Abstract
Advanced age is characterized by impairments in wound healing, and evidence is accumulating that this may be due in part to a concomitant increase in oxidative stress. Extended exposure to reactive oxygen species (ROS) is thought to lead to cellular dysfunction and organismal death via the destructive oxidation of intra-cellular proteins, lipids and nucleic acids. Extracellular superoxide dismutase (ecSOD/SOD3) is a prime antioxidant enzyme in the extracellular space that eliminates ROS. Here, we demonstrate that reduced SOD3 levels contribute to healing impairments in aged mice. These impairments include delayed wound closure, reduced neovascularization, impaired fibroblast proliferation and increased neutrophil recruitment. We further establish that SOD3 KO and aged fibroblasts both display reduced production of TGF-β1, leading to decreased differentiation of fibroblasts into myofibroblasts. Taken together, these results suggest that wound healing impairments in ageing are associated with increased levels of ROS, decreased SOD3 expression and impaired extracellular oxidative stress regulation. Our results identify SOD3 as a possible target to correct age-related cellular dysfunction in wound healing.
Keywords: ageing, myofibroblast, oxidative stress, superoxide dismutase, wound healing
Introduction
Cutaneous wound healing is an intricate process defined by three overlapping but distinct molecular phases: inflammation, proliferation and remodelling (1). To achieve functional wound healing, a dynamic sequence of interactions between several cell types, the extracellular matrix (ECM) and numerous cytokines is required (1). With advanced age, aberrations in these physiological processes occur and result in impaired tissue repair and increased rates of chronic wounds characterized by insufficient neovascularization, stromal deposition and delayed epithelialization (2–6).
Chronic wounds represent both a major health burden for elderly patients as well as an increasing economic strain on the healthcare systems worldwide. With approximately 40 million patients aged 65 and older in the United States alone and an estimated 55 million patients by 2020, impaired wound healing will continue to remain a substantial healthcare challenge, affecting patients' quality of life and costing billions annually (7). Gaining insight into the mechanisms accountable for defective healing in the setting of advanced age is of fundamental importance for the development of efficacious therapeutics for chronic wounds (5).
Recent evidence links the dysfunctions that occur in the setting of advanced age to the effects of oxidative stress (8–10). Reactive oxygen species (ROS) accumulate in aged tissues, a phenomenon which has been linked to a reduced antioxidant activity of aged cells (11). Cells exist in an oxygenated environment which contains ROS physiologically generated as by-products of molecular oxygen (12). To maintain cellular ROS homoeostasis, enzymes such as the superoxide dismutase family (SODs) consume superoxide anion (O2•−). There are 3 SOD isoforms, which are contained in the intra-cellular space and the extracellular matrix. SOD1 and SOD2 are intra-cellular with SOD1 being primarily found in the cytoplasm and SOD2 being restricted to the mitochondria. SOD3 is the only extracellular enzyme (13,14). Under physiological conditions, cells are exposed intermittently to low levels of ROS, which initiate numerous inter- and intra-cellular signalling cascades (15). In advanced age, it is believed that sustained presence of high levels of ROS leads to cellular attrition, dysfunction and eventual organismal death (16–19) because of structural damage to proteins, lipids, and DNA and subsequent activation of cell death pathways (20).
SOD3 is the main SOD isoenzyme expressed in the arterial wall (21), lungs (22) and circumventricular regions in the brain (23) where, in addition to scavenging superoxide, it upregulates nitric oxide bioavailability. By reducing superoxide levels, SOD3 inhibits the rapid reaction of nitric oxide with superoxide to form peroxynirite, resulting in increased nitric oxide activity and improved homoeostasis of blood vessel contractility (24,25). Loss of SOD3 activates perivascular inflammation and causes atherosclerosis (21), acute lung damage (22) and peripheral hypertension (23). More recently, SOD3 was found in the epidermis and dermis (26) and appears to have a substantial role in limb ischaemia, being expressed in arterioles postischaemia and promoting neovasculariazation (27). Given the accumulating evidence that reduced neovascularization may be a major underlying factor in the decreased healing response observed in aged tissues (28), we hypothesized that there is a link between SOD3 dysfunction and wound healing impairments in advanced age.
Here, we investigate the effects of ageing and SOD3 activity on cutaneous wound healing. We further explore impaired SOD3 expression as a potential mechanism for cellular dysfunction in the setting of advanced age.
Materials and methods
Animals
All animal experiments were conducted in accordance with a protocol approved by the Stanford Administrative Panel on Laboratory Animal Care (APLAC) in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International) accredited animal care facility. Male mice homozygous for the targeted mutation of SOD3 (B6.129P2-Sod3tm1Mrkl/J) and male wild-type mice (C57BL/6) 8 weeks of age were obtained from Jackson laboratory (Bar Harbor, ME). Aged male mice (C57BL/6, 22 months) were obtained from the National Institute of Aging rodent colony.
Excisional wound healing model
Two 6-mm circular full-thickness wounds were created as described previously (29) on the dorsum of three groups of mice: young, aged and SOD3 knockout (n = 6). Silicone splints were sewn around the wounds to prevent contraction, and an occlusive dressing was used to cover the wounds (Tegaderm, 3M, St. Paul, MN, USA). Wounds were photographed every other day until closure, and ImageJ software (NIH, Bethesda, MD, USA) was used to quantify wound area. The percent of original wound area was defined as: wound area on day 0 – wound area on day ‘X’/(wound area on day 0) × 100. At days 0, 3 and 7 postwounding, animals were euthanized and wounds were harvested (n = 3 mice; 6 wounds per time point). Half of each wound was used for either histology or snap frozen in dry ice and stored at −80°C for transcriptional and protein analysis.
qRT-PCR
RNA was isolated from wound lysates of young, aged and SOD3 knockout mice at 0, 3 and 7 days postwounding using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Reverse transcription was performed to obtain cDNA (Superscript First-Strand Synthesis Kit, Invitrogen, Grand Island, NY, USA). For PCR, we used TaqMan® Assays-on-Demand™ Gene Expression Products from Applied Biosystems (Foster City, CA, USA): collagen III, assay ID Mm01254476_m1; alpha-smooth muscle actin, assay ID Mm01546133_m1; β-actin, assay ID Mm01205647_g1. All qRT-PCRs were run in triplicate for all samples. Levels of β-actin were quantified in parallel as an internal control, and gene expression was normalized accordingly.
ELISA
Total protein of wounds was isolated using RIPA buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). After homogenization of tissue in the supplemented RIPA buffer, samples were centrifuged at 10 000×g for 10 min at 4°C. Protein was quantified using the Quick Start Bradford Protein Assay Kit (Bio-Rad, Hercules, CA, USA). 4-hydroxynonenal (4-HNE) is a common by-product of lipid peroxidation during oxidative stress and is a reliable indicator of oxidative stress index. Therefore, levels of 4-HNE bound to protein were measured using the Mouse HNE adduct ELISA Kit (STA-338, Cell Biolabs, Minneapolis, MN, USA) according to the manufacturer's instructions. Furthermore, TGF-β1 levels in medium of cultured fibroblasts were evaluated using a Quantikine ELISA Kit (MB100B, Mouse/Rat/Porcine/Canine TGFβ1 Quantikine ELISA Kit Second Generation, R&D Systems, Minneapolis, MN, USA).
Histology
Wound tissue was harvested, fixed in 4% paraformaldehyde in PBS solution overnight, and dehydrated in a 30% sucrose solution. The samples were fixed in OCT compound (Sakura Finetek USA, Torrance, CA, USA) and cryosectioned as previously described (30). Antigen retrieval was performed using 1% SDS in PBS solution, and samples were blocked with PowerBlock in PBST for 1 h. Anti-CD31 (ab28364; Abcam, Cambridge, MA, USA), anti-alpha-smooth muscle actin (ab5694, Abcam) or antineutrophil (ab2557, Abcam) primary antibody was added overnight at a 1:200 concentration, followed by a secondary antibody. Slides were DAPI-stained for nuclei, mounted with Vectastain, and photomicrographs were taken. Quantification of CD31 intensity by ImageJ analysis was used to determine blood vessel density per high-powered field. Similarly, ImageJ was used to quantify relative fluorescent intensity for alpha-smooth muscle actin from wound tissue of young, aged and SOD3 knockout mice at day 7 and for neutrophils at day 3. Four images per group were analysed for each quantification.
Cell culture
All fibroblasts used in experiments were obtained from intact dorsal murine skin. The dermal fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum (FBS), 44 mM sodium bicarbonate, 30 mM HEPES, 100 U/ml penicillin and 100 μg/ml streptomycin, in a humidified incubator at 37°C with 5% CO2. The fibroblasts were used after three passages in all experiments and serum starved for 12 h before each experiment.
Proliferation assay
Cell proliferation in vitro was assessed using a BrdU assay. Fibroblasts were harvested from the dorsal skin of young, aged or SOD3 knockout mice and cultured with no treatment, exposure to 2 mmol/l xanthine oxidase to generate superoxide anion (31) or the same concentration of xanthine oxidase supplemented with recombinant SOD3 (Catalogue #H00006649-Q01 Abnova, Taipei, Taiwan). Cells were labelled with BrdU for 24 h, fixed, DNA denatured and an anti-BrdU-peroxidase antibody was added to bind intra-cellularly to incorporated BrdU. The difference in absorbance at 370 and 492 nm revealed the amount of newly synthesized DNA as a means of assessing proliferation.
Western blotting
Wound samples were homogenized in 500 μl of RIPA lysis buffer (Sigma-Aldrich) with proteinase inhibitor cocktail (p8340; Sigma-Aldrich) and centrifuged. The aqueous layer was collected, and protein concentration was determined using the BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Equal amounts of protein (20 μg) were loaded into each lane of a gel. The separated proteins were transferred to a nitrocellulose membrane, and immunostaining was performed using rabbit anti-α-smooth muscle actin (α-SMA) (ab5694, Abcam) antibody. β-Actin signal served as internal control. Band densities were quantified with ImageJ software (NIH) (n = 3).
Statistical analysis
All values are expressed as mean ± SD. Statistical significance was determined using one-way ANOVA testing. A P value <0.05 was considered statistically significant. Error bars are representative of standard deviation.
Results
Aged wounds display impaired healing and increased ROS levels similar to SOD3 KO wounds
We first sought to confirm our previous findings of delayed wound healing in aged mice compared to young controls in our model of murine wound healing (32). We verified that wounds in aged mice healed significantly slower compared to younger controls with significant differences in wound size at days 3, 5, 7, 9, 11 and 13 postwounding (Fig. 1a). We next looked at the differences in SOD3 protein levels within wounds at day 3 as this was the time point where we first saw significant differences in wound size. We found significantly reduced levels of SOD3 in day 3 wounds of aged mice compared to young controls (Fig. 1b). Given this difference in SOD3 levels, we next looked at wound healing in SOD3 KO mice compared to aged mice. We found a very similar phenotype in the SOD3 KO mice compared to aged mice with nearly identical wound closure curves (Fig. 1c). HNE analysis of wound lysates at postwounding day 3 also showed very similar level of oxidative stress in aged and SOD3 KO mice, which were significantly higher than the young controls (Fig. 1d). These results suggest that reduced levels of cutaneous SOD3 in aged mice may contribute to the impaired wound healing response in aged skin.
Figure 1.
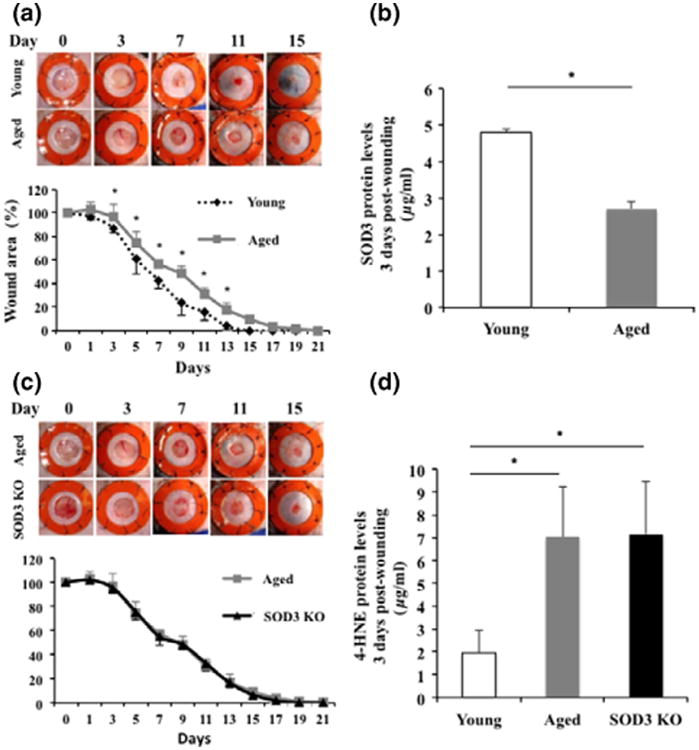
Aged mice display wound healing impairments similar to SOD3 KO mice. (a) Aged wild-type mice show significantly delayed wound healing when compared to young controls. Young mouse wounds were completely healed by day 17, whereas aged mice took 21 days to completely heal. (b) Wound SOD3 protein concentration was significantly reduced in aged mice compared to young mice at 3 days postwounding. (c) SOD3 KO mice showed almost identical healing curve to aged wild-type mice with no significant difference in wound size at any time point measured. (d) Wound 4-HNE adduct protein levels were significantly increased in aged and SOD3 KO mice compared to young mice at 3 days postwounding. *P < 0.05.
Aged mice exhibit impaired neovascularization during wound healing similar to SOD3 KO mice
Sufficient neovascularization is crucial for functional wound healing, and there is emerging evidence that ROS and oxidative stress directly inhibit angiogenesis (33). Consistent with the higher ROS burden, we observed impaired neovascularization in both aged and SOD3 KO mice. Tissue sections of excisional wounds at day 7 postwounding of aged and SOD3 KO mice revealed reduced neovascularization upon complete healing compared to young mice, based on CD31 immunostaining (Fig. 2a). Quantification of vascular density revealed a fourfold drop in neovascularization in aged and SOD3 KO mice compared to young controls at day 7 postwounding (Fig. 2b).
Figure 2.
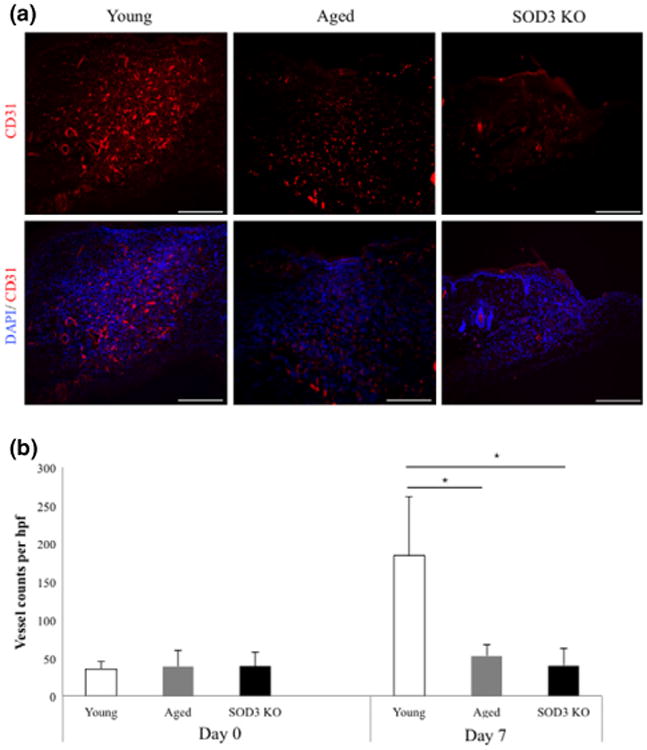
SOD3 KO and aged mice show similar deficits in neovascularization. (a,b) CD31 staining and quantification of blood vessels per high power field demonstrated that both SOD3 KO and aged mice have a lower number of newly-formed blood vessels than young mice at 7 days postwounding. *P < 0.05. Scale bar = 200 μm.
Aberrant fibroblast proliferation and neutrophil infiltration in aged wounds correlate to deficiencies observed in SOD3 KO wounds
In addition to adequate vascular supply, physiological wound healing is characterized by carefully orchestrated stromal and inflammatory cell behaviour (1). Neutrophil recruitment during the early inflammatory response is necessary for the clearance of bacteria and cellular debris from injured tissue (34). However, excess inflammation has deleterious effects on the course of wound healing as inflammatory cells also have tissue-damaging effects (35). SOD3 has been demonstrated to play a role in inflammatory cell recruitment (36) and has specifically been linked to the regulation of neutrophil infiltration (37). Consistent with the described role of SOD3 as a suppressor of local inflammation (37), immunostaining of wound sections at postoperative day 3 revealed an increased infiltration of neutrophils in aged mice similar to SOD3 KO mice (Fig. 3a).
Figure 3.
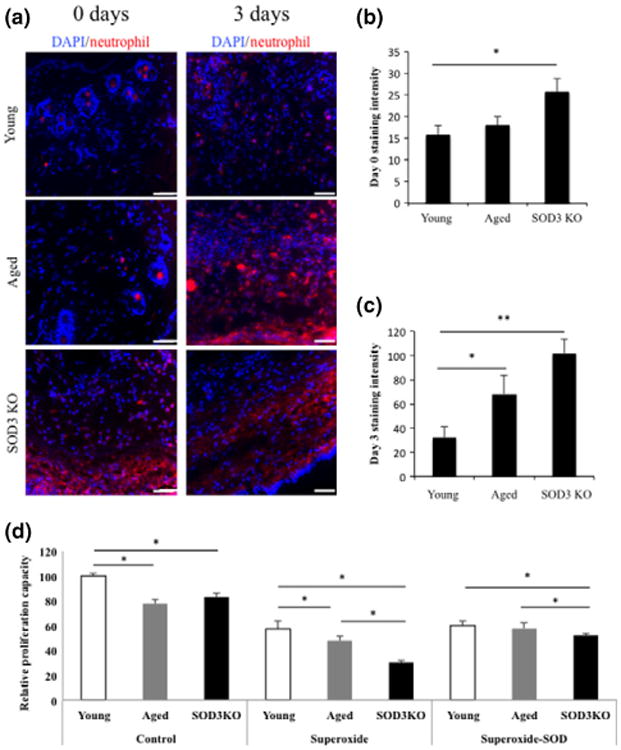
Aged and SOD3 KO wounds display comparable aberrations in fibroblast proliferation and neutrophil infiltration. (a) Neutrophil staining 3-days postwounding showed an increased number of neutrophils in both aged and SOD3 KO mice. (b) Quantification of antineutrophil staining intensity at day 0 postwounding shows a significant increase in neutrophil recruitment in SOD3 KO wounds compared to wounds in young mice. (c) There is a significant increase in the number of neutrophils seen in aged and SOD3 KO wounds at day 3 compared to day 3 wounds in young mouse controls. (d) Fibroblasts from SOD3 KO and aged wounds showed significantly decreased proliferation compared to those from young mice as measured by BrdU incorporation. With addition of superoxide, the proliferation rate of SOD3 KO fibroblasts was significantly decreased from both young and aged mouse dermal fibroblasts. When compared to young fibroblasts, aged fibroblasts continued to show significantly decreased proliferation rates as well. In the setting of increased oxidative stress, aged fibroblast proliferation rates were rescued with the addition of recombinant SOD3. *P < 0.05, **P < 0.01 Scale bar = 100 μm.
Fibroblasts, the primary dermal stromal cell and workhorses of wound healing, are responsive to reactive oxygen species (38), and their functionality has been shown to be, at least in part, regulated by oxidative stress (39). Investigating the influence of increased ROS stress on cutaneous fibroblasts isolated from young, aged and SOD3 KO mice, we found that both aged and SOD3 KO fibroblasts exhibited lower rates of proliferation compared to their young counterparts. This was true for cells grown in standard culture conditions and when stimulated via a previously described xanthine oxidase system to induce superoxide radical stress (31) (Fig. 3b). The contribution of SOD3 to dysfunction in aged fibroblast proliferation could be confirmed by the addition of recombinant SOD3 to fibroblasts stimulated with xanthine oxidase. Replenishing the missing enzyme fully rescued the proliferative capacity of aged fibroblasts, but only partially rescued SOD3 KO fibroblasts (Fig. 3b).
Wound healing deficiencies in aged and SOD3 KO mice are partly due to reduced differentiation of fibroblasts into myofibroblasts
Normal fibroblast function is critical for normal wound healing (40,41). Along with proliferation and migration, fibroblasts promote normal healing through collagen deposition and extracellular matrix remodelling (42). During wound healing, fibroblasts are stimulated by TGF-β1 (43), which stimulates some cells to differentiate into myofibroblasts, expressing smooth muscle actin and synthesizing a new collagen-rich matrix essential for wound closure (44). Given the emerging evidence regarding the impact of oxidative stress on fibroblast collagen synthesis (39), we evaluated how superoxide deregulation affects the fibroblast to myofibroblast transition. First we assessed transcriptional changes of genes involved in ECM deposition within wound samples and found reduced collagen type-III alpha-I expression in both aged and SOD3 KO mice 7 days postwounding (Fig. 4a). This decrease in collagen expression corresponded with reduced transcription (Fig. 4b) and protein expression (Fig. 4c, d) of the myofibroblast marker alpha-smooth muscle actin (43), suggesting a reduced myofibroblast presence in aged and SOD3 KO wounds. We also found that fibroblasts isolated from SOD3 KO and aged mice secreted significantly reduced amounts of TGF-β1 into the cell culture medium, both under normal conditions and in response to stimulation with superoxide compared to young fibroblast controls (Fig. 4e). This suggests myofibroblast differentiation is impaired in the setting of elevated ROS stress in ageing, due in part to reduced SOD3 activity via TGF-β1 signalling.
Figure 4.
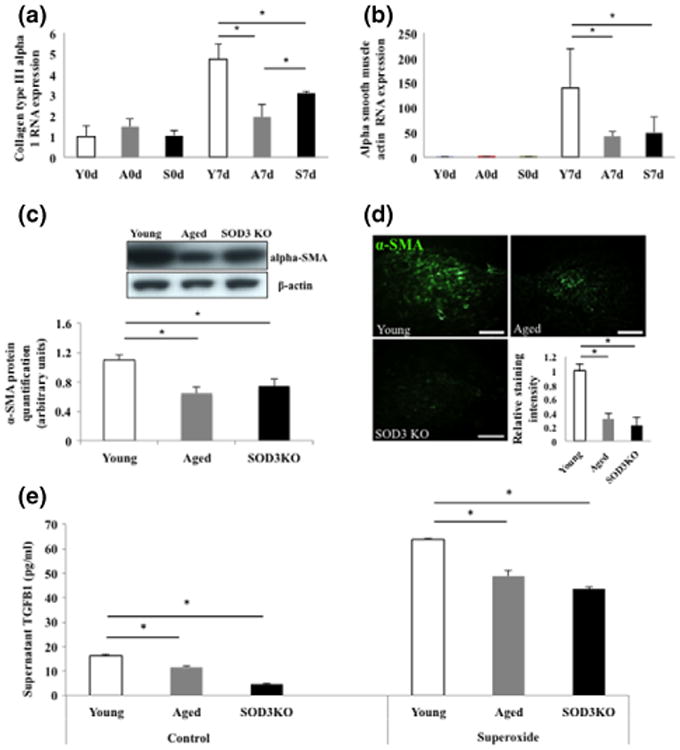
Reduced fibroblast to myofibroblast transition in aged and SOD3 KO mice. (a, b) Both collagen type-III alpha-I and alpha-smooth muscle actin (alpha-SMA) RNA expression are similar at baseline on the day of wounding but were decreased in aged and SOD3 KO wounds at 7 days postwounding when compared to young controls. Y = young mice, A = aged mice, S = SOD3 KO mice (c, d) Western blot and immunohistochemistry demonstrated significant reduction of alpha-SMA in aged and SOD3 KO wounds on a protein level. (e) In both control conditions and when exposed to xanthine oxidase, fibroblasts from aged and SOD3 KO mice release significantly lower levels of TGFβ-1, compared to fibroblasts from young mice. *P < 0.05. Scale bar = 100 μm.
Together, these results indicate that defective reactive oxygen scavenging in the setting of ageing leads to a variety of cellular dysfunctions within the wound microenvironment. Specifically, SOD3 deficiency leads to reduced neovascularization, abnormal recruitment of neutrophils, decreased fibroblast proliferation and impaired fibroblast to myofibroblast differentiation. All of these may contribute to the compromised wound healing phenotype observed in aged skin.
Discussion
Ageing leads to substantial adverse changes in metabolic and repair mechanisms, ultimately interfering with the organism's natural wound healing capabilities (45,46). Ageing skin is typically characterized by aberrations of the dermal matrix composition resulting in increased atrophy, wrinkle formation and compromised wound healing (47,48). Managing non-healing wounds in the elderly is difficult, and, unfortunately, therapies often fail, which makes chronic wounds a significant public health burden. A better understanding of how ageing interferes with physiological wound healing may help with the development of effective therapeutic strategies to combat chronic wounds in an ageing society.
Evidence is accumulating that advanced age leads to impairments in the dermal microenvironment, which is driven by increased oxidative stress (49). In ageing, dysfunctional ROS neutralizing systems (50–52) permit the concentration of ROS to exceed the neutralization capacity, resulting in increased concentrations of ROS. While a low level of ROS are important for the clearance of pathogens and intra-cellular signalling during early wound healing (53), an excess of oxidative stress causes an inhibition of cellular proliferation and migration and a perpetuation of inflammation, which results in tissue damage and chronic non-healing wounds (54). Importantly, it has been previously demonstrated that application of high concentrations of H2O2 to excisional wounds in mice inhibits wound closure (55).
The main effector cells of cutaneous repair are fibroblasts (56), and the intermediate phase of wound healing is dependent upon fibroblast proliferation. We show here inhibition of fibroblast proliferation with SOD3 knockout in mice as well as in ageing. In a model of SOD3 gene transfer into rat muscle, SOD3 was found to induce cell proliferation through Ras-ERK activation with downstream stimulation of mitogenic signalling pathways (57). In our study, SOD3 inhibition via transgenic knockout and the deficit of SOD3 seen in ageing likely leads to downregulation of these mitogenic pathways and consequently decreased proliferation. When we performed our rescue experiments looking at proliferation in aged and SOD3 KO fibroblasts, while we found a complete rescue of the deficit in proliferation in the aged cells, the addition of recombinant SOD3 to SOD3 KO fibroblasts improved but did not lead to completely normal proliferation capacity. This may be secondary to the fact that the aged fibroblasts still have some SOD3 activity and the addition of recombinant protein is enough to rescue the deficit in proliferation. The SOD3 KO fibroblasts have a complete knockout of SOD3 activity and thus the amount of recombinant protein added may not have been enough to completely rescue the phenotype. Additionally, it is possible there are unknown downstream effects of permanent knockout of SOD3 that may have long-term effects on proliferation despite attempt at rescue with recombinant protein.
It is thought that some fibroblasts acquire a myofibroblastic phenotype to maximize contraction and migration essential for wound healing. Myofibroblasts combine the extracellular matrix – synthesizing capacity of fibroblasts with cytoskeletal characteristics of contractile smooth muscle cells (58). In the context of wound healing in particular, fibroblast to myofibroblast conversion is governed by mechanical stress, TGF-β signalling and interaction with ECM proteins (56). The mechanisms underlying the impaired differentiation of fibroblasts into myofibroblasts with age is poorly understood, but is thought to involve TGF-β1-dependent fibroblast activation (59). In this study, we suggest that ROS–SOD3 interactions might impact TGF-β1 signalling in the setting of advanced age. In addition to Ras-ERK activation, SOD3 has been shown to phosphorylate and simulate activation of the downstream O-box subfamily of the forkhead transcription factors (FOXO) (60). FOXO1 has been shown to be upregulated after wounding and plays an important role in wound healing through the stimulation of TGF-β-mediated keratinocyte migration and regulation of antioxidant genes (61,62) FOXO1 stimulates TGF-β promoter activity causing increased TGF-β expression (62). This potential SOD3–FOXO–TGF-β pathway may provide a possible explanation for the decreased levels of TGF-β1 seen in aged and SOD3 KO fibroblasts in our study when stimulated by superoxide.
We demonstrate that reduced levels of extracellular SOD3 may contribute to the age-related defects in cutaneous wound healing. SOD3 deletion recapitulates the gross morphological and cellular defects in cutaneous wound healing that are observed in the setting of ageing. We observed that defective neovascularization, neutrophil infiltration, fibroblast migration and myofibroblast activation were present in both SOD3 KO and aged wounds. Interestingly, the fibroblast to myofibroblast transition was actually more impaired in aged mice compared to SOD3 KO mice, which is consistent with our findings regarding the role of SOD1 in this process (63). Consequently, our findings suggest that dysfunctions in ROS regulation and particularly SOD3 may be potential targets to counteract wound healing deficits in ageing. Others have shown that genetically modified mice with extracellular SOD3 overexpression and subsequent protection against oxidative stress-induced damage do not display an age-related decline in learning and memory function (64,65) and that increased levels of extracellular SOD3 result in enhanced survival and reduced pulmonary damage in a murine sepsis model (66). Further studies are needed to test whether a therapeutic elevation of extracellular SOD3 might improve oxidative stress regulation and wound healing in the aged.
Acknowledgments
The authors thank Yujin Park for her assistance with tissue processing. Funding for this research was provided by the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine, the National Institute of Ageing (R01-AG025016), and the Oak Foundation. TF was supported by Grant-in-Aid for Researchers, Hyogo College of Medicine 2013. MR was supported by the Wound Healing Society Foundation 3M Fellowship and ZNM by the Plastic Surgery Foundation Research Fellowship Grant.
Footnotes
Author contributions: TF and GCG designed the research study. TF and ZM performed the experiments. DD, KCR, RK, MR, AJW and MJ analysed the data. DD, KCR, RK, MR, AJW, MJ, ZM and GCG wrote and edited the manuscript.
Conflicts of interest: The authors have declared no conflicting interests.
References
- 1.Gurtner GC, Werner S, Barrandon Y, et al. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft GS, Horan MA, Ferguson MW. Lab Invest. 1998;78:47–58. [PubMed] [Google Scholar]
- 3.Chang EI, et al. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 4.Duscher D, et al. Sci Rep. 2014;4:7144. doi: 10.1038/srep07144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould L, et al. Wound Repair Regen. 2014;23:1–13. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt DR, et al. Surgery. 1992;112:293–297. discussion 297–298. [PubMed] [Google Scholar]
- 7.Sen CK, et al. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohlgemuth SE, Calvani R, Marzetti E. J Mol Cell Cardiol. 2014;71:62–70. doi: 10.1016/j.yjmcc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Romano AD, Serviddio G, de Matthaeis A, et al. J Nephrol. 2010;23(Suppl 15):S29–S36. [PubMed] [Google Scholar]
- 10.Figueiredo PA, Mota MP, Appell HJ, et al. Biogerontology. 2008;9:67–84. doi: 10.1007/s10522-007-9121-7. [DOI] [PubMed] [Google Scholar]
- 11.Shalini S, et al. Cell Death Differ. 2012;19:1370–1380. doi: 10.1038/cdd.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkel T, Holbrook NJ. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 13.Foresman EL, Miller FJ., Jr Redox Biol. 2013;1:292–296. doi: 10.1016/j.redox.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukui M, Zhu BT. Free Radic Biol Med. 2010;48:821–830. doi: 10.1016/j.freeradbiomed.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Autreaux B, Toledano MB. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 16.Sohal RS, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabrizio P, Pozza F, Pletcher SD, et al. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 18.Harman D. Ann NY Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 19.Nemoto S, Finkel T. Nature. 2004;429:149–152. doi: 10.1038/429149a. [DOI] [PubMed] [Google Scholar]
- 20.Dai DF, Chiao YA, Marcinek DJ, et al. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luoma JS, et al. Arterioscler Thromb Vasc Biol. 1998;18:157–167. doi: 10.1161/01.atv.18.2.157. [DOI] [PubMed] [Google Scholar]
- 22.Gongora MC, et al. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lob HE, et al. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Z, Reszka KJ, Fukai T, et al. Transl Res. 2008;151:68–78. doi: 10.1016/j.trsl.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavez MD, Lakshmanan N, Kavdia M. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1022–1026. doi: 10.1109/IEMBS.2007.4352468. [DOI] [PubMed] [Google Scholar]
- 26.Kwon MJ, Kim B, Lee YS, et al. J Dermatol Sci. 2012;67:81–87. doi: 10.1016/j.jdermsci.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim HW, Lin A, Guldberg RE, et al. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 28.Edelberg JM, Reed MJ. Front Biosci. 2003;8:s1199–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- 29.Galiano RD, Michaels Jt, Dobryansky M, et al. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 30.Suga H, et al. Stem Cells. 2014;32:1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aitken RJ, Buckingham D, Harkiss D. J Reprod Fertil. 1993;97:441–450. doi: 10.1530/jrf.0.0970441. [DOI] [PubMed] [Google Scholar]
- 32.Loh SA, et al. Plast Reconstr Surg. 2009;123(Suppl):65S–75S. doi: 10.1097/PRS.0b013e318191bdf4. [DOI] [PubMed] [Google Scholar]
- 33.Perveen S, et al. PLoS ONE. 2012;7:e51945. doi: 10.1371/journal.pone.0051945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MH, et al. J Invest Dermatol. 2008;128:1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tidball JG. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 36.Folz RJ, Abushamaa AM, Suliman HB. J Clin Investig. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurila JP, Laatikainen LE, Castellone MD, et al. PLoS ONE. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vile GF, Basu-Modak S, Waltner C, et al. Proc Natl Acad Sci USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siwik DA, Pagano PJ, Colucci WS. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 40.Walmsley GG, et al. Plast Reconstr Surg. 2015;135:907–917. doi: 10.1097/PRS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 41.Duscher D, et al. J Biomech. 2014;47:1997–2005. doi: 10.1016/j.jbiomech.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tettamanti G, et al. Biol Cell. 2004;96:443–455. doi: 10.1016/j.biolcel.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Hinz B. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 44.Martin P. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 45.Sharpless NE, DePinho RA. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 46.Hayflick L. Ann NY Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 47.Quan T, Shao Y, He T, et al. J Invest Dermatol. 2010;130:415–424. doi: 10.1038/jid.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher GJ, Varani J, Voorhees JJ. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Rando TA. J Cell Biol. 2011;193:257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Raamsdonk JM, Hekimi S. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unlu ES, Koc A. Ann NY Acad Sci. 2007;1100:505–509. doi: 10.1196/annals.1395.055. [DOI] [PubMed] [Google Scholar]
- 52.Wallace DC, Melov S. Nat Genet. 1998;19:105–106. doi: 10.1038/448. [DOI] [PubMed] [Google Scholar]
- 53.Schafer M, Werner S. Pharmacol Res. 2008;58:165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Clark RA. J Invest Dermatol. 2008;128:2361–2364. doi: 10.1038/jid.2008.257. [DOI] [PubMed] [Google Scholar]
- 55.Roy S, Khanna S, Nallu K, et al. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vedrenne N, Coulomb B, Danigo A, et al. Pathol Biol. 2012;60:20–27. doi: 10.1016/j.patbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Laurila JP, et al. Mol Ther. 2009;17:448–454. doi: 10.1038/mt.2008.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinz B, et al. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson RM, et al. Am J Pathol. 2010;176:1215–1228. doi: 10.2353/ajpath.2010.090802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laatikainen LE, et al. PLoS ONE. 2011;6:e24456. doi: 10.1371/journal.pone.0024456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ponugoti B, et al. J Cell Biol. 2013;203:327–343. doi: 10.1083/jcb.201305074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hameedaldeen A, Liu J, Batres A, et al. Int J Mol Sci. 2014;15:16257–16269. doi: 10.3390/ijms150916257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujiwara T, et al. Age Associated Superoxide Dismutase Deficiency Impairs TGF-β1 Mediated Myofibroblast Function During Wound Healing. 2015 [Google Scholar]
- 64.Levin ED. Curr Alzheimer Res. 2005;2:191–196. doi: 10.2174/1567205053585710. [DOI] [PubMed] [Google Scholar]
- 65.Levin ED, et al. Behav Genet. 2002;32:119–125. doi: 10.1023/a:1015201823417. [DOI] [PubMed] [Google Scholar]
- 66.Ueda J, et al. Free Radic Biol Med. 2008;45:897–904. doi: 10.1016/j.freeradbiomed.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


