Abstract
Background
Experience with neoadjuvant chemoradiation (CXRT) has raised questions regarding the additional benefit of surgery after locally advanced esophageal adenocarcinoma patients achieve a clinical response to CXRT. We sought to quantify the value of surgery by comparing the overall (OS) and disease-free survival (DFS) of trimodality eligible patients treated with definitive CXRT versus CXRT followed by esophagectomy.
Methods
We identified 143 clinical stage III esophageal adenocarcinoma patients that were eligible for trimodality therapy. All patients successfully completed neoadjuvant CXRT and were considered appropriate candidates for resection. Patients that were medically inoperable were excluded. Cox regression models were used to identify significant predictors of survival.
Results
Among the 143 patients eligible for surgery after completing CXRT, 114 underwent resection and 29 did not. Poorly differentiated tumors (HR=2.041, 95% CI 1.235–3.373) and surgical resection (HR=0.504, 95% CI 0.283–0.899) were the only independent predictors of OS. Patients treated with surgery had a 50% and 54% risk reduction in overall and cancer-specific mortality, respectively. Median OS (41.2 months vs. 20.3 months, p=0.012) and DFS (21.5 months vs. 11.4 months, p=0.007) were significantly improved with the addition of surgery compared to definitive CXRT.
Conclusions
Surgery provides a significant survival benefit to trimodality-eligible esophageal adenocarcinoma patients with locally advanced disease.
Keywords: esophageal adenocarcinoma, trimodality therapy, neoadjuvant chemoradiation, esophagectomy, selective surgery
Introduction
The majority of esophageal adenocarcinoma patients present with locally advanced disease [1, 2]. This represents a significant disease burden at high risk of local and regional recurrence unless aggressive treatment is received. Trimodality therapy with neoadjuvant concurrent chemoradiation (CXRT) (with or without induction chemotherapy) followed by esophagectomy is currently considered the optimal treatment as a means to achieve a complete resection, reduce recurrence, and improve disease-free survival in medically able patients [3–5].
Although surgery has traditionally been a central therapeutic component of trimodality therapy, increased experience with neoadjuvant CXRT has raised questions regarding the appropriate role of surgery after patients achieve a clinical response to CXRT [6]. Further, salvage esophageal resection has emerged as a viable option for select patients that present with locoregional recurrent disease after definitive CXRT [7, 8]. Perhaps because of these findings, we have noted a practice trend toward bimodality therapy with definitive CXRT in patients with locally advanced adenocarcinoma. Definitive CXRT treatment strategies, however, have not been rigorously evaluated in exclusively adenocarcinoma populations. In light of disparate data, medically able patients that are eligible for trimodality therapy are increasingly treated with definitive CXRT [9].
Strategies are needed to further identify groups of patients that may benefit from the addition of surgery (vs. observation) after successful completion of CXRT. We sought to quantify the benefit of surgery by comparing overall survival (OS) and disease-free survival (DFS) of trimodality eligible patients treated with definitive CXRT versus CXRT followed by surgery. Specifically, we hypothesized that surgery provides an additional OS and DFS benefit to locally advanced esophageal adenocarcinoma patients that successfully complete CXRT.
Methods
Study Population
We identified patients treated consecutively for esophageal adenocarcinoma at the University of Texas MD Anderson Cancer Center (MD Anderson) using a prospectively maintained database. This research was approved by the Institutional Review Board at MD Anderson (DR11-0298). Patients were diagnosed between January 2002 and December 2008, treated with neoadjuvant CXRT with or without induction chemotherapy, and all treatment was delivered at MD Anderson. Patients with synchronous cancers or cancer of the cervical esophagus were excluded.
All patients included in this study were staged as clinical stage (cStage) III (T3N1). Clinical staging was determined as follows: endoscopic ultrasound (EUS) for tumor depth, positron emission tomography (PET) scan and /or computed tomography (CT) scan and/or EUS for regional nodal involvement, and PET-CT scan for distant metastasis. Patients were classified according to the American Joint Committee on Cancer (AJCC) Staging Manual, 6th Edition [10].
Patients that met inclusion criteria must have also been considered eligible for trimodality therapy both before and after neoadjuvant therapy. Medically inoperable patients with significant comorbidities or patients with medical contraindications to surgery were excluded. All patients were evaluated in a prospective multidisciplinary setting and were recommended as candidates for curative resection at the time of initial staging and physiologic assessment. After completing neoadjuvant therapy, patients underwent restaging evaluations and were reassessed for surgery. Any patient with suspected or proven distant metastasis (i.e., disease progression) or that died during neoadjuvant therapy was further excluded. The resulting study population consisted exclusively of trimodality eligible patients that successfully completed neoadjvuant therapy and were considered appropriate surgical candidates.
Predictor Variables
Patient, tumor, and treatment related variables were used in all survival analyses. Patient characteristics included age at diagnosis (<55, 55–64, 65–69, ≥70), sex (male, female), race/ethnicity (White, non-White) and comorbidity. Patient comorbidity severity was evaluated by calculating a Charlson score [11]. Primary cancer and treatment associated complications for the index cancer were not included in the comorbidity score.
Tumor and treatment related variables included tumor length, tumor grade (well/moderately differentiated, poorly differentiated), CXRT sequence (induction chemotherapy followed by concurrent CXRT, concurrent CXRT), radiation dose, pre- and post-treatment standard uptake value (SUV) of the primary tumor on pre- and post-CXRT PET scans, and treatment with surgery. Recursive partitioning was used to determine appropriate cut-offs for PET SUV.
Statistical Analysis
Pearson chi-square tests or Fisher exact tests were used to compare categorical characteristics, and t-tests or Wilcoxon rank sum tests were used to compare continuous characteristics of the study population.
Univariate and multivariable Cox hazard regression models were used to identify significant predictors of OS and DFS. Univariate factors with a p-value of less than 0.25 were entered into a multivariable stepwise Cox proportional hazard regression model. Wald’s stepwise selection with p=0.10 was used as entry and removal probability until the final model for the data set was obtained. Final regression models were age-adjusted to account for potential differences between the two treatment groups.
We also compared median OS and DFS using the Kaplan-Meier method. OS was calculated from the day of first treatment until the last known date of follow-up or date of death, and DFS from the day of first treatment until the first known date of disease recurrence or last known date of follow-up or date of death. Log-rank tests were used to compare the survival distributions.
Finally, propensity score matching was used to correct for baseline differences between treatment groups. This statistical methodology controls for potential selection bias associated with observational data by reducing bias related to the non-randomized assignment of treatment [12]. The propensity score reflects an individual patient’s predicted probability of receiving treatment. Patients were matched on age, comorbidity score, tumor length, tumor grade, post-CXRT biopsy, and post-CXRT SUV.
Subgroup and Sensitivity Analysis
In addition to comparing OS and DFS of patients treated with definitive CXRT and CXRT followed by surgery, we performed a subgroup analysis on patients considered clinical responders to CXRT. Clinical response was evaluated using the following criteria: 1) no evidence of disease progression on post-CXRT regional or distant PET; 2) negative post-CXRT biopsy; and 3) decrease in local pre- and post-CXRT SUV on PET scan ≥ 35% [13]. Criteria were applied hierarchically. Any patients with evidence of physiologic change (e.g., radiation pneumonitis, esophagitis) and/or local SUV ≤ 5 on post-CXRT PET were evaluated on a case by case basis. Patients were classified as either responders or nonresponders. Any patient with missing pre- or post-CXRT PET data or post-CXRT biopsy was excluded from the subgroup analysis. We compared median OS of definitive CXRT responders and surgery responders using the Kaplan-Meier method.
We also performed a sensitivity analysis that included salvage resections as part of the definitive CXRT treatment group. Median OS of definitive CXRT (including salvage patients) and CXRT followed by surgery was compared using the Kaplan-Meier method.
Statistical calculations were performed using the Statistical Package for Social Sciences (SPSS, Chicago, IL). Statistical significance was accepted as p of 0.05 or less.
Results
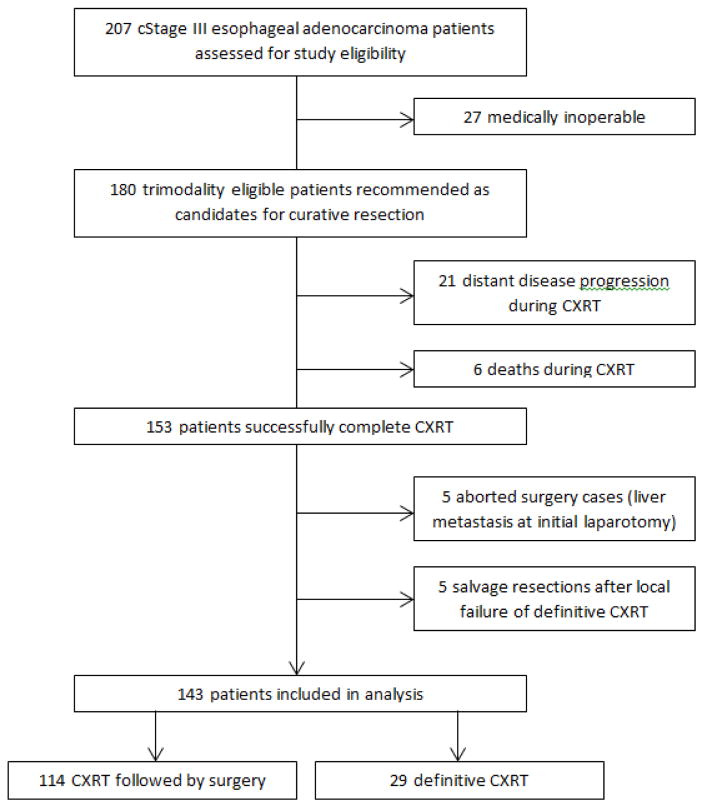
We identified 207 potentially eligible cStage III esophageal adenocarcinoma patients treated with neoadjuvant CXRT with or without induction chemotherapy at our institution. After examining for eligibility, we excluded patients that were medically inoperable (n=27), had distant disease progression (n=21) or died (n=6) during neoadjuvant therapy. These 153 patients successfully completed chemoradiation, and were considered candidates for curative resection after restaging and evaluation. We further excluded definitive chemoradiation patients that later underwent salvage surgery for local recurrence (n=5) and surgery cases that were terminated upon discovery of liver metastasis at initial laparotomy (n=5). As shown in Figure 1, a remaining 143 patients were included in the analysis.
Figure 1.
Treatment flow diagram of locally advanced esophageal adenocarcinoma patients treated with definitive chemoradiation or chemoradiation followed by esophagectomy (n=143)
Treatment
Among the 143 patients included in the analysis, 114 underwent surgery and 29 did not. The most common reasons for refusing surgery were patient choice and physician choice (e.g., physician preference for observation and/or no referral to surgeon) [9]. Most patients that did not undergo surgery had a favorable response to therapy and were considered complete clinical responders. Patients received a median radiation dose of 50.4 Gy (range 36–50.4 Gy) (Table 1). Chemotherapy regimens were most commonly a platinum doublet consisting of 5-flourouracil plus cisplatin or oxaliplatin versus a taxane/platinum combination. A small majority of patients (80, 55.9%) received induction chemotherapy followed by concurrent CXRT compared to concurrent CXRT (63, 44.1%) (Table 1).
Table 1.
Patient demographic and tumor characteristics of trimodality eligible patients with locally advanced esophageal adenocarcinoma, according to treatment groupa
| Characteristic | Chemoradiation and Surgery (n=114) | Definitive Chemoradiation (n=29) |
|---|---|---|
| Sex—no. (%) | ||
| Male | 108 (95) | 26 (90) |
| Female | 6 (5) | 3 (10) |
| Age*** | ||
| Median | 60 | 70 |
| Range | 27–78 | 48–83 |
| Race/Ethnicity—no. (%) | ||
| White | 103 (90) | 26 (90) |
| Non-White | 11 (10) | 3 (10) |
| Tumor Location—no. (%) | ||
| Upper/middle | 1 (0) | 0 (0) |
| Lower/GEJ | 113 (99) | 29 (100) |
| Tumor Length—cm | ||
| Median | 5 | 6 |
| Range | 2–16 | 2–13 |
| Tumor Grade—no. (%) | ||
| Well/Moderately Differentiated | 40 (35) | 12 (41) |
| Poorly Differentiated | 74 (65) | 17 (59) |
| CXRT Sequence—no. (%) | ||
| Chemo/XRT | 48 (42) | 15 (52) |
| Chemo→Chemo/XRT | 66 (58) | 14 (48) |
| XRT Dose—no. (%) *** | ||
| <50.4 Gyb | 43 (38) | 2 (7) |
| 50.4 Gy | 71 (62) | 27 (93) |
| Post-Treatment Local SUV—no. (%) | ||
| <3.45 | 32 (29) | 7 (29) |
| 3.45–8.75 | 69 (63) | 13 (54) |
| ≥8.75 | 8 (7) | 4 (17) |
| missingc | 5 | 5 |
| Charlson Comorbidity Score—no. (%) | ||
| 0 | 78 (68) | 19 (66) |
| 1 | 31 (33) | 6 (21) |
| ≥2 | 5 (4) | 4 (14) |
| Surgical technique—no. (%) | ||
| Right transthoracic (Ivor Lewis) | 74 (65) | |
| Transhiatal | 11 (10) | |
| Total (three field technique) | 11 (10) | |
| Minimally invasive | 18 (16) | |
NOTE: GEJ = gastroesophageal junction; CXRT = chemoradiation; Chemo = chemotherapy; XRT = radiation; Gy = gray; SUV = standard uptake value
for p < 0.05,
for p < 0.01, and
for p < 0.001
Percentages may not add up to 100 because of rounding
Patients treated with <50.4 Gy typically received 45 Gy
Categorical variable cut-off for post-treatment SUV determined with recursive partitioning
Missing values not included in χ2 significance test
Esophagectomy was typically performed 6–8 weeks after completion of CXRT. The majority of patients (74/114, 64.9%) underwent a right transthoracic esophagectomy (Table 1). Perioperative mortality was low, with only 5 (4.4%) treatment related deaths within 90 days of surgery. Operative morbidity was also low. Among the surgery patients, 21 (18.4%) had a major pulmonary event (defined as postoperative pneumonia and/or reintuabtion and/or pulmonary embolus and/or acute respiratory distress syndrome). There were 13 (11.4%) anastomotic leaks, the majority of which were grade I (5/13, 35.7%) or grade II (5/13, 35.7%) that required no or mild intervention (e.g., drain, stent), respectively. Nineteen (16.7%) patients had a pathologic complete response.
Patient Characteristics
Patients in the two treatment groups had similar demographic and tumor characteristics with the exception of age (Table 1). Definitive CXRT patients were more likely to be older with a median age of 70 (range 48–83); however, patients in both groups had similar comorbidity scores.
Overall and Disease-Free Survival
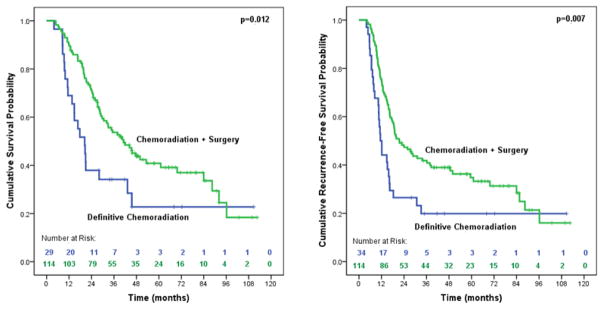
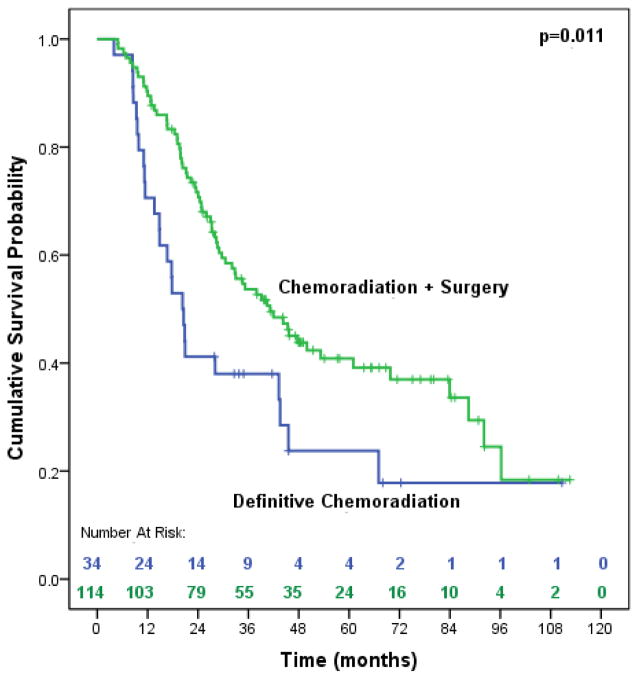
As shown in Table 2, poorly differentiated tumors and treatment with surgery were the only significant predictors of OS. Patients treated with surgery saw a 50% risk reduction in overall mortality. Median OS was significantly improved (41.2 months vs. 20.3 months, p=0.012) among patients treated with surgery (Figure 2). This finding did not change in the sensitivity analysis when salvage resections (n=5) were added to the definitive CXRT group (41.2 months vs. 20.3 months, p=0.011) (Figure 3).
Table 2.
Multivariable Cox proportional regression of predictors of overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma treated with chemoradiation followed by surgery or definitive chemoradiation (n=143)
| Predictor | n | Univariate Cox Regressiona | Multivariable Cox Regressionb | ||||
|---|---|---|---|---|---|---|---|
| p-value | HR | 95% CI | p-value | HR | 95% CI | ||
| Agec | 143 | 0.088 | 1.017 | 0.997–1.037 | 0.469 | 1.008 | 0.987—1.1029 |
| Sex | |||||||
| Male | 134 | 1.000 | |||||
| Female | 9 | 0.698 | 1.179 | 0.513—2.708 | |||
| Race/Ethnicity | |||||||
| White | 129 | 1.000 | |||||
| Non-White | 14 | 0.963 | 0.984 | 0.491—1.972 | |||
| Comorbidity Score | |||||||
| 0 | 97 | 1.000 | |||||
| ≥1 | 46 | 0.273 | 1.282 | 0.822—2.001 | |||
| Tumor Length | 143 | 0.338 | 0.963 | 0.893—1.040 | |||
| Tumor Grade | |||||||
| Well/Moderately Differentiated | 52 | 1.000 | |||||
| Poorly Differentiated | 91 | 0.005 | 2.028 | 1.242—3.310 | 0.005 | 2.041 | 1.235—3.373 |
| CXRT Sequence | |||||||
| Chemo/XRT | 63 | 1.000 | |||||
| Induction Chemotherapy → Chemo/XRT | 80 | 0.702 | 0.919 | 0.597—1.415 | |||
| XRT Dose | |||||||
| <50.4 Gy | 45 | 1.000 | |||||
| 50.4 Gy | 98 | 0.238 | 1.317 | 0.834—2.080 | |||
| Pre-Treatment Local SUV | 0.984 | ||||||
| ≤6.8 | 29 | 1.000 | |||||
| 6.8–10.1 | 38 | 0.700 | 1.128 | 0.611—2.083 | |||
| 10.1–15.8 | 34 | 0.893 | 1.045 | 0.547—1.998 | |||
| ≥15.8 | 37 | 0.900 | 1.041 | 0.553—1.963 | |||
| missing | 5 | 0.769 | 1.179 | 0.393—3.537 | |||
| Post-Treatment Local SUV | 0.051 | 0.097 | |||||
| <3.45 | 39 | 1.000 | |||||
| 3.45–8.75 | 82 | 0.187 | 1.417 | 0.844—2.378 | 0.298 | 1.320 | 0.782—2.228 |
| ≥8.75 | 12 | 0.007 | 2.844 | 1.325—6.105 | 0.012 | 2.686 | 1.237—5.830 |
| missing | 10 | 0.139 | 1.923 | 0.808—4.577 | 0.453 | 1.432 | 0.560—3.663 |
| Surgical Resection | |||||||
| No | 29 | 1.000 | |||||
| Yes | 114 | 0.014 | 0.539 | 0.329—0.881 | 0.020 | 0.504 | 0.283—0.899 |
NOTE: CXRT = chemoradiation; Chemo = chemotherapy; XRT = radiation; Gy = Gray; SUV = standard uptake value
Any variable with a univariate association of p<0.25 (bolded in table) was carried forward to multivariable analysis
Final model of multivariable regression displayed in the table. Wald’s stepwise selection with p=0.10 was used as entry and removal probability until the final model for the data set was obtained.
Final multivariable model age-adjusted
Figure 2.
Median overall survival and disease-free survivala of trimodality eligible patients with locally advanced esophageal adenocarcinoma of treated with chemoradiation followed by surgery compared to definitive chemoradiation (n=148). aFor the disease-free survival analysis, we included 5 definitive chemoradiation patients that were later treated with salvage resections to fully account for local recurrences in this treatment group.
Figure 3.
Median overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma treated with chemoradiation followed by surgery compared to definitive chemoradiation, including salvage resections (n=148)
Poorly differentiated tumors and treatment with surgery were also significant predictors of DFS, as was a SUV≥8.75 on post-treatment PET (Table 3). DFS was significantly improved with the addition of surgery (21.5 months vs. 11.4 months, p=0.007) (Figure 2). Surgery provided a 46% reduced risk of cancer-specific mortality.
Table 3.
Multivariable Cox proportional regression of predictors of disease-free survivala of trimodality eligible patients with locally advanced esophageal adenocarcinoma treated with chemoradiation followed by surgery or definitive chemoradiation (n=148)
| Predictor | n | Univariate Cox Regressionb | Multivariable Cox Regressionc | ||||
|---|---|---|---|---|---|---|---|
| p-value | HR | 95% CI | p-value | HR | 95% CI | ||
| Aged | 148 | 0.245 | 1.011 | 0.993—1.029 | 0.751 | 0.997 | 0.977—1.017 |
| Sex | |||||||
| Male | 139 | ||||||
| Female | 9 | 0.363 | 1.429 | 0.662—3.084 | |||
| Race/Ethnicity | |||||||
| White | 133 | ||||||
| Non-White | 15 | 0.860 | 0.945 | 0.504—1.771 | |||
| Comorbidity Score | |||||||
| 0 | 100 | ||||||
| ≥1 | 48 | 0.322 | 1.230 | 0.816—1.854 | |||
| Tumor Length | 148 | 0.320 | 0.965 | 0.900—1.035 | |||
| Tumor Grade | |||||||
| Well/Moderately Differentiated | 54 | ||||||
| Poorly Differentiated | 94 | 0.008 | 1.784 | 1.167—2.728 | 0.006 | 1.848 | 1.195—2.857 |
| CXRT Sequence | |||||||
| Chemo/XRT | 68 | ||||||
| Induction Chemotherapy → Chemo/XRT | 80 | 0.213 | 0.780 | 0.528—1.153 | |||
| XRT Dose | |||||||
| <50.4 Gy | 46 | ||||||
| 50.4 Gy | 102 | 0.100 | 1.426 | 0.934—2.177 | |||
| Pre-Treatment Local SUV | 0.989 | ||||||
| ≤6.8 | 31 | ||||||
| 6.8–10.1 | 38 | 0.797 | 1.074 | 0.623—1.852 | |||
| 10.1–15.8 | 36 | 0.908 | 0.967 | 0.546—1.174 | |||
| ≥15.8 | 38 | 0.828 | 0.939 | 0.529—1.664 | |||
| missing | 5 | 0.857 | 1.103 | 0.378—3.216 | |||
| Post-Treatment Local SUV | 0.008 | 0.006 | |||||
| <3.45 | 40 | ||||||
| 3.45–8.75 | 85 | 0.339 | 1.257 | 0.787—2.008 | 0.467 | 1.192 | 0.742—1.913 |
| ≥8.75 | 13 | 0.001 | 3.290 | 1.644—6.582 | 0.001 | 3.340 | 1.650—6.763 |
| missing | 10 | 0.332 | 1.485 | 0.668—3.306 | 0.937 | 1.035 | 0.440—2.437 |
| Surgical Resection | |||||||
| No | 34 | ||||||
| Yes | 114 | 0.008 | 0.553 | 0.356—0.858 | 0.004 | 0.466 | 0.278—0.779 |
NOTE: CXRT = chemoradiation; Chemo = chemotherapy; XRT = radiation; Gy = Gray; SUV = standard uptake value
For the disease-free survival analysis, we included 5 definitive chemoradiation patients that were later treated with salvage resections to fully account for local recurrences in this treatment group
Any variable with a univariate association of p<0.25 (bolded in table) was carried forward to multivariable analysis
Final model of multivariable regression displayed in the table. Wald’s stepwise selection with p=0.10 was used as entry and removal probability until the final model for the data set was obtained.
Final multivariable model age-adjusted
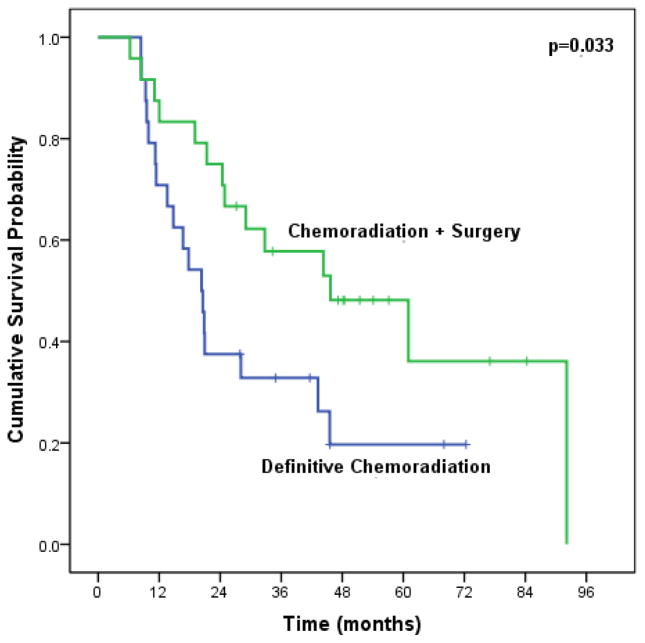
Using propensity score matching to match patients on age, comorbidity score, tumor length, tumor grade, post-CXRT biopsy, and post-CXRT SUV, median OS was significantly better among surgery patients (45.7 months vs. 20.3 months, p=0.033) (Figure 4). Due to patients with missing post-treatment SUV, propensity matching was done on 48 patients (24 CXRT + surgery, 24 definitive CXRT).
Figure 4.
Median overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma treated with chemoradiation followed by surgery compared to definitive chemoradiation, using propensity score matchinga (n=48) aPatients matched on age, comorbidity, tumor length, tumor grade, post-treatment biopsy, and post-treatment SUV
Clinical Responders
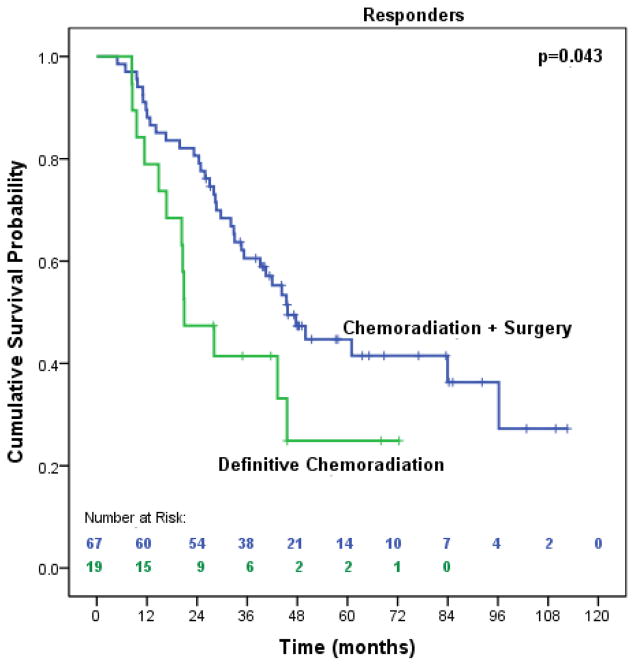
The majority of patients (125, 87.4%) had pre- and post-CXRT PET scans available to evaluate clinical response to CXRT. We identified 86 responders (19 definitive CXRT, 67 surgery) and 39 nonresponders (all treated with surgery). Ten definitive CXRT patients did not have evaluable PET scans or biopsy results to classify them according to our response algorithm. These patients were often considered clinical responders by the treating physician (thus prompting observation vs. further treatment with surgery). Among clinical responders (n=86), patients treated with surgery had significantly improved median OS (45.7 months vs. 20.9 months, p=0.043) (Figure 5).
Figure 5.
Median overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma that achieved a clinical response to neoadjuvant therapy treated with chemoradiation followed by surgery compared to definitive chemoradiation (n=86)
Discussion
Our study is the first to compare treatment with definitive CXRT to CXRT followed by surgery in exclusively adenocarcinoma patients that are eligible for trimodality therapy. Although there are other studies that appear similar to ours [14–16], by contrast, these studies have included patients that were not considered surgical candidates or had progression of disease during neoadjuvant CXRT. Because inclusion criteria may have biased the results of these studies in favor of surgery, an analysis of properly selected patients was critical to establishing a survival benefit. We found that in a cohort of trimodality eligible patients with locally advanced esophageal adenocarcinoma, patients treated with CXRT followed by surgery saw significant advantages in median OS and DFS compared to patients treated with definitive CXRT.
These findings clarify a previously undefined clinical benefit and transform the clinical discussion that takes place after a patient successfully completes CXRT. What is the potential value of additional locoregional therapy with surgery after response to CXRT? Patients with locally advanced, resectable esophageal adenocarcinoma (cStage III) frequently receive treatment with neoadjuvant CXRT, and the majority demonstrates a favorable response to therapy [7, 16–18]. To date, we lacked adequate data to estimate the additional benefit of surgery after successful completion of CXRT. Existing evidence promoted surgery as an integral component of successful trimodality therapy [20], and established that surgery significantly improves locoregional control as compared to definitive CXRT [21–23]. However, an inability to reference the specific survival contribution of surgery may have encouraged exclusion of resection as part of multimodality therapy. This study adds further evidence that the additional benefit in locoregional control afforded by surgery may translate into significant gains in overall survival. For patients contemplating further treatment with surgery after CXRT, knowledge of a specific mortality reduction with the addition of surgery will allow physicians and patients to make informed decisions regarding therapy options.
Our data stands in sharp contrast to two previously published prospective randomized trials. In the FFCD 9102 trial [24], patients were treated with CXRT followed by randomization to further medical or surgical therapy based on barium swallow findings of response to treatment. The study enrolled almost exclusively esophageal squamous cell carcinoma (SCCA) patients (11% glandular cancer), and reported no benefit in overall survival with the addition of surgery. However, a significant number of patients were eliminated from the randomization, and patients that were later treated with salvage surgery were analyzed on intent-to-treat. Similarly, a German collaborative trial [25] randomized patients to treatment with induction chemotherapy and concurrent CXRT with or without surgery. Among patients with SCCA (adenocarcinoma patients were primarily excluded), there was no reported benefit for overall survival in the surgery arm compared to definitive CXRT. Criticism of this trial would suggest that it was underpowered; there is a clear trend in overall survival favoring the surgery arm that does not reach significance, and disease-free survival was significantly improved among surgery patients. Treatment related mortality in both trials was excessive in the surgery arm, negating the potential benefit of surgery. Despite these shortcomings and potential dissimilarities between adenocarcinoma and SCCA, trials describing definitive therapy for esophageal cancer have likely influenced the utilization of definitive CXRT for locally advanced adenocarcinoma in non-protocol settings. Yet, given the observed differences in response rates to CXRT [20], we must consider the treatment of adenocarcinoma as distinct from that of SCCA.
Although our study may be the first to quantify the additional benefit from surgery, several limitations and cautions should be noted. First, this is a retrospective observational study with a limited sample size. These results reflect the experience of a single institution with high surgical volume and a multidisciplinary service dedicated to esophageal cancer. Our findings may not be generalizable to clinical settings that have higher rates of perioperative morbidity and mortality, which may reduce or negate the benefit of surgery we observed in our study. Validation of our results in a larger, multi-institutional setting is ideal.
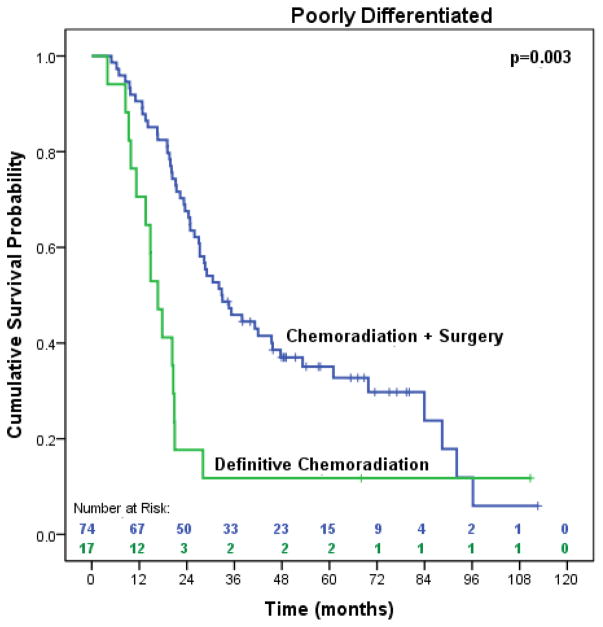
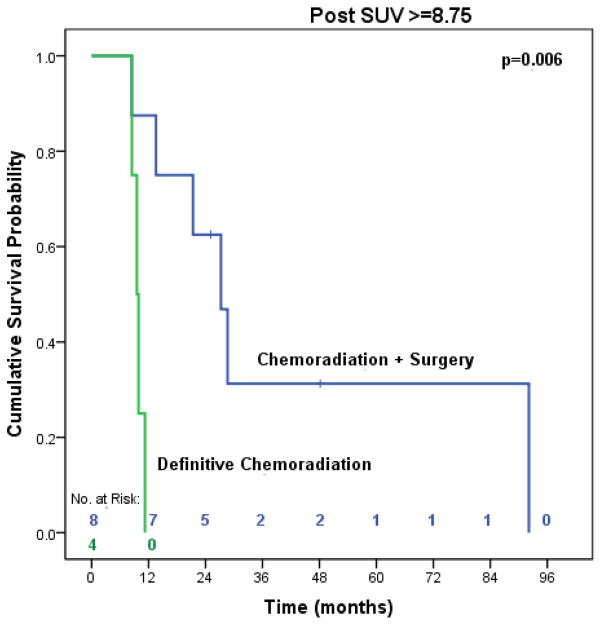
Patients in our study were not randomized to treatment with bimodality or trimodality therapy, which may have introduced a selection bias. To address this limitation, we made every effort to include only patients that were considered surgical candidates after completing neoadjuvant therapy and used important prognostic variables in the propensity matched analysis. However, we should not ignore the possibility that there may be subgroups of patients with aggressive tumor biology that will not benefit from additional locoregional therapy with surgery. In both the OS and DFS multivariable analyses, poorly differentiated tumors and high post-treatment SUV were independently associated with poorer survival. When we stratified the analysis by post-treatment SUV, surgery still provided significant improvements in overall survival (27.3 months vs. 9.5 months, p=0.006) in the subgroup of patients with the highest SUV (≥8.75) (data available in online supplement). Although the number of patients in this subgroup may be too small to reach valid conclusions, the findings are consistent with our previous work indicating that surgery improves the outcomes of patients with high SUV after CXRT [26]. In addition, we compared the overall survival of patients with poorly differentiated tumors, which demonstrated significant survival advantages among patients treated with surgery (32.8 months vs. 16.7 months, p=0.003) (data available in online supplement). Despite the tendency to consider the influence of high SUV and/or poorly differentiated tumors as overwhelming the potential benefit of surgery, these exploratory analyses suggest that surgery still provides significant improvements in survival for patients with aggressive tumor biology. We must consider the results cautiously, though, as our study was not designed or adequately powered to examine the interaction of prognostic variables on survival in subgroups of patients that did or did not undergo surgery. This requires a more complex or full factorial trial design with a much larger sample size.
Finally, our findings also do not preclude selective surgery for patients that do well with CXRT. Patients treated with definitive CXRT in our study reached an OS and DFS plateau at 20%, suggesting that some patients may be destined for complete pathological response and are ultimately cured without surgery. Although we found that there was a benefit to surgery in both matched and unmatched patient populations, as well as the subgroup analysis of clinical responders, there may be a point at which the additional risk of surgery may outweigh the potential benefit for an individual patient. There may be several indications for initially considering trimodality therapy with surgery but later electing to stop treatment after completing CXRT. Neoadjuvant therapy may serve as a physiologic challenge for patients that are considered “borderline” candidates for surgery at initial staging and evaluation. Planning for trimodality therapy utilizes CXRT as an additional assessment of risk that may avoid unnecessarily resecting patients that cannot tolerate surgery. In certain cases, salvage resection could be used after locoregional failure of definitive CXRT if performance status is adequate. This selective approach to resection may prove to be a versatile algorithm to therapy, but this decision must take place within a multidisciplinary setting that includes surgical input. Further study is necessary to better characterize precisely which patients should be considered for initial resection and which would benefit from selective approaches to surgery.
Conclusion
Surgery should be considered an integral component of effective trimodality therapy for locally advanced esophageal adenocarcinoma. Further prospective study is warranted prior to adopting treatment regimens that omit surgery, either entirely or selectively.
Figure 6.
Median overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma with poorly differentiated tumors treated with chemoradiation followed by surgery compared to definitive chemoradiation (n=91)
Figure 7.
Median overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma with post-treatment SUV ≥ 8.75 treated with chemoradiation followed by surgery compared to definitive chemoradiation (n=12)
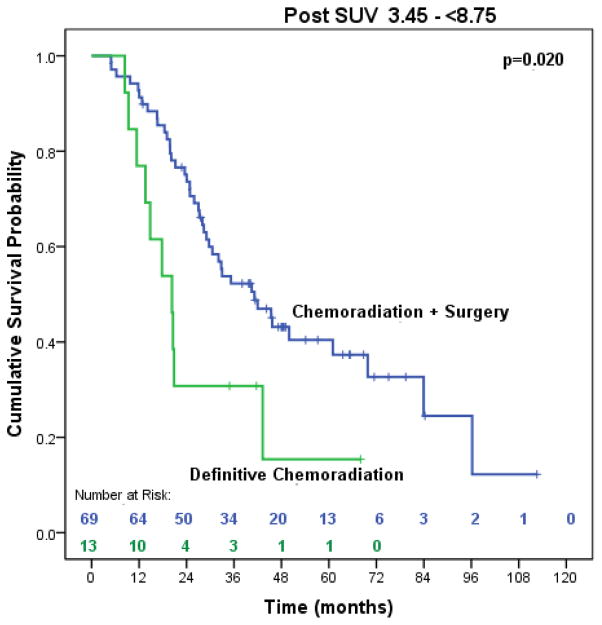
Figure 8.
Median overall survival of trimodality eligible patients with locally advanced esophageal adenocarcinoma with post-treatment SUV 3.45–<8.75 treated with chemoradiation followed by surgery compared to definitive chemoradiation (n=82)
Acknowledgments
Funding: none
The authors would like to thank the generous support of the Stuart & Flora Mason Family Foundation and the Edwards Family Esophageal Research Fund.
Footnotes
Author conflicts of interest: none
Author financial disclosures: none
References
- 1.Cen P, Banki F, Cheng L, Khali K, Du XL, Fallon M, Amaot RJ, Kaiser LR. Changes in age, stage distribution, and survival of patients with esophageal adenocarcinoma over three decades in the United States. Ann Surg Oncol. 2012;19(5):1685–1691. doi: 10.1245/s10434-011-2141-1. [DOI] [PubMed] [Google Scholar]
- 2.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 3.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 4.Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, Falchi AM, Craxi A, Camma C. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB, Ajani JA, Dolormente M, Francisco R, Komaki RR, Lara A, Martin F, Rice DC, Sarabia AJ, Smythe WR, Vaporciyan AA, Walsh GL, Roth JA. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236(3):376–84. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JY, Hofstetter WL. Esophagectomy after chemoradiation: who and when to operate. Sem Thoracic Cardiovasc Surg. 2012 doi: 10.1053/j.semtcvs.2012.10.005. in press. [DOI] [PubMed] [Google Scholar]
- 7.Swisher SG, Winter KA, Komaki RU, Ajani JA, Wu TT, Hofstetter WL, Konski AA, Willett CG. A Phase II study of a paclitaxel-based chemoradiation regimen with selective surgical salvage for resectable locoregionally advanced esophageal cancer: initial reporting of RTOG 0246. Int J Radiat Oncol Biol Phys. 2012;82(5):1967–1972. doi: 10.1016/j.ijrobp.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks JL, Hofstetter W, Correa AM, Mehran RJ, Rice D, Roth J, Walsh G, Vaporciyan A, Erasmus J, Chang J, Maru D, Lee JH, Lee J, Ajani JA, Swisher SG. Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg. 2012;94(4):1126–32. doi: 10.1016/j.athoracsur.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CC, Hofstetter WL, Correa AM, Ajani JA, Komaki RU, Swisher SG. Utilization of surgery in trimodality eligible patients with locally advanced adenocarcinoma in a non-protocol setting. Dis Esophagus. 2013 doi: 10.1111/dote.12019. [DOI] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6. Philadelphia: Lippincott-Raven; 2002. pp. 91–98. [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–385. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, Lorenzen S, Schuster T, Wieder H, Hermann K, Bredenkamp R, Hofler H, Fink U, Peschel C, Schwaiger M, Siewert JR. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 14.Tougeron D, Scotte M, Hamidou H, Di Fiore F, Paillot B, Michot F, Michel P. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol. 2012;105:761–766. doi: 10.1002/jso.22157. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie S, Mailey B, Artinyan A, Metchikian M, Shibata S, Kernstine K, Kim J. Improved outcomes in the management of esophageal cancer with the addition of surgical resection to chemoradiation therapy. Ann Surg Oncol. 2011;18:551–558. doi: 10.1245/s10434-010-1314-7. [DOI] [PubMed] [Google Scholar]
- 16.Merkow RP, Bilimoria KY, McCarter MD, Chow WB, Gordon HS, Stewart AK, Ko CY, Bentrem DJ. Effect of histologic subtype on treatment and outcomes for esophageal cancer in the United States. Cancer. 2012;118(13):3268–3276. doi: 10.1002/cncr.26608. [DOI] [PubMed] [Google Scholar]
- 17.Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschlager G, Busch R, Siewert JR, Schwaiger M, Fink U. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19(12):3058–3065. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- 18.Downey RJ, Akhurst T, Ilson D, Ginsberg R, Bains MS, Gonen M, Koong H, Gollub M, Minsky BD, Zakowski M, Turnbull A, Larson SM, Rusch V. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21(3):428–432. doi: 10.1200/JCO.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Willis J, Cooper GS, Isenberg G, Sivak MV, Levitan N, Clayman J, Chak A. Correlation of EUS measurement with pathologic assessment of neoadjuvant therapy response in esophageal carcinoma. Gastrointest Endosc. 2002;55(6):655–661. doi: 10.1067/mge.2002.123273. [DOI] [PubMed] [Google Scholar]
- 20.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 21.Al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, Cooper J, Byhardt R, Davis L, Emami B. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15(1):277–284. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01) JAMA. 1999;281(17):1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 23.Herskovic A, Martz K, Al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Eng J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 24.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 25.Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, Klump B, Budach W, Teichmann R, Schmitt M, Schmitt G, Franke C, Wilke H. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 26.Patnana SV, Murthy SB, Xiao L, Rohren E, Hofstetter WL, Swisher SG, Liao Z, Lee JH, Bhutani MS, Macapinlac HA, Wang X, Ajani JA. Critical role of surgery in patients with gastroesophageal carcinoma with a poor prognosis after chemoradiation as defined by positron emission tomography. Cancer. 2010;116(19):4487–4494. doi: 10.1002/cncr.25431. [DOI] [PubMed] [Google Scholar]