dunnett's T3 test was used for heterogeneity. P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of the model of rats with CHF
Partial abdominal aortic constriction was employed for 70 male SD rats to establish the HF model, and abdominal aortas were isolated without banding for 30 rats in the sham-operated group. Post-operation, 18 rats in the model group died, and the mortality rate was 26%; and 1 rat in the sham-operated group died, and the mortality rate was 3%. The rats in the HF model group had symptoms of loss of appetite and weight, low spirit, low activity, fluffy and lackluster coat of hair, tachypnea after surgery, and the 27 rats conforming to the criteria of HF in color Doppler ultrasonography after 12 weeks, were randomly divided into groups. No symptoms were identified in the sham-operated group, and 27 of 29 rats were randomly selected for ultrasonography, and randomly divided into groups. The results of color Doppler ultrasonography after 12 weeks showed that compared with the sham-operated group, LVEDD and LVESD of rats in the HF group significantly increased (P<0.05), while FS and EF significantly decreased (P<0.05; Table I).
Table I.
Comparison of cardiac ultrasound indexes between heart failure and sham-operated rats, 12 weeks after operation.
| Groups | Cases | LVEDD, mm | LVESD, mm | FS, % | EF, % |
|---|---|---|---|---|---|
| Sham | 27 | 5.93±0.68 | 3.25±0.72 | 46.22±6.28 | 81.59±5.20 |
| HF | 27 | 8.78±0.80a | 6.15±0.95a | 26.30±3.17a | 55.96±5.05a |
Compared with sham-operated group
P<0.05. HF, heart failure; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; FS, shortening fraction; EF, ejection fraction.
Results of myocardial cell survival rate
In total, 27 rats conformed to the criteria of HF. Following myocardial cell isolation, the rats were randomly divided into the HF control group (HF), EGFP-transfected group (HF+EGFP) and β2-AR-EGFP-transfected group (HF+β2), with 9 cases in each group. Similarly, after myocardial cell isolation, 27 rats in the sham-operated group were randomly divided into the control group (sham), EGFP-transfected group (sham+EGFP) and β2-AR-EGFP-transfected group (sham+β2), with 9 cases in each group. In addition, 9 normal rats were selected for myocardial cell isolation as the normal control group (control).
In comparison to the control group, after 48 h of incubation the myocardial cell survival rates in the HF, HF+EGFP and HF+β2 groups significantly decreased (P<0.05). Compared with the sham group, after 48 h the myocardial cell survival rates in the sham+EGFP and sham+β2 groups did not show any significance. Compared with the HF group, the 48-h myocardial cell survival rates in the HF+EGFP and HF+β2 groups had no significant difference (Table II).
Table II.
Comparison of 48 h myocardial cell survival rates of rats in each group.
| Groups | Cases | 48-h survival rates, % |
|---|---|---|
| Control | 9 | 63.93±4.82 |
| Sham | 9 | 63.45±5.76 |
| Sham+EGFP | 9 | 62.15±4.59 |
| Sham+β2 | 9 | 62.68±7.55 |
| HF | 9 | 54.48±7.70a |
| HF+EGFP | 9 | 52.33±7.22a |
| HF+β2 | 9 | 52.79±7.90a |
Control group compared with sham group
P<0.05. HF, heart failure; EGFP, enhanced green fluorescent protein.
Myocardial cells transfected with adenovirus with β2-AR or EGFP genes
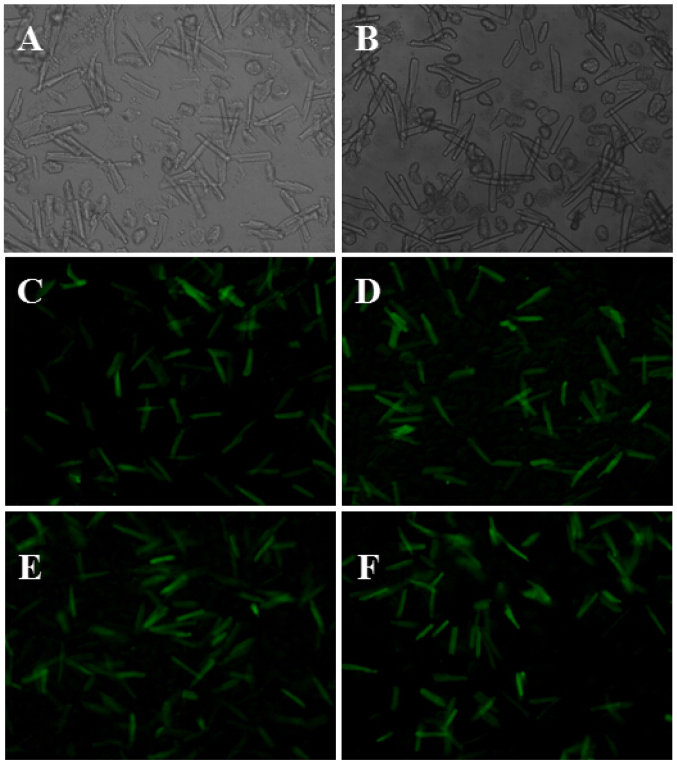
At 48 h after myocardial cells of rats were transfected with adenovirus with β2-AR or EGFP genes, green fluorescence was observed under an inverted fluorescence microscope, and the transfection efficiency was calculated as 80% (Fig. 1).
Figure 1.
Expression of green fluorescent protein after 48 h myocardial cell culture, detected by a fluorescence microscope. Myocardial cells in: (A) Sham group (×100 magnification); (B) HF group (×100 magnification); (C) sham+β2 group (×100 magnification); (D) HF+β2 group (×100 magnification); (E) sham+EGFP group (×100 magnification); and (F) HF+EGFP group (×100 magnification).
β2-AR expression of myocardial cells of rats transfected with adenovirus with β2-AR or EGFP genes
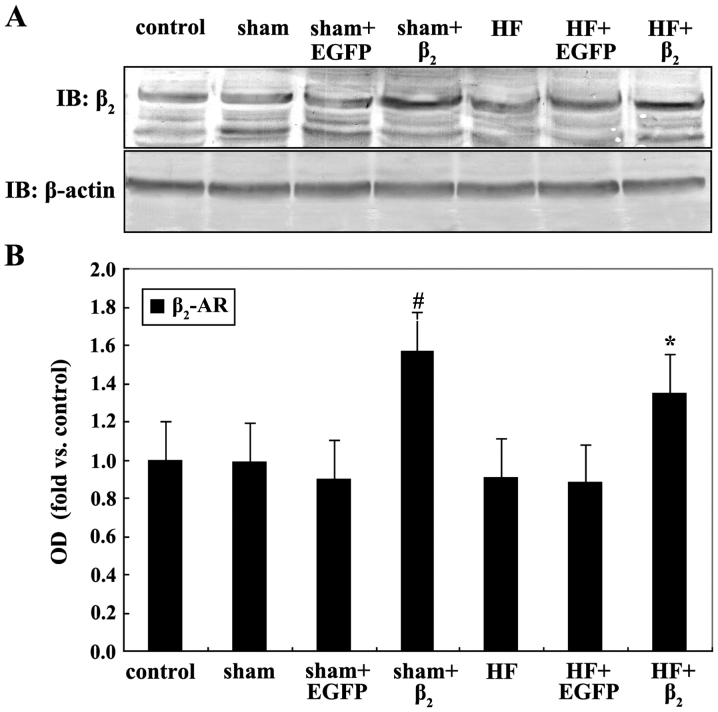
Compared with the control or sham group, β2-AR expression in the HF group had no significant difference. The content of β2-AR of myocardial cells in the HF+β2 group was significantly higher than that of the HF+EGFP and HF groups (P<0.05), and the β2-AR expression content of the sham+β2 group was higher than that of the sham+EGFP and sham groups (P<0.05; Fig. 2).
Figure 2.
(Α) Western blotting for β2-adrenergic receptor (AR) protein expression in each group after 48 h culture of myocardial cells, compared with (Β) sham+EGFP, #P<0.05; compared with HF+EGFP, *P<0.05 (n=4). EGFP, enhanced green fluorescent protein; OD, optical density.
Results of myocardial cell IL-10 level
Compared with the control and sham group, IL-10 content in the HF group was slightly higher (P<0.05). The IL-10 content in the HF+β2 group was significantly higher than that of the HF+EGFP and HF groups (in both conditions, P<0.05), while the differences between IL-10 content in the sham+β2 group and that of the sham+EGFP and sham groups are of no statistical significance (in either condition, P>0.05; Table III).
Table III.
Comparison of IL-10 content in each group released by myocardial cells of rats.
| Groups | Cases | IL-10, pg/ml |
|---|---|---|
| Control | 9 | 18.91±5.87 |
| Sham | 9 | 21.60±5.89 |
| Sham+EGFP | 9 | 21.05±3.76 |
| Sham+β2 | 9 | 22.51±5.67 |
| HF | 9 | 39.84±5.96a |
| HF+EGFP | 9 | 42.23±4.74a |
| HF+β2 | 9 | 63.11±5.62a,b |
Compared with control group and sham group
P<0.05; compared with HF group
P<0.05. HF, heart failure; EGFP, enhanced green fluorescent protein.
Discussion
Heart failure (HF) is a complex clinical syndrome, its basic process is myocardial remodeling, and its performance includes hypertrophy and apoptosis of myocardial cells and fibrosis of mesenchyme (1). Recent findings have shown that inflammatory cytokines participate in the development and progression of HF. According to its biological effects, multiple cytokines that are involved in CHF can be generally divided into (8,9): The pro-inflammatory cytokines, including IL-1, IL-6, TNF-α and CRP, the most reported in literature (10–12); and the anti-inflammatory cytokines, such as IL-10, IL-4 and transforming growth factor-β (8–12).
IL-10 is one of the most important anti-inflammatory cytokines which is mainly generated by B cells, monocytes/macrophages and Th2 cells (13). In addition, other types of cells including CD8+ cells, keratinocytes, mastocytes and myocardial cells can also generate IL-10 (14). The main biological activity of IL-10 involves immunosuppressive effects, which can inhibit multiple cytokines including interferon-γ, IL-2, IL-3, IL-1β, IL-6, and TNF-α. IL-10 has been identified to have immunomodulatory effects, which can facilitate cellular immunity, stimulate B cells to activate hyperplasia and release antibodies, and facilitate the proliferation and maturity of T cells (8,15). Based on the biological characteristics of IL-10, it is believed that IL-10 is a protective cytokine in CHF (16). Verma et al (17) reported that isoproterenol (ISO) was utilized to induce the myocardial hypertrophy HF model of wild-type and IL-10 gene knockout mice, and it was identified that the myocardial hypertrophic remodeling and cardiac function conditions of IL-10 gene knockout mice are more deteriorated than controls. In spite of the introduction of IL-10 into IL-10 gene knockout mice through genetic recombination, the results have shown that IL-10 inhibits or even reverses ISO-induced myocardial hypertrophic remodeling and fibrosis, as well as improve cardiac function (17). The results suggest that IL-10 can inhibit myocardial remodeling and improve cardiac function, and has protective effects on myocardium. However, results of IL-10 level changes in CHF, are inconsistent. Changes in the IL-10 level may increase significantly (18), increase insignificantly, while some even slightly decrease (19). The results of the present study showed that the IL-10 level of HF rats was higher than the normal rats, and the probable causes were: i) In HF, due to the increase and stimulation of multiple pro-inflammatory cytokines, IL-10 increase is compensatory; and ii) in HF, the level of TNF-α increases, which stimulates the expression of IL-10 in the transcriptional level, and makes IL-10 composited and released by myocardial cell increase.
The decrease of number and function of selective β1-AR is accompanied by HF, and an interesting ‘substitution effect’ phenomenon was generated between β1-AR and β2-AR (20). Especially after the overexpression of β2-AR, under the background of β1-AR loses its power, the advantages of β2-AR gradually become evident and it overtakes. Dorn et al (21) reported that the overexpression of β2-AR in rats can improve cardiac contractile and relaxant function and reverse myocardial hypertrophy. The results of early laboratory investigations of the present study also show that after the β2-AR overexpression of myocardial cells of rats with HF, basic contractile function of myocardiac cells can be improved (22). Previous findings have shown that β2-AR mediates effects such as cardiac contractile and relaxant function, and leads to many changes of numerous inflammatory cytokines after the excitement of β2-AR. Using lipopolysaccharide to induce inflammatory reactions and excite β2-AR by specific β2-AR agonist, it was identified that β2-AR can inhibit the generation of IL-18 and IL-12 (23). There are results of animal experiments showing that the changes of β2-AR expression can adjust inflammatory reactions of cytokines such as IL-12 (24).
HF itself is an inflammatory reaction, thus in this study, we assessed whether the increase of β2-AR expression of myocardial cells of HF have the effects of adjusting inflammatory reactions by influencing the release of IL-10. We found that in comparison to normal rats, β2-AR expression on the surface of myocardial cells of rats with HF has no significant changes, which concurs with earlier reports (25). We transferred β2-AR gene into the myocardial cells of rats with HF by the carrier of recombinant adenovirus to overexpress β2-AR, thus, the impact of β2-AR on myocardial cells anti-inflammatory cytokine IL-10 can be studied, and the relationship between β2-AR and HF inflammatory reaction can be preliminarily studied. We identified that IL-10 level of myocardial cells of rats with HF was slightly higher than that of normal rats. Through gene transfection technology, we enhanced the myocardial cell surface expression of β2-AR, and IL-10 level of myocardial cells of the HF-transfected β2-AR group further increased in comparison with the HF control group, and IL-10 level of myocardial cells of the sham-operated-transfected β2-AR group has no significant decrease compared with the sham-operated control group. This result shows that the increase of β2-AR expression can increase the IL-10 level of myocardial cells of rats with HF, while it has no significant impact on IL-10 level of myocardial cells of normal rats. It suggests that introducing β2-AR into myocardial cells by gene transfection can also be achieved by increasing the IL-10 level of myocardial cells anti-inflammatory cytokine, which can play certain roles of anti-inflammatory immunoregulation, and can become a new therapeutic target for the treatment of HF.
The present study excluded the whole body influences including nerve and body fluid, and the impact of β2-AR overexpression on the IL-10 release of myocardial cells of rats with HF was observed at the in vitro cellular level. However, understanding the relationship between them with the combination of the impact of in vivo level β2-AR on cytokines would be beneficial. Currently, the mechanism of the impact of β2-AR overexpression on HF myocardial cell IL-10 release and molecular signaling pathways remain to be determined and require further investigation. In addition, this study selected pro-inflammatory factor IL-10 as the object of investigation in order to understand its relationship with β2-AR. Therefore, future studies should select other inflammatory factors as the focus of study to more comprehensively understand the relationship between β2-AR and inflammatory reaction in HF.
Acknowledgements
The present study was partly financed by the Jiangsu Provincial Special Program of Medical Science (no. BL2012019).
References
- 1.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. doi: 10.1016/j.ijcard.2012.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Chen Q, Sun S, Yu H, Zhang Z. Overexpression of beta(2)AR improves contractile function and cellular survival in rabbit cardiomyocytes under chronic hypoxia. Biochem Biophys Res Commun. 2010;398:383–388. doi: 10.1016/j.bbrc.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 4.Bolger AP, Sharma R, von Haehling S, Doehner W, Oliver B, Rauchhaus M, Coats AJ, Adcock IM, Anker SD. Effect of interleukin-10 on the production of tumor necrosis factor-alpha by peripheral blood mononuclear cells from patients with chronic heart failure. Am J Cardiol. 2002;90:384–389. doi: 10.1016/S0002-9149(02)02494-3. [DOI] [PubMed] [Google Scholar]
- 5.Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–225. doi: 10.1161/01.CIR.103.2.220. [DOI] [PubMed] [Google Scholar]
- 6.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of β2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26:2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fragaki K, Kileztky C, Trentesaux C, Zahm JM, Bajolet O, Johnson M, Puchelle E. Downregulation by a long-acting beta2-adrenergic receptor agonist and corticosteroid of Staphylococcus aureus-induced airway epithelial inflammatory mediator production. Am J Physiol Lung Cell Mol Physiol. 2006;291:L11–L18. doi: 10.1152/ajplung.00488.2005. [DOI] [PubMed] [Google Scholar]
- 8.Padda RS, Gkouvatsos K, Guido M, Mui J, Vali H, Pantopoulos K. A high-fat diet modulates iron metabolism but does not promote liver fibrosis in hemochromatotic Hjv¯/¯ mice. Am J Physiol Gastrointest Liver Physiol. 2015;308:G251–G261. doi: 10.1152/ajpgi.00137.2014. [DOI] [PubMed] [Google Scholar]
- 9.Saghazadeh A, Gharedaghi M, Meysamie A, Bauer S, Rezaei N. Proinflammatory and anti-inflammatory cytokines in febrile seizures and epilepsy: Systematic review and meta-analysis. Rev Neurosci. 2014;25:281–305. doi: 10.1515/revneuro-2013-0045. [DOI] [PubMed] [Google Scholar]
- 10.Hirota H, Izumi M, Hamaguchi T, Sugiyama S, Murakami E, Kunisada K, Fujio Y, Oshima Y, Nakaoka Y, Yamauchi-Takihara K. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart Vessels. 2004;19:237–241. doi: 10.1007/s00380-004-0770-z. [DOI] [PubMed] [Google Scholar]
- 11.Kurdi M, Booz GW. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of interleukin-6-type cytokine signaling. J Cardiovasc Pharmacol. 2007;50:126–141. doi: 10.1097/FJC.0b013e318068dd49. [DOI] [PubMed] [Google Scholar]
- 12.Padda RS, Shi Y, Lo CS, Zhang SL, Chan JS. Angiotensin-(1-7): A novel peptide to treat hypertension and nephropathy in diabetes? J Diabetes Metab. 2015;6:1–6. doi: 10.4172/2155-6156.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, Hanawa H, Toba K, Watanabe H, Watanabe R, Yoshida K, Abe S, Kato K, Kodama M, Aizawa Y. Expression of immunological molecules by cardiomyocytes and inflammatory and interstitial cells in rat autoimmune myocarditis. Cardiovasc Res. 2005;68:278–288. doi: 10.1016/j.cardiores.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy - review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 16.Domínguez Rodríguez A, Abreu González P, García González MJ, Ferrer Hita J. Association between serum interleukin 10 level and development of heart failure in acute myocardial infarction patients treated by primary angioplasty. Rev Esp Cardiol. 2005;58:626–630. (In Spanish) [PubMed] [Google Scholar]
- 17.Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, Thal M, Mackie A, Hoxha E, Ramirez V, et al. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-κB. Circulation. 2012;126:418–429. doi: 10.1161/CIRCULATIONAHA.112.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wykretowicz A, Furmaniuk J, Smielecki J, Deskur-Smielecka E, Szczepanik A, Banaszak A, Wysocki H. The oxygen stress index and levels of circulating interleukin-10 and interleukin-6 in patients with chronic heart failure. Int J Cardiol. 2004;94:283–287. doi: 10.1016/j.ijcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD. Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci (Lond) 2003;105:45–50. doi: 10.1042/CS20020359. [DOI] [PubMed] [Google Scholar]
- 20.Lamba S, Abraham WT. Alterations in adrenergic receptor signaling in heart failure. Heart Fail Rev. 2000;5:7–16. doi: 10.1023/A:1009885822076. [DOI] [PubMed] [Google Scholar]
- 21.Dorn GW, II, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proc Natl Acad Sci USA. 1999;96:6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong H, Lv Q, Lei W. The relationship and mechanism between the expression of myocardial cells β2-AR of rats with heart failure and cardiac function changes. Prog Mod Biomed. 2009;9:1024–1027. [Google Scholar]
- 23.Mizuno K, Takahashi HK, Iwagaki H, Katsuno G, Kamurul HA, Ohtani S, Mori S, Yoshino T, Nishibori M, Tanaka N. Beta2-adrenergic receptor stimulation inhibits LPS-induced IL-18 and IL-12 production in monocytes. Immunol Lett. 2005;101:168–172. doi: 10.1016/j.imlet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Itoh CE, Kizaki T, Hitomi Y, Hanawa T, Kamiya S, Ookawara T, Suzuki K, Izawa T, Saitoh D, Haga S, et al. Down-regulation of beta2-adrenergic receptor expression by exercise training increases IL-12 production by macrophages following LPS stimulation. Biochem Biophys Res Commun. 2004;322:979–984. doi: 10.1016/j.bbrc.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 25.Shizukuda Y, Buttrick PM. Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:823–831. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]