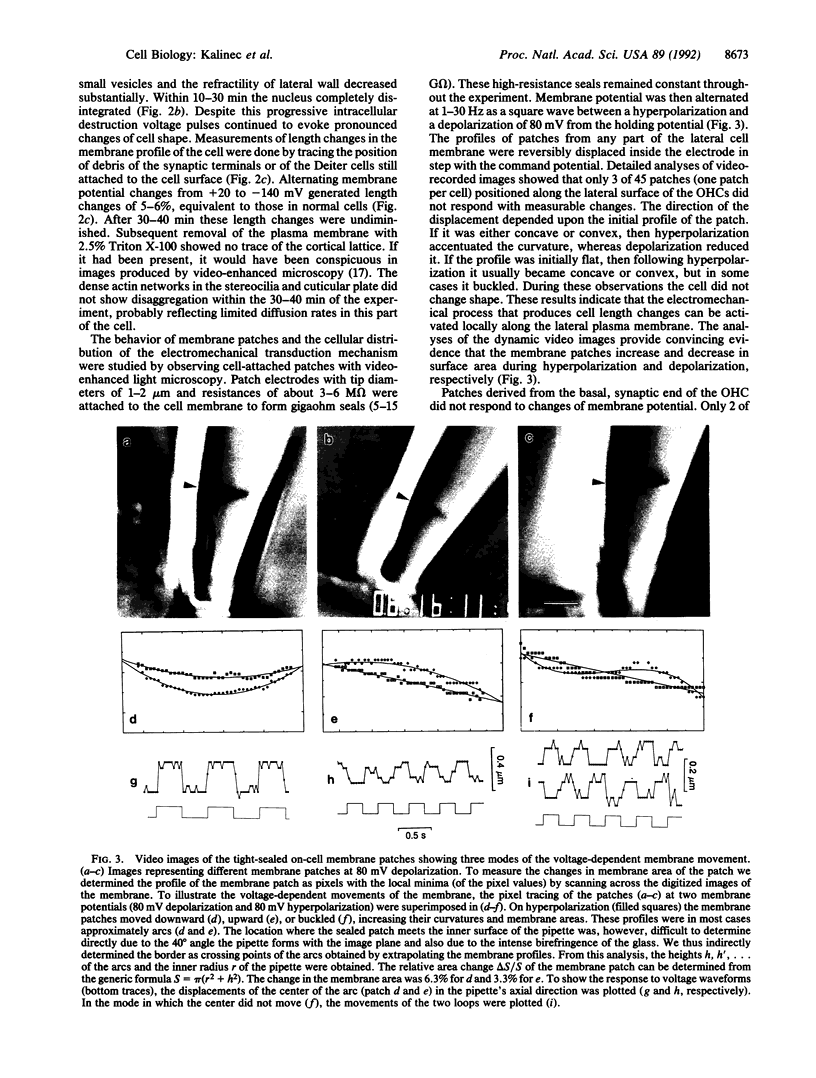
Abstract
Auditory outer hair cells can elongate and shorten at acoustic frequencies in response to changes of plasma membrane potential. We show that this fast bidirectional contractile activity consists of an electromechanical transduction process that occurs at the lateral plasma membrane and can be activated and analyzed independently in small membrane patches inside a patch electrode. Bidirectional forces are generated by increases and decreases in membrane area in response to hyperpolarization and depolarization, respectively. We suggest that the force generation mechanism is driven by voltage-dependent conformational changes within a dense array of large transmembrane proteins associated with the site of electromechanical transduction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Kuraoka A., Toriya R., Shibata Y., Uemura T. Quick-freeze, deep-etch visualization of the 'cytoskeletal spring' of cochlear outer hair cells. Cell Tissue Res. 1991 Jan;263(1):91–97. doi: 10.1007/BF00318403. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister L. H., Dodson H. C., Astbury A. R., Douek E. E. The cortical lattice: a highly ordered system of subsurface filaments in guinea pig cochlear outer hair cells. Prog Brain Res. 1988;74:213–219. doi: 10.1016/s0079-6123(08)63016-2. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Stühmer W. Quantal charge redistributions accompanying the structural transitions of sodium channels. Eur Biophys J. 1989;17(2):53–59. doi: 10.1007/BF00257102. [DOI] [PubMed] [Google Scholar]
- Corwin J. T., Warchol M. E. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–333. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- Dallos P., Evans B. N., Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991 Mar 14;350(6314):155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Dieler R., Shehata-Dieler W. E., Brownell W. E. Concomitant salicylate-induced alterations of outer hair cell subsurface cisternae and electromotility. J Neurocytol. 1991 Aug;20(8):637–653. doi: 10.1007/BF01187066. [DOI] [PubMed] [Google Scholar]
- Délèze J. Cell-to-cell communication in the heart: structure-function correlations. Experientia. 1987 Oct 15;43(10):1068–1075. doi: 10.1007/BF01956041. [DOI] [PubMed] [Google Scholar]
- Evans B. N. Fatal contractions: ultrastructural and electromechanical changes in outer hair cells following transmembraneous electrical stimulation. Hear Res. 1990 May;45(3):265–282. doi: 10.1016/0378-5955(90)90126-a. [DOI] [PubMed] [Google Scholar]
- Flock A., Flock B., Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243(2):83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Furness D. N., Hackney C. M. Comparative ultrastructure of subsurface cisternae in inner and outer hair cells of the guinea pig cochlea. Eur Arch Otorhinolaryngol. 1990;247(1):12–15. doi: 10.1007/BF00240941. [DOI] [PubMed] [Google Scholar]
- Gulley R. L., Reese T. S. Regional specialization of the hair cell plasmalemma in the organ of corti. Anat Rec. 1977 Sep;189(1):109–123. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. Spectrin, actin and the structure of the cortical lattice in mammalian cochlear outer hair cells. J Cell Sci. 1990 Jun;96(Pt 2):283–291. doi: 10.1242/jcs.96.2.283. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Kalinec F., Kachar B. Structure of the cortical cytoskeleton in mammalian outer hair cells. J Cell Sci. 1992 Jul;102(Pt 3):569–580. doi: 10.1242/jcs.102.3.569. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Kachar B. Fast in vitro movement of outer hair cells in an external electric field: effect of digitonin, a membrane permeabilizing agent. Hear Res. 1989 Jul;40(3):247–254. doi: 10.1016/0378-5955(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Jen D. H., Steele C. R. Electrokinetic model of cochlear hair cell motility. J Acoust Soc Am. 1987 Nov;82(5):1667–1678. doi: 10.1121/1.395158. [DOI] [PubMed] [Google Scholar]
- Kachar B., Bridgman P. C., Reese T. S. Dynamic shape changes of cytoplasmic organelles translocating along microtubules. J Cell Biol. 1987 Sep;105(3):1267–1271. doi: 10.1083/jcb.105.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Brownell W. E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986 Jul 24;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Liman E. R., Hess P., Weaver F., Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991 Oct 24;353(6346):752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- Ruggero M. A., Rich N. C. Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991 Apr;11(4):1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of guinea-pig organ of Corti: subsurface cisternae and lamellar bodies in the outer hair cells. Cell Tissue Res. 1983;229(3):467–481. doi: 10.1007/BF00207692. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J. P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988 Sep 15;35(2-3):143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991 Oct;11(10):3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Identification of proteolytically resistant domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5673–5677. doi: 10.1073/pnas.77.10.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]