Abstract
Flavonoids have been demonstrated to have cytotoxic activities toward numerous human cancer cells, whereas they have little or no effect on normal cells. The numerous flavonoids in traditional Chinese herbs may be promising candidates for the development of novel anti-cancer drugs. Our previous study demonstrated that CH2Cl2 and 95% ethanol eluate (EE) fractions have the strongest cytotoxic activities against human cancer cell lines of the 9 fractions separated from Artemisia sacrorum Ledeb., which is widely used to prevent and treat diverse diseases in Northeast China. In the present study, 8 flavonoids were isolated from the 95% EE fraction of Artemisia sacrorum Ledeb. The chemical structures of the compounds were elucidated by extensive spectroscopic analyses. The following 5 flavonoids were isolated for the first time from this plant: Jaceosidin, kaempferol, quercetin, luteolin and quercitrin. A total of 2 flavonoids from the CH2Cl2 fraction and 8 flavonoids from the 95% EE fraction were examined to evaluate their cytotoxic activities against human SK-HEP-1 hepatoma cancer cells and human HeLa cervical cancer cells, respectively. The results revealed that 2 flavonoids had marked cytotoxic activities against HeLa cells.
Keywords: Artemisia sacrorum Ledeb., flavonoids, cytotoxic activities, human cancer cell lines
Introduction
The majority of approved anticancer drugs are either natural products or have been developed based on knowledge gained from natural products, which have been important in the search for novel anticancer drugs compared with other areas of drug development (1–5). Traditional Chinese medicine has been historically used to treat disease using natural products. Although certain traditional Chinese herbs should no longer be used, other traditional Chinese herbs that have been proven to be effective have been gradually incorporated into modern medicine (6).
Among these traditional Chinese medicinal plants, Artemisia sacrorum Ledeb (ASL; Compositae) is of particular interest as it is widely used to prevent and treat diverse diseases in the Yanbian area of Northeast China (7,8). Previous studies have indicated that the water-soluble parts of the A. sacrorum extract were protective against carbon tetrachloride and acetaminophen-induced hepatotoxicity in mice, and the underlying mechanisms have been investigated (9–11). In a previous study, A. sacrorum was demonstrated to prevent N-acetyl-p-aminophenol (APAP)-induced apoptosis and necrosis, as indicated by liver histopathological and immunohistochemical analysis, and DNA laddering (9–11). According to the results from a western blot analysis, the ethanol eluate precipitation (EEP) decreased APAP-induced caspase-3 and −8 protein expression levels in mouse livers (9–11). Furthermore, ASL was able to inhibit adipocyte differentiation and adipogenesis through the activation of 5′-adenosine monophosphate-activated protein kinase (AMPK) in 3T3-L1 adipocytes and hepatocellular carcinoma (HepG2) cells, respectively (12,13). Petroleum ether fraction of A. sacrorum Ledeb, another extract from A. sacrorum, inhibited glucose production via the AMPK-glycogen synthase kinase-cAMP response element binding protein signaling pathway in HepG2 cells (14). Notably, the cytotoxicities of the 9 fractions separated from A. sacrorum via the two extraction methods was investigated in our previous study, and the results demonstrated that the CH2Cl2 and 95% ethanol eluate (EE) fractions revealed the strongest cytotoxic activities against 3 human cancer cell lines. In addition, a subsequent study demonstrated that 10 compounds, of which 2 were flavonoids, were isolated from the CH2Cl2 fraction (15).
The present study aimed to investigate the isolation and structure of the compounds from the 95% EE fraction of A. sacrorum. The results demonstrated that this fraction was markedly rich in flavonoids. Flavonoids are cancer-preventive agents, and have been shown to be particularly important (16–18). Therefore, the present study was further conducted to evaluate the cytotoxic activities of all flavonoids from the CH2Cl2 and 95% EE fractions.
Materials and methods
General experimental procedures
The nuclear magnetic resonance (NMR) spectra of compounds 1, 2, 3, 5, 6, and 7 were recorded on a Bruker 500 MHz NMR spectrometer (Bruker Corporation, Billerica, MA, USA), operating at a frequency of 500 MHz for 1H and 125 MHz for 13C nuclei at room temperature, and with TMS as the internal standard. The NMR spectra of compounds 4 and 8 were recorded on a Bruker 300 MHz NMR spectrometer, operating at 300 MHz for 1H and 75 MHz for 13C nuclei at room temperature with tetramethylsilane (TMS) as the internal standard. Chemical shifts (δ) were expressed in parts per million (ppm) relative to TMS. The chemical shifts (δ) and coupling constant values were reported in ppm and Hz, respectively. Compounds 1, 2, 3, 4, 6, and 7 were dissolved in CD3OD, 5 was dissolved in dimethyl sulfoxide (DMSO), and 8 was dissolved in acetone-d6. Column chromatography (CC) was performed on a Sephadex LH-20 (18–111 µm; GE Healthcare Biosciences, Pittsburgh, PA, USA), YMC-GEL octadecyl (ODS)-A (50 µm; YMC Co., Ltd., Kyoto, Japan) and silica gel (200–300 mesh, Qingdao Marine Chemical, Ltd., Qingdao, China) columns. D-101 macroporous absorption resin was purchased from Tianjin Haiguang Chemical Co., Ltd. (Tianjin, China). Silica gel 60 F254 plates (20×20 cm; 0.2 mm thick; Merck, Darmstadt, Germany) and silica gel GF 254 (Qingdao Marine Chemical Co., Ltd.) were used for thin-layer chromatography (TLC) analysis. The CH3OH used for column chromatography was high performance liquid chromatography-grade (Jiangsu Hanbang Science and Technology Co., Ltd., Jiangsu, China). All other chemicals and reagents used in the present study were of analytical grade.
Plant material
The aerial parts of ASL (Compositae) were collected in July 2008 from Xidong, Yanji in Jilin. The plant was taxonomically identified and authenticated by Professor Huizi Lv (College of Pharmacy, Yanbian University of China, Yanji, China). A specimen of the plant was deposited at the College of Pharmacy, Yanbian University.
Extraction and isolation
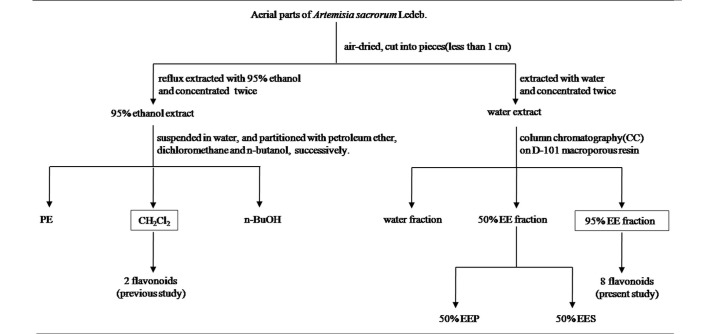
A total of 5 kg of the aerial parts of A. sacrorum were collected, air-dried in the shade, and dissolved in boiling water twice (Figs. 1 and 2). The extract was then separated on a D-101 macroporous absorption resin to elute water, 50% EE and 95% EE fractions. From this separation, the 95% EE fraction (16.03 g) was chromatographed on a silica-gel column (200–300 mesh) with petroleum ether-EtOAc in a stepwise gradient elution (50:1-0:100). The eluates were combined based on the TLC results in order to produce 8 fractions (1–8).
Figure 1.
Extraction scheme for the isolation of 10 flavonoids from the 2 most cytotoxic fractions of Artemisia sacrorum Ledeb. By shade-dried we mean that the aerial parts of the herb were air-dried in the shade. EE, ethanol eluate; PE, petroleum ether; EEP, ethanol eluate precipitation; EES, ethanol eluate supernatant.
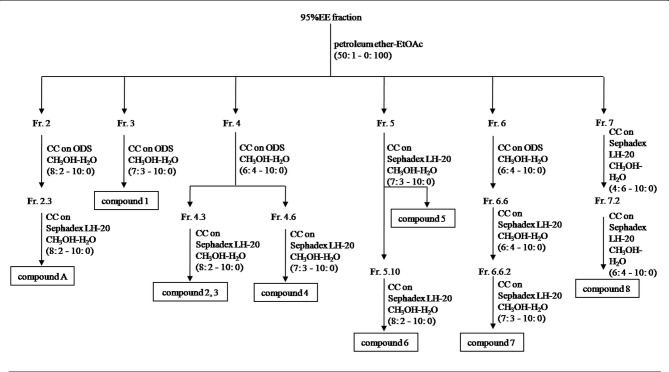
Figure 2.
Extraction and separation of 8 flavonoids and 1 coumarin from the 95% EE fraction. Fr, fraction; EE, ethanol eluate.
The fractions were chromatographed as follows: Fraction 2 was chromatographed on an ODS column by eluting with gradient mixtures of CH3OH and H2O (8:2 to 10:0) in order to obtain 7 subfractions (2.1–7). Subfraction 2.3 was rechromatographed on a Sephadex LH-20 column by eluting with gradient mixtures of CH3OH and H2O (8:2 to 10:0) to obtain compound A (3.0 mg). Fraction 3 was rechromatographed on an ODS column by eluting with gradient mixtures of CH3OH and H2O (7:3 to 10:0) to afford compound 1 (23.2 mg). Fraction 4 was rechromatographed on an ODS column by eluting with gradient mixtures of CH3OH and H2O (6:4 to 10:0) to obtain 11 subfractions (4.1–11). Subfraction 4.3 was rechromatographed on a Sephadex LH-20 column by eluting with gradient mixtures of CH3OH and H2O (8:2 to 10:0) to obtain compound 2 (2.6 mg) and compound 3 (1.5 mg). Subfraction 4.6 was subjected to a Sephadex LH-20 column by eluting with gradient mixtures of CH3OH and H2O (7:3 to 10:0) to obtain compound 4 (8.2 mg). Fraction 5 was rechromatographed on a Sephadex LH-20 column by eluting with CH3OH-H2O (7:3 to 10:0) to obtain compound 5 (2.0 mg) and 12 subfractions (5.1–12). Subfraction 5.10 was subjected to a Sephadex LH-20 column by eluting with gradient mixtures of CH3OH and H2O (8:2 to 10:0) to obtain compound 6 (1.5 mg). Fraction 6 was rechromatographed on an ODS column by eluting with gradient mixtures of CH3OH and H2O (6:4 to 10:0) to obtain eight subfractions (6.1–8). Subfraction 6.6 was subjected to a Sephadex LH-20 column repeatedly to obtain compound 7 (2.6 mg). Fraction 7 was rechromatographed on a Sephadex LH-20 column by eluting with gradient mixtures of CH3OH-H2O (4:6 to 10:0) to produce 5 subfractions (7.1–5). Finally, subfraction 7.2 was subjected to a Sephadex LH-20 column once more by eluting with gradient mixtures of CH3OH and H2O (6:4 to 10:0) to afford compound 8 (6.0 mg).
Cell culture and MTS assay
The two human cancer cell lines (SK-HEP-1 and HeLa) and one normal human cell line (HEK293) used in the cytotoxic assay were purchased from American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 mg/ml streptomycin and kept at 37°C in a humidified atmosphere containing 5% CO2.
A 200-µl aliquot of adherent cells was used to seed 96-well cell culture plates at 3×104 cells/well and allowed to adhere for 6 h prior to drug addition. Each cell line was treated with 0, 5, 10, 25, 50, 100 and 200 µM of flavonoids for 48 h. Subsequently, 20 µl MTS reagent was added into each well and incubated for 1 h. The cell viability was detected by the CellTiter 96 AQueous One solution Cell Proliferation Assay kit (Promega Corporation, Madison, WI, USA).
Results and Discussion
To observe the cytotoxic activity of the herb, A. sacrorum was extracted and isolated by 95% EtOH and water, as described in our previous study (15). Following extraction with 95% EtOH extract with various solvents, and separation of the water extract with a D-101 macroporous resin column, 9 fractions of A. sacrorum were obtained, of which the 95% EE and CH2Cl2 fraction revealed the strongest cytotoxity against 3 human cancer cell lines. In a subsequent study, 2 flavonoids were isolated from the CH2Cl2 fraction (15). In the present study, 9 compounds were isolated from the 95% EE fraction.
Compound 1 was isolated as yellow, needle-like crystals (acetone). The 1H-NMR and 13C-NMR spectra of the compound revealed the characteristic signals of a flavonoid. The 1H-NMR spectrum exhibited two proton signals at δ 6.56 (1H; s) and 6.61 (1H; s), which represented the proton signals of C3-H and C8-H of the A ring, respectively. Signals at δ 7.45 (1H; d; J=2.0 Hz), 6.92 (1H; d; J=8.35 Hz) and 7.49 (1H; dd; J=2.0; 8.35 Hz) represented the proton signals of C2′-H, C5′-H and C6′-H of the B ring. δ 3.96 (3H; s) and 3.88 (3H; s) corresponded to the proton signals of the 2 methoxy groups. The 13C-NMR spectrum showed 17 carbon signals of which δC 184.23 was the carbonyl signal, whereas 56.71 and 60.94 were assigned to 2 methoxy groups. By analyzing these data and comparing them with those in the literature (19), the structure of compound 1 was determined as jaceosidin (Fig. 3).
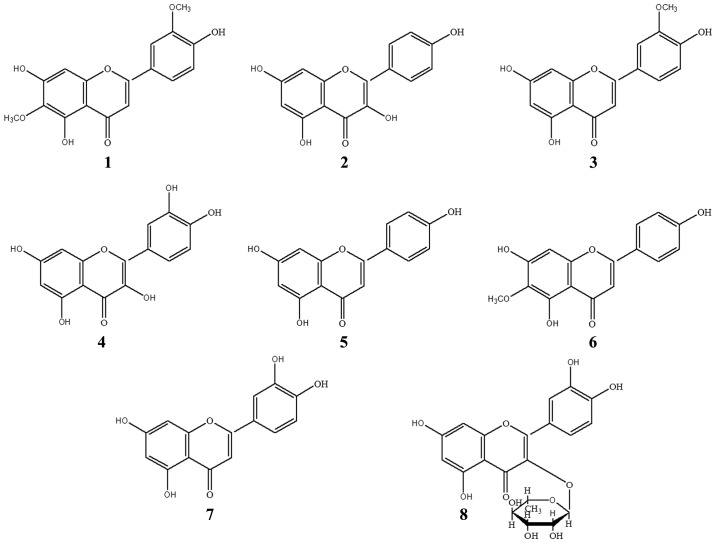
Figure 3.
Chemical structures of 8 flavonoids from the 95% EE fraction from Artemisia sacrorum Ledeb. 1–8 represent the compound numbers. EE, ethanol eluate.
Compound 2 was obtained as a yellow powder (MeOH). The 1H-NMR spectrum demonstrated 2 meta-coupled proton signals at δ 6.18 (1H; d; J=2.0 Hz; H-6) and 6.40 (1H; d; J=2.0 Hz; H-8) of the A ring. Two groups of meta-coupled proton signals at δ 6.90 (2H; d; J=9.0 Hz; H-3′; H-5′) and 8.09 (2H; d; J=9.0 Hz; H-2′; H-6′) were assigned to 4 protons in the B ring. The 13C-NMR spectrum revealed carbon signals of δC 148.15, 137.17, 177.44, 158.35, 99.38, 165.77, 94.55, 162.53, and 104.56 for the A ring, and 123.81, 130.69, 116.35, 160.57, 116.35, and 130.69 for the B ring. Based on the above spectral data and comparison with a previous study (20), the chemical structure of compound 2 was determined as kaempferol.
Compound 6 was obtained as yellow, needle-like crystals (MeOH). The 1H-NMR spectrum revealed 2 isolated proton signals at δ 6.58 (1H; s; H-3) and 6.67 (1H; s; H-8), which were attributed to H-3 (C-ring) and H-8 (A-ring), respectively. Two groups of meta-coupled proton signals at δ 6.92 (2H; d; J=8.75 Hz; H-3′; H-5′) and 7.84 (2H; d; J=8.75 Hz; H-2′; H-6′) were assigned to 4 protons of the B ring. The 13C-NMR spectrum showed δC values of 164.46, 102.46, 184.31, 152.69, 131.70, 157.66, 94.17, 152.69, and 104.87 for the A ring, and 122.14, 128.45, 116.15, 161.32, 116.15, and 128.45 for the B ring. These spectroscopic data were characteristic of a flavonoid. Based on the above spectral data and comparison with previous studies (21,22), the chemical structure of compound 6 was determined as hispidulin.
Compound 8 was obtained as a yellow powder (acetone). 1H-NMR revealed, in the aromatic region, 2 meta-coupled proton signals at δ 6.25 (1H; d; J=2.07 Hz; H-6) and 6.46 (1H; d; J=2.07 Hz; H-8) for the A ring. 1H-NMR also demonstrated 2 meta-coupled proton signals at δ 7.49 (1H; d; J=2.07 Hz; H-2′), 7.39 (1H; dd; J=2.07; 8.34 Hz; H-6′), and 1 isolated proton signal at δ 6.98 (1H; d; J=8.34 Hz; H-5′) for the B ring. These 3 proton signals exhibited the typical three-spin system of the 1′,3′,4′-trisubstituted B ring. δ 5.28 (1H; d; J=1.5 Hz; H-1′′) was the anomeric proton signal of sugar. The 13C-NMR spectrum revealed a carbonyl proton signal at δC 179.18, which was assigned to C-4, and 17.63–102.57 ppm for a group of 6-carbon sugar signals revealing the presence of an aglycone moiety. Based on the above spectral data and a comparison with previous studies (23,24), the chemical structure of compound 8 was determined as quercitrin.
In addition to the above-mentioned compounds, 5 other compounds were also identified as chrysoeriol (compound 3) (25), quercetin (compound 4) (26), apigenin (compound 5) (27), luteolin (compound 7) (28,29) and scopoletin (compound A) (30). These were identified by interpretation of their spectroscopic data and comparison of the data with the reported values. Among them, compounds 1–8 were flavonoids, and flavonoids 1, 2, 4, 7 and 8 were isolated for the first time from A. sacrorum. The structures of the 8 flavonoids are depicted in Fig. 3 and all 13C-NMR spectra data were listed in Table I.
Table I.
13C-NMR spectra data of compounds 1–8 (δ, ppm; compounds 4 and 8 were detected at 75 MHz, others at 125 MHz; compounds 1, 2, 3, 4, 6 and 7 dissolved in CD3OD, compound 5 dissolved in DMSO, and compound 8 dissolved in acetone-d6; OMe, OCH3).
| Compound no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 2 | 166.24 | 148.15 | 160.89 | 147.99 | 164.17 | 164.46 | 164.73 | 158.15 |
| 3 | 103.80 | 137.17 | 104.15 | 137.24 | 103.04 | 102.46 | 104.62 | 135.63 |
| 4 | 184.23 | 177.44 | 183.79 | 177.34 | 181.77 | 184.31 | 183.66 | 179.18 |
| 5 | 154.70 | 158.35 | 163.13 | 158.23 | 157.48 | 152.69 | 162.33 | 163.06 |
| 6 | 132.97 | 99.38 | 123.75 | 99.22 | 98.90 | 131.70 | 99.78 | 99.35 |
| 7 | 158.90 | 165.77 | 166.09 | 162.52 | 163.88 | 157.66 | 165.61 | 164.77 |
| 8 | 95.38 | 94.55 | 95.31 | 94.39 | 94.16 | 94.17 | 94.75 | 94.35 |
| 9 | 154.70 | 162.53 | 156.19 | 165.58 | 161.22 | 152.69 | 158.25 | 157.63 |
| 10 | 105.78 | 104.56 | 105.39 | 104.52 | 103.77 | 104.87 | 103.89 | 105.75 |
| 1′ | 123.78 | 123.81 | 124.69 | 148.77 | 121.38 | 122.14 | 122.31 | 122.43 |
| 2′ | 110.75 | 130.69 | 110.70 | 115.98 | 128.56 | 128.45 | 114.22 | 115.99 |
| 3′ | 149.52 | 116.35 | 149.58 | 124.14 | 116.05 | 116.15 | 146.78 | 145.68 |
| 4′ | 152.13 | 160.57 | 152.52 | 146.22 | 161.06 | 161.32 | 150.71 | 148.85 |
| 5′ | 116.81 | 116.35 | 116.86 | 116.22 | 116.05 | 116.15 | 116.01 | 116.56 |
| 6′ | 121.76 | 130.69 | 121.77 | 121.67 | 128.56 | 128.45 | 120.46 | 122.73 |
| OMe | 56.71 | 56.72 | 59.85 | |||||
| OMe | 60.94 | |||||||
| 1′′ | 102.57 | |||||||
| 2′′ | 71.26) | |||||||
| 3′′ | 71.97) | |||||||
| 4′′ | 72.86) | |||||||
| 5′′ | 71.19 | |||||||
| 6′′ | 17.63 |
The table values represent 13C-NMR spectra data of compounds 1–8. OMe represents OCH3. NMR, nuclear magnetic resonance; DMSO, dimethyl sulfoxide.
A total of 8 flavonoids isolated from the 95% EE fraction of A. sacrorum were designated as flavonoids 1–8, and 2 flavonoids (genkwanin and acacetin) previously isolated from the CH2Cl2 fraction were designated as flavonoids 9 and 10. Although these 10 flavonoids were not novel compounds, their possible biological activities should not be ignored because they have previously been identified. Furthermore, not every novel compound is examined for all its activities (even in vitro experiments before it is considered to be old). Conversely, the majority of novel compounds have only undergone in vitro activity tests.
In the present study, the cytotoxic activities against the 10 flavonoids on SK-HEP-1, HeLa and HEK293 cell growth were determined using an MTS assay (31). Their IC50 values were presented in Table II, in which apigenin (flavonoid 5) and luteolin (flavonoid 7) exhibited strong cytotoxic activities against human cervical cancer HeLa cells. Conversely, they had no cytotoxic activity against normal human embryonic kidney HEK293 cells.
Table II.
Cytotoxities of 10 flavonoids isolated from Artemisia sacrorum (IC50, µM).
| Flavonoids | SK-HEP1 | HeLa | HEK293 |
|---|---|---|---|
| 1 | >50 | >50 | 14.1380 |
| 2 | >50 | 45.809 | >50 |
| 3 | 47.19 | >50 | >50 |
| 4 | 45.96 | >50 | >50 |
| 5 | 46.56 | 6.092 | >50 |
| 6 | 49.93 | >50 | >50 |
| 7 | >50 | 16.254 | >50 |
| 8 | >50 | >50 | >50 |
| 9 | >50 | 47.834 | >50 |
| 10 | >50 | >50 | >50 |
All compounds with IC50 values >50 µM were considered inactive.
The structures of the 10 flavonoids are similar. However, certain flavonoids revealed different cytotoxic activity against the two human liver cancer SK-HEP-1 and human cervical cancer HeLa cells. Although certain flavonoids have similar chemical structures, there are still compound-specific effects on certain neoplasms that are relevant to adjust specific biochemical processes to treat certain neoplasms differentially (32,33). In the present study, the majority of the 10 flavonoids demonstrated no cytotoxic activity against normal human embryonic kidney cells.
Flavonoids may serve as clinically significant chemotherapeutic agents in the treatment of cancer. Indeed, flavonoids have been demonstrated to reveal cytotoxic activities towards numerous human cancer cells, whilst having little or no effect on normal cells. This has led to interest in the development of potential flavonoid-based chemotherapeutics for anticancer treatment (34–37). Traditional Chinese herbs, which depend predominantly on empirical medication, have been used for thousands of years in the treatment of numerous diseases, often with few adverse effects. Furthermore, many of the active flavonoids in traditional Chinese herbs have been the subject of studies aiming to develop novel anti-cancer drugs by demonstrating their activity through in vivo and in vitro experiments (38–40). The diverse flavonoids in these herbal medicines may be promising candidates in the development of novel anti-cancer drugs.
Acknowledgements
The present study was supported by the Natural Science Foundation of China (grant no. 81260669). The authors are grateful to Mr. Guang-Hao Zheng and Mr. Zhi-Yong Li for their support during the extraction and fractionation procedures. We also thank Dr. Ming-Shan Zheng for her help in the chemical structure elucidation of several compounds.
References
- 1.LiWeber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics (Sao Paulo) 2011;66:1073–1079. doi: 10.1590/S1807-59322011000600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tundis R, Loizzo MR, Menichini F, Bonesi M, Colica C, Menichini F. In vitro cytotoxic activity of extracts and isolated constituents of Salvia leriifolia Benth. against a panel of human cancer cell lines. Chem Biodivers. 2011;8:1152–1162. doi: 10.1002/cbdv.201000311. [DOI] [PubMed] [Google Scholar]
- 4.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. Natural products as source of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 6.Dong J. The relationship between traditional chinese medicine and modern medicine. Evid Based Complement Alternat Med. 2013;2013:153148. doi: 10.1155/2013/153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piao MJ, Cui SN, Zhang DY. Korean ethnic Materia Medica in China. Yanji, China: Yanbian People Press; 2012. pp. 244–245. Yan Bian Ren Min Chu Ban She. [Google Scholar]
- 8.Jia MR, Li XW. Chinese ethnic Materia Medica. Zhong Guo Yi Yao Ke Ji Chu Ban She. 2005;69 [Google Scholar]
- 9.Piao GC, Quan YC. Protective effects of extracts of Artemisia sacrorum Ledeb. on acute hepatic injury in mice. Shi Zhen Guo Yi Guo Yao. 2007;18:1646–1647. (In Chinese) [Google Scholar]
- 10.Yuan HD, Jin GZ, Piao GC. Protective effects of the supernatant of ethanol eluate from Artemisia sacrorum Ledeb. against acetaminophen-induced liver injury in mice [corrected] Biol Pharm Bull. 2009;32:1683–1688. doi: 10.1248/bpb.32.1683. [DOI] [PubMed] [Google Scholar]
- 11.Yuan HD, Jin GZ, Piao GC. Hepatoprotective effects of an active part from Artemisia sacrorum Ledeb. against acetaminophen-induced toxicity in mice. J Ethnopharmacol. 2010;127:528–533. doi: 10.1016/j.jep.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Yuan HD, Piao GC. An active part of Artemisia sacrorum Ledeb. inhibits adipogenesis via the AMPK signaling pathway in 3T3-L1 adipocytes. Int J Mol Med. 2011;27:531–536. doi: 10.3892/ijmm.2011.620. [DOI] [PubMed] [Google Scholar]
- 13.Yuan HD, Yuan HY, Chung SH, Jin GZ, Piao GC. An active part of Artemisia sacrorum Ledeb. attenuates hepatic lipid accumulation through activating AMP-activated protein kinase in human HepG2 cells. Biosci Biotechnol Biochem. 2010;74:322–328. doi: 10.1271/bbb.90651. [DOI] [PubMed] [Google Scholar]
- 14.Yuan HD, Piao GC. An active part of Artemisia sacrorum Ledeb. inhibits adipogenesis via the AMPK signaling pathway in 3T3-L1 adipocytes. Int J Mol Med. 2011;27:531–536. doi: 10.3892/ijmm.2011.620. [DOI] [PubMed] [Google Scholar]
- 15.Piao GC, Li YX, Yuan HD, Jin GZ. Cytotoxic fraction from Artemisia sacrorum Ledeb. against three human cancer cell lines and separation and identification of its compounds. Nat Prod Res. 2012;26:1483–1491. doi: 10.1080/14786419.2011.565473. [DOI] [PubMed] [Google Scholar]
- 16.Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 17.Chung CS, Jiang Y, Cheng D, Birt DF. Impact of adenomatous polyposis coli (APC) tumor supressor gene in human colon cancer cell lines on cell cycle arrest by apigenin. Mol Carcinog. 2007;46:773–782. doi: 10.1002/mc.20306. [DOI] [PubMed] [Google Scholar]
- 18.Takagaki N, Sowa Y, Oki T, Nakanishi R, Yogosawa S, Sakai T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int J Oncol. 2005;26:185–189. [PubMed] [Google Scholar]
- 19.Wang QH, Wu XL, Wang JH. Study on chemical constituents of Artemisia frigida (II) Chin Tradit Herb Drug. 2011;42:1075–1078. [Google Scholar]
- 20.Yu DQ, Yang JS, editors. Analytical Chemistry Handbook, Vol 7. 2nd edition. Beijing Chemical industry Press; Beijing: 1999. p. 820. 820. [Google Scholar]
- 21.Hazekamp A, Verpoorte R, Panthong A. Isolation of a bronchodilator flavonoid from the Thai medicinal plant Clerodendrum petasites. J Ethnopharmacol. 2001;78:45–49. doi: 10.1016/S0378-8741(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LD, Yu JG, Guo J, Yang SL. Compounds from roots of Chirita fimbrisepala Hand.-Mazz. Zhongguo Zhong Yao Za Zhi. 2001;26:114–117. (In Chinese) [PubMed] [Google Scholar]
- 23.Wang HP, Cao F, Yang XW. Chemical constituents in aerial parts of Polygonum capitatum. Zhong Cao Yao. 2013;44:24–30. [Google Scholar]
- 24.Lu J, Kong LY. Studies on the Constituents of Hypericum japonicum Thunb. Ex Murray. Zhong Guo Xian Dai Zhong Yao. 2007;9:12–13. [Google Scholar]
- 25.Wei JH, Chen J, Cai SF, Lu RM, Lin SW. Chemical constituents in whole herb of Cardiospermum halicacabum (I) Zhong Cao Yao. 2011;42:1509–1511. [Google Scholar]
- 26.Luo QH, Bu XY, Liu HW, Fan M, Ding AS, Yao XS. Studies on the anti-hypoxia active Constituents of Artemisia scoparia. Zhong Cao Yao Zeng Kan. 2006;37:199–201. [Google Scholar]
- 27.Nan HH, Zhang S, Wu J. Chemical constituents from Clerodendrum inerme. Zhong Cao Yao. 2005;36:492–494. [Google Scholar]
- 28.Wei TM, Yan YN, Guan XL, Liu YF, Wei DH. Analysis of the chemical constituents of the ground part of Sedum sarmentosum. J B Univ TCM. 2003;26:59–61. [Google Scholar]
- 29.Xu ZH, Wu HX, Wei XY, Feng SX, Hu TM. Flavonoids from Lespedeza davurica. Acta Bot Boreal-Occident Sin. 2010;30:1485–1489. [Google Scholar]
- 30.Xie T, Liang JY, Liu J, Wang M, Wei XL, Yang CH. Chemical Study on Artemisia scoparia. J China Pharm Univ. 2004;35:401–403. [Google Scholar]
- 31.Kometani T, Yoshino I, Miura N, Okazaki H, Ohba T, Takenaka T, Shoji F, Yano T, Maehara Y. Benzo[a]pyrene promotes proliferation of human lung cancer cells by accelerating the epidermal growth factor receptor signaling pathway. Cancer Lett. 2009;278:27–33. doi: 10.1016/j.canlet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Bonham M, Posakony J, Coleman I, Montgomery B, Simon J, Nelson PS. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin Cancer Res. 2005;11:3905–3914. doi: 10.1158/1078-0432.CCR-04-1974. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, Sakata K, Matsumoto Y, Sayama Y, Mori H. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128:539–546. doi: 10.1007/s00432-002-0373-y. [DOI] [PubMed] [Google Scholar]
- 34.Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang Y, Liu B. Baicalin induces human mucoepidermoid carcinoma Mc3 cells apoptosis in vitro and in vivo. Invest New Drugs. 2011;29:637–645. doi: 10.1007/s10637-010-9402-x. [DOI] [PubMed] [Google Scholar]
- 35.Chen WY, Hsieh YA, Tsai CI, Kang YF, Chang FR, Wu YC, Wu CC. Protoapigenone, a natural derivative of apigenin, induces mitogen-activated protein kinase-dependent apoptosis in human breast cancer cells associated with induction of oxidative stress and inhibition of glutathione S-transferase TT. Invest New Drugs. 2011;29:1347–1359. doi: 10.1007/s10637-010-9497-0. [DOI] [PubMed] [Google Scholar]
- 36.Plochmann K, Korte G, Koutsilieri E, Richling E, Riederer P, Rethwilm A, Schreier P, Scheller C. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch Biochem Biophys. 2007;460:1–9. doi: 10.1016/j.abb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 37.BenSghaier M, Skandrani I, Nasr N, Franca MG, ChekirGhedira L, Ghedira K. Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: A structure-activity relationship study. Environ Toxicol Pharmacol. 2011;32:336–348. doi: 10.1016/j.etap.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, Sun G, Dong X, Wang M, Qin M, Yu Y, Sun X. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS One. 2015;10:e0120259. doi: 10.1371/journal.pone.0120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Zhang B, Yuan X, Yang F, Liu J, Zhao H, Liu L, Wang Y, Wang Z, Zheng Q. Isoliquiritigenin-induced differentiation in mouse melanoma B16F0 cell line. Oxid Med Cell Longev. 2012 Dec 10; doi: 10.1155/2012/534934. (Epub ahead of print) doi: 10.1155/2012/534934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B. General research on effects of flavonoids ingredients on Chinese herbs on anti-cancer. Zhe Jiang Zhong Yi Yao Da Xue Xue. 2012;36:838–840. (In Chinese) [Google Scholar]