Abstract
The mortality rate associated with oral cancer is estimated at approximately 12,300 deaths per year, and the survival rate is only 40% to 50% for diagnosed patients and is closely related to the duration of time between disease perception and its diagnosis and treatment. Socioeconomic risk factors are determinants of the incidence and mortality related to oral cancer. We conducted a retrospective, cross-sectional study of 573 records of patients with oral cancer at Haroldo Juaçaba Hospital – Cancer Institute of Ceará from 2000 to 2009 to evaluate the influence of socioeconomic factors on survival and epidemiological behavior of this neoplasia in a Brazilian population. In this study, patients with oral cancer were males greater than 60 years of age, presented squamous cell carcinoma in the floor of mouth and were characterized by low education levels. A total of 573 lesions were found in oral cavities. Cox proportional hazards regression model showed that the histological type, tumor stage, and low degree of education significantly influenced survival. A lower patient survival rate was correlated with a more advanced stage of disease and a worse prognosis. Squamous cell carcinoma is associated with a higher mortality when compared with other histological types of malign neoplasia.
INTRODUCTION
Cancer is a disease of great concern worldwide due to its high incidence and mortality. The World Health Organization (WHO)1 has estimated that in 2030, 27 million new cases and 75 million people will be with cancer. The greatest effect of this increased focus on oral cancer in underdeveloped countries is that various neoplasms predominate.2 Oral cancer is 1 of the 10 most frequently occurring cancers globally, and its incidence displays an increasing trend worldwide.2–4 In Brazil, there is an estimated risk of 11.54 and 3.92 new cases in every 100 thousand men and women, respectively.1
Studies show that men over 50 years of age are more affected by oral cancer. The primary tumor site is the tongue, and the most common histological type is squamous cell carcinoma (SCC).5 With an estimated 12,300 deaths per year, oral cancer has a 5-year survival rate of approximately 40% to 50%.6 Despite being heavily influenced by the tumor stage, the survival of oral cancer patients is influenced by many factors of a social nature such as the time between disease perception, its diagnosis and treatment, access to health-care services, educational level and occupation of the patient, behavioral/cultural factors, exposure to risk factors such as chewing tobacco and some specific topographical distributions.7,4,8–12
Characterization of survival of patients with oral cancer and the contributing factors is based on data on the incidence of the disease and mortality. This data should be constantly updated to provide managers and health planners, with new information regarding the disease, frequency, and distribution.10 Wong et al10 (Thailand) showed that socio-demographic factors such as marital status and religious belief may influence survival and prognosis of patients with oral cancer independent of other clinical factors. Although several studies evaluated the influence of these social factors on survival of oral cancer,7,4,8–12 the studies do not make an assessment investigating the relationship between their variables.
Thus, the objective of this study is to determine whether clinical features, histopathology, and socio-economic status influences the survival of patients with oral cancer treated at a tertiary institution in Brazil.
MATERIALS AND METHODS
Study Design
In this observational, retrospective, cross-sectional, quantitative study, we reviewed the medical records of 573 patients with oral malignant neoplasms treated at the Haroldo Juaçaba Hospital (Cancer Institute of Ceará).
Description of Variables
During the period between 2000 and 2009, a convenience sample was obtained that included patients with oral cancer.
Socio-demographic data such as age, sex, education, race/color, type of admission to hospital (private health system or public health system) and clinical features and histopathology data, such as the tumor histological type, location in the oral cavity, tumor size lymph node metastasis, stage of the tumor, and survival following treatment completion were assessed. The level of education was obtained through direct interviews with the patients noted in medical records held at the time of entry in the hospital.
The location of the primary tumor was classified according to WHO13 criteria (lip, gingiva, anterior third of the tongue, hard palate, floor of the mouth and other parts, and parts not identified in the mouth) and the histological types were grouped into 2 groups: SCC and non-SCC (see results).
The stage of the tumor was defined according to the proposition of WHO:13 T is related to the tumor size, N indicates the lymph node involvement, and M is distant metastases. Regarding stages, it was considered stage I, stage II, stage III, or stage IV encompassing the IVA, IVB, and IVC stages.
Survival (months) was determined based on the difference between the date of the start of treatment and the date of death.
Statistical Analysis
Data were analyzed using statistical software SPSS (Statistical Package for Social Sciences) for Windows version 17.0, with a confidence level of 95% (P < 0.05).
Categorical data were analyzed by a Chi-square test with 95% confidence intervals. Survival analysis was investigated by Chi-square test, Kaplan–Meier method, and Cox regression model of survival.
Ethical Correlations
This study conformed to ethical principles and was accepted by the ethics committee under protocol 011/2011.
RESULTS
Exploratory Analysis
Within the period evaluated, 573 malignant lesions were determined. Of these, 63.9% occurred in males and 207 (36.1%) in females. The male:female ratio (1.8:1.0) was statistically significant (P < 0.001). The age distribution revealed a significantly greater proportion of patients in the 61 to 70-year-old age group (n = 144, 25.1%); (P < 0.001), and there were no cases of malignant tumor of the mouth in patients younger than 21 years. The 2 main sites of involvement were the mouth floor (n = 153, 26.7%) and tongue (n = 145, 25.3%) (P < 0.001) (Table 1).
TABLE 1.
Clinical, Demographic, Socioeconomic, and Therapeutic Profile of Patients With Oral Cancer Treated at the Haroldo Juaçaba Hospital (Cancer Institute of Ceará) (2000–2009)
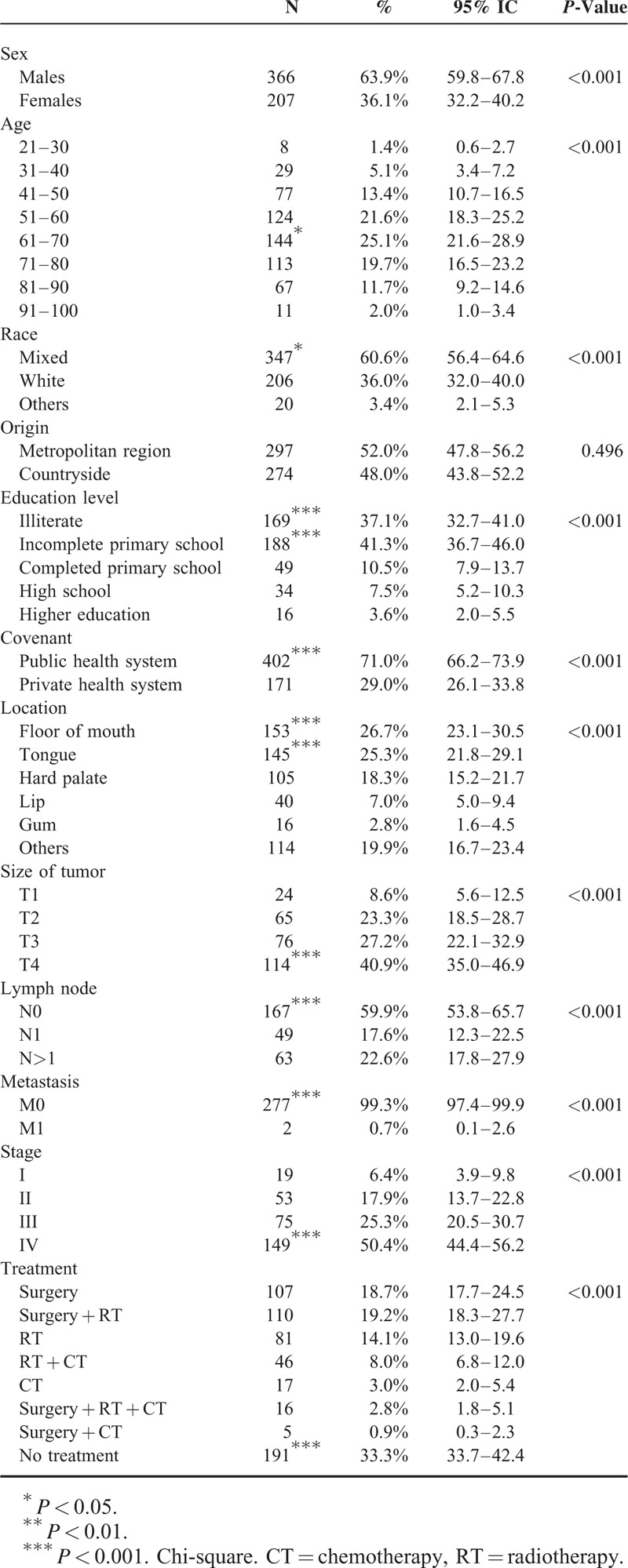
The racial distribution showed a higher prevalence of lesions in patients of mixed race (n = 347, 60.6%) (P < 0.001). There was no difference regarding the number of patients in the metropolitan area compared with countryside (P = 0.496) (Table 1).
Regarding education level, 169 (n = 37.1%) patients said they were illiterate and 188 (n = 41.3%) had incomplete primary school. These values were significantly higher compared with groups categorized by level of education (P < 0.001). The public health system (n = 402, 71.0%) was the main agreement by which patients were admitted to the hospital (P < 0.001) (Table 1).
The majority of the tumors were diagnosed in floor of mouth (n = 153, 26.7%) and tongue (n = 145, 25.3%) (P < 0.001), as size T4 (n = 114, 40.9%) (P < 0.001), with no lymph nodes (N0) affected (n = 167, 59.9%) (P < 0.001) and no metastasis (M0) identified (n = 277, 99.3%) (P < 0.001). The most prevalent clinical stage was stage IV with 149 (50.4%) cases (P < 0.001). Noncompletion of treatment (n = 191, 33.3%) was the approach most significantly adopted (P < 0.001) (Table 1).
SCC (n = 524, 91.4%) was the main histological type (P < 0.001) in relation to non-SCC (n = 49, 8.6%). This group comprised adenoid cystic carcinoma (n = 18, 3.2%), adenocarcinomas (n = 6, 1.1%), cystadenocarcinomas (n = 4, 0.7%), undifferentiated neoplastic malignancy (n = 5, 0.8%), malignant melanoma and clear cell adenocarcinoma (n = 3, 0.5%), malignant large B-cell lymphoma, mucinous adenocarcinoma (n = 2, 0.3%), and acinic cell carcinoma, epithelial-myoepithelial carcinoma, carcinosarcoma, myoepithelioma, malignant non-Hodgkin lymphoma, and T-cell lymphoma (n = 1, 0.2%).
Ten-Year Survival
The 10-year survival rate was 56.5%. We observed that variables non-SCC (n = 27, 84.4%, P = 0.001), patients in the 3rd decade of life (n = 5, 100.0%, P = 0.037), multiracial (P = 0.018), surgery alone (n = 53, 67.9%, P = 0.002), or surgery combined with radiotherapy (RT, n = 48, 70.6%, P = 0.001) showed average survival rate higher compared with other variables (Chi-square test). Clinically, patients with T1 tumor (n = 20, 90.9%, P = 0.001), stage I (n = 16, 94.1%, P < 0.001) showed significantly higher survival percentages. The lymph node involvement only influenced the survival rate (P = 0.049), and the presence of distant metastases was not related to the survival percentage (P = 0.294) (Table 2, Fig. 1).
TABLE 2.
Ten-year Survival of Patients Diagnosed With Oral Cancer in Haroldo Juaçaba Hospital (Cancer Institute of Ceará) (2000–2009)
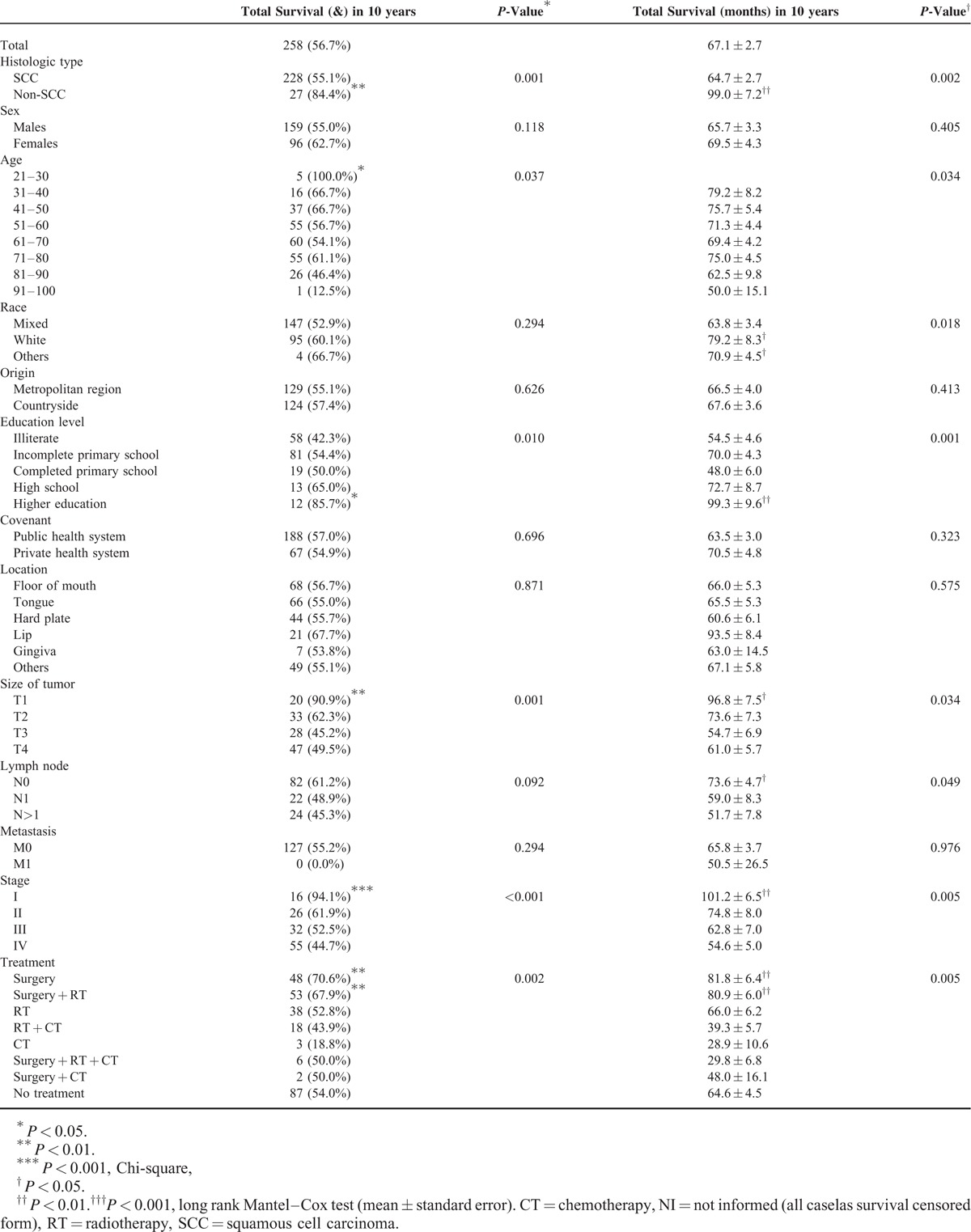
FIGURE 1.
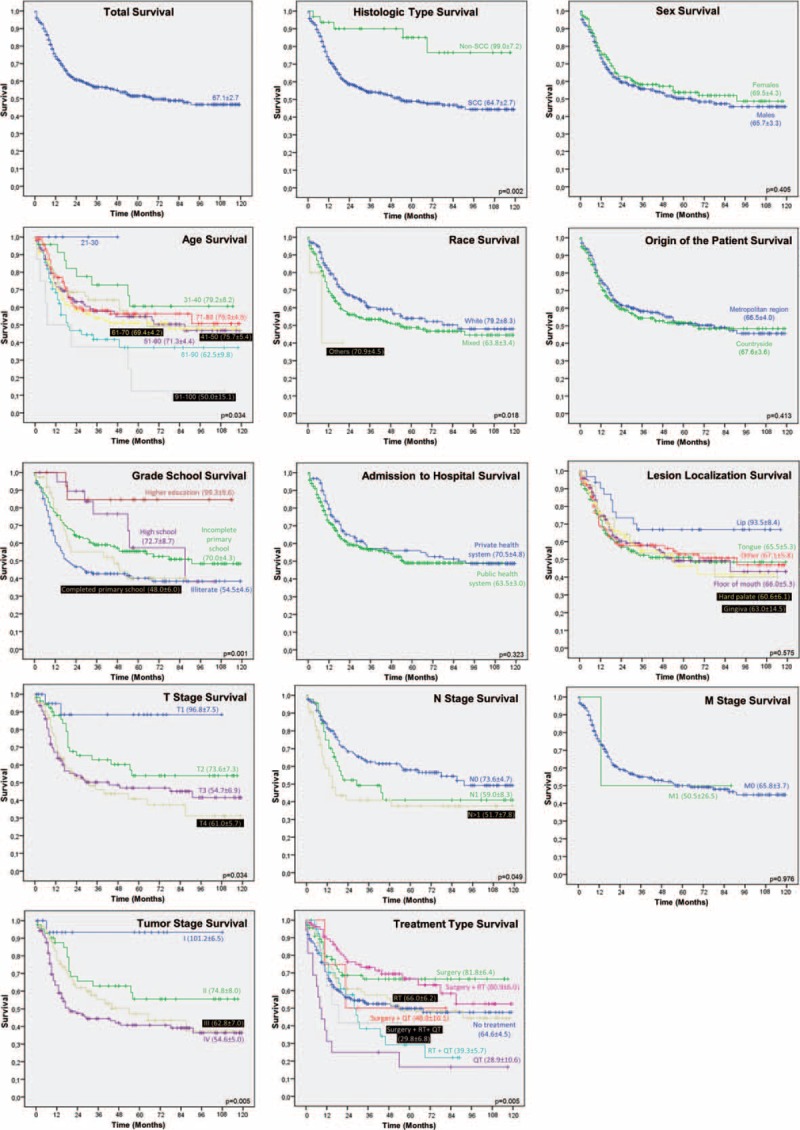
Ten-year survival of patients diagnosed with oral cancer in Haroldo Juaçaba Hospital (2000–2009) (long-rank Mantel–Cox).
The long-rank Mantel–Cox test showed that the variables resulting in higher survival rates were non-SCC patients (99.0 ± 7.2) (P = 0.002) in the 3rd decade of life (P = 0.034) and multiracial patients (79.2 ± 8.3) (P = 0.018). Other factors producing higher survival rates included only surgical treatment (81.8 ± 6.4) (P = 0.005), surgery combined with RT (80.9 ± 6.0) (P = 0.005), T1 tumor gradation (96.8 ± 7.5) (P = 0.034), and stage I classification (101.2 ± 6.5) (P = 0.005).
In a Cox proportional hazards regression model, categorization factors that significantly influenced survival were histological type (P = 0.017), education level of the patient (P = 0.022), and tumor stage (P < 0.001). Sex (P = 0.336), age (P = 0.106), race (P = 0.126), origin (P = 0.379), covenant (P = 0.157), location (P = 0.587), size of tumor (P = 0.211), lymph node metastasis (P = 0.080), distant metastasis (P = 0.217), and treatment (P = 0.854) did not influence survival.
Evaluation of Histological Type
The number of SCCs in men (n = 349, 66.6%) was 3.7 (IC95% = 2.0–6.9) times greater than the number of SSC in women (n = 175, 33.4%) (P < 0.001). The non-SCC were diagnosed specifically at the age groups of 21 to 30 (n = 6, 12.2%) and 31 to 40 (n = 10, 20.4%) (P < 0.001) (Table 3).
TABLE 3.
Influence of Histological Type of Oral Cancer in the Clinical, Demographic, Socioeconomic, and Therapeutic Profile of Patients Treated at the Haroldo Juaçaba Hospital (Cancer Institute of Ceará)
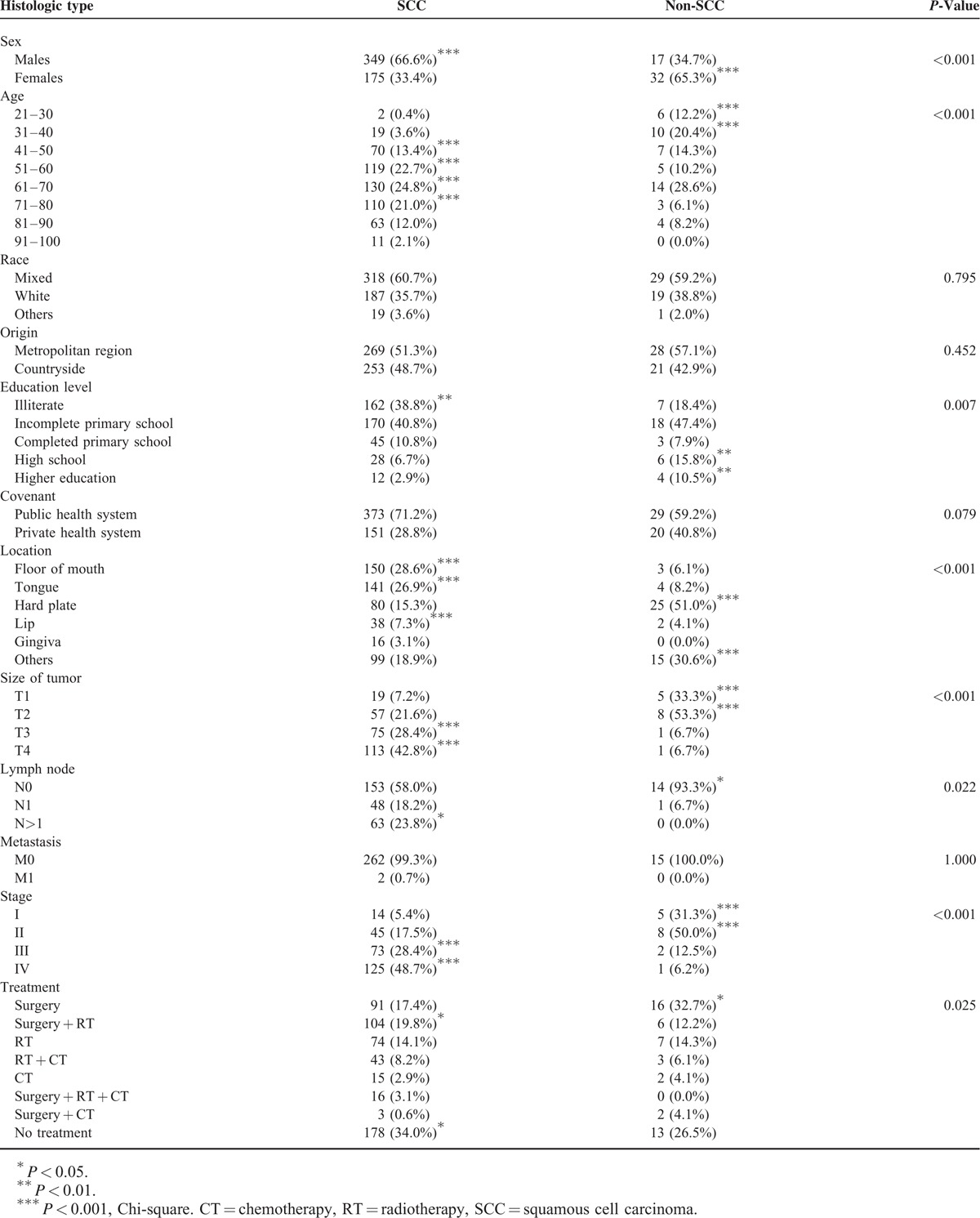
The race (P = 0.795), the origin (P = 0.452), and the agreement of admission to hospital did not significantly influence the distribution of these 2 histological types. However, in relation to education, SCC (n = 162, 38.8%) was diagnosed significantly more than non-SCC (n = 7, 18.4%) in illiterate individuals (P = 0.007) (Table 3).
Clinically, the SCC showed a higher prevalence in the floor of the mouth (n = 150, 28.6%), tongue (n = 141, 26.9%), and lip (n = 38, 7.3%), while the non-SCC showed high prevalence in the hard palate (n = 25, 51.0%) and other sites (n = 15, 30.6%) (P < 0.001) (Table 3).
The SSC was diagnosed significantly more often as size T3 (n = 75, 28.4%) and T4 (n = 113, 42.8%) (P < 0.001). The involvement of more than 1 lymph node showed a greater association with SSC (n = 63, 23.8%) than non-SCC (n = 0, 0.0%) (P = 0.022), but the presence of metastases was not associated with the histological type (P = 1.000). The non-SCC had a higher prevalence of diagnosis in stage I (n = 5, 31.3%) and II (n = 8, 50.0%). In turn, SCC tumors were diagnosed more commonly in stage III (n = 73, 28.4%) and IV (n = 125, 48.7%) (P < 0.001) (Table 3).
Evaluation of Education
Regarding schooling, female patients showed a high prevalence among illiterate individuals (n = 82, 48.5%) and completion of only grade school (n = 20, 41.7%). The proportion of men was significantly greater among individuals with incomplete primary school (n = 136, 72.3%), high school (n = 24, 75.0%), and higher education (n = 12, 75.0%) (P = 0.001) (Table 4).
TABLE 4.
Influence of Education in the Clinical, Demographic, Socioeconomic, and Therapeutic Profile of Patients Treated at Haroldo Juaçaba Hospital (Cancer Institute of Ceará) (2000–2009)
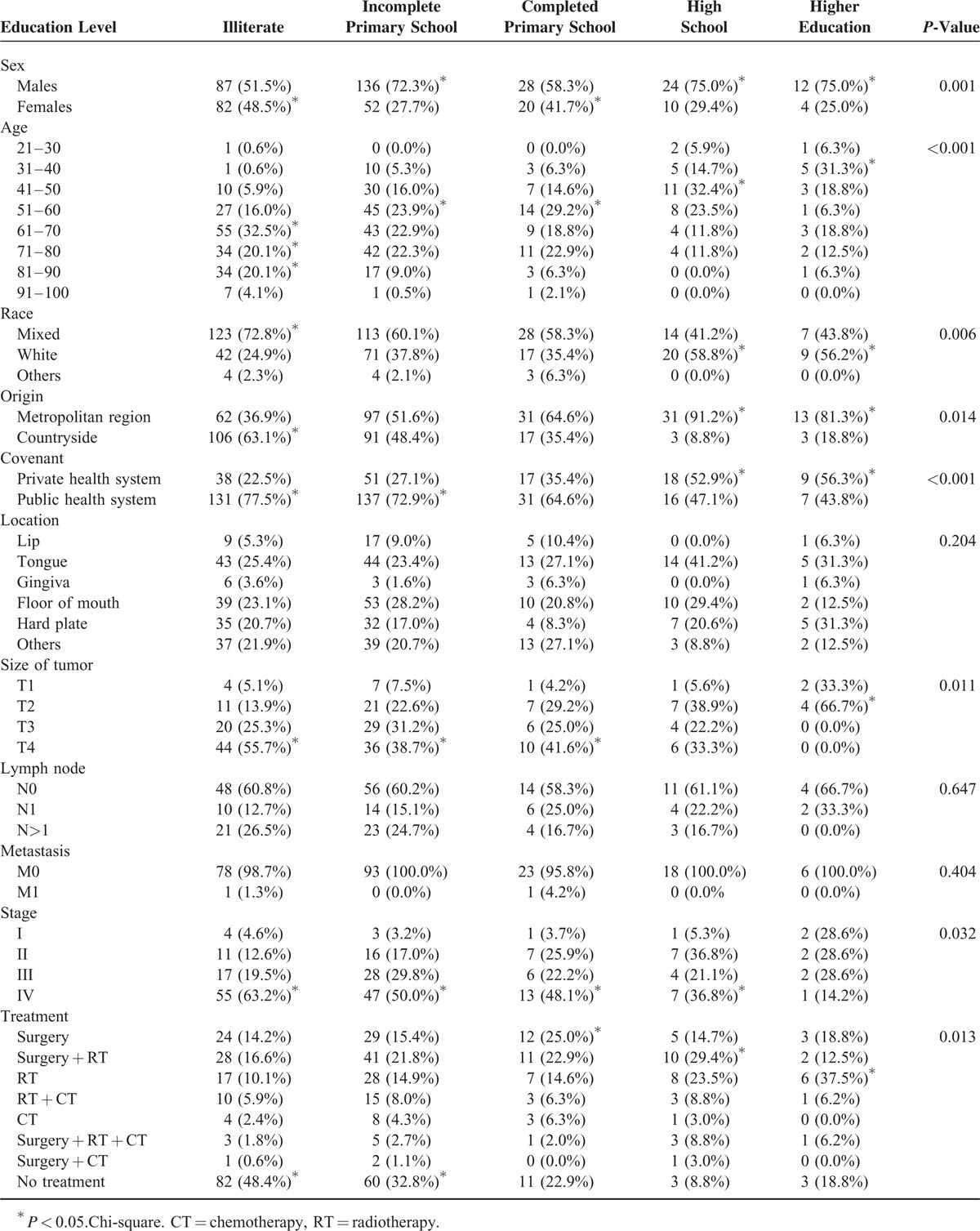
The age of the patient showed an inverse association with the degree of schooling (P < 0.001). The number of multiracial patients was significantly larger in the illiterate group (n = 123, 72.8%) (P < 0.001), and the patients from countryside were illiterate (n = 106, 63.1%) (P = 0.014) (Table 4).
There was a higher prevalence of public health system patients among the illiterate individuals (n = 131, 77.5%) and the group with an incomplete primary school (n = 137, 72.9%) (P < 0.001) (Table 4).
The tumor size was inversely associated with the level of education. Illiterate patients (n = 44, 55.7%), with an incomplete primary school level (n = 36, 38.7%) and completed primary school (n = 10, 41.6%) showed an increased proportion of T4 tumors, while patients with a higher education level (n = 0, 0.0%), showed a high frequency of tumors with sizes of T2 (n = 4, 66.7%) and T1 (n = 2, 33.3%) (P = 0.011) (Table 4).
Tumor stage of IV was significantly more prevalent in all educational levels except the group of higher education (P = 0.032) (Table 4).
The main therapeutic decision in illiterate patients (n = 82, 48.4%) and incomplete primary school (n = 60, 32.8%) was therapeutic abstention, whereas patients with completed primary school were mainly treated with surgery (n = 12, 25.0%). High school patients were treated significantly more with surgery and RT (n = 10, 29.4%), and higher education patients were treated only with RT (n = 4, 37.5%) (P = 0.013) (Table 4).
Assessment of Stage
Female patients were diagnosed mainly as stage I (n = 13, 68.4%), while men were diagnosed significantly more often as stage II (n = 39, 73.6%), III (n = 45, 60.0%), and IV (n = 99, 66.4%) (P = 0.009). There was a direct association between age and stage of the tumor (P = 0.002) (Table 5).
TABLE 5.
Influence of the Stage of Oral Cancer in Patients Diagnosed at Haroldo Juaçaba Hospital (Cancer Institute of Ceará) (2000–2009)
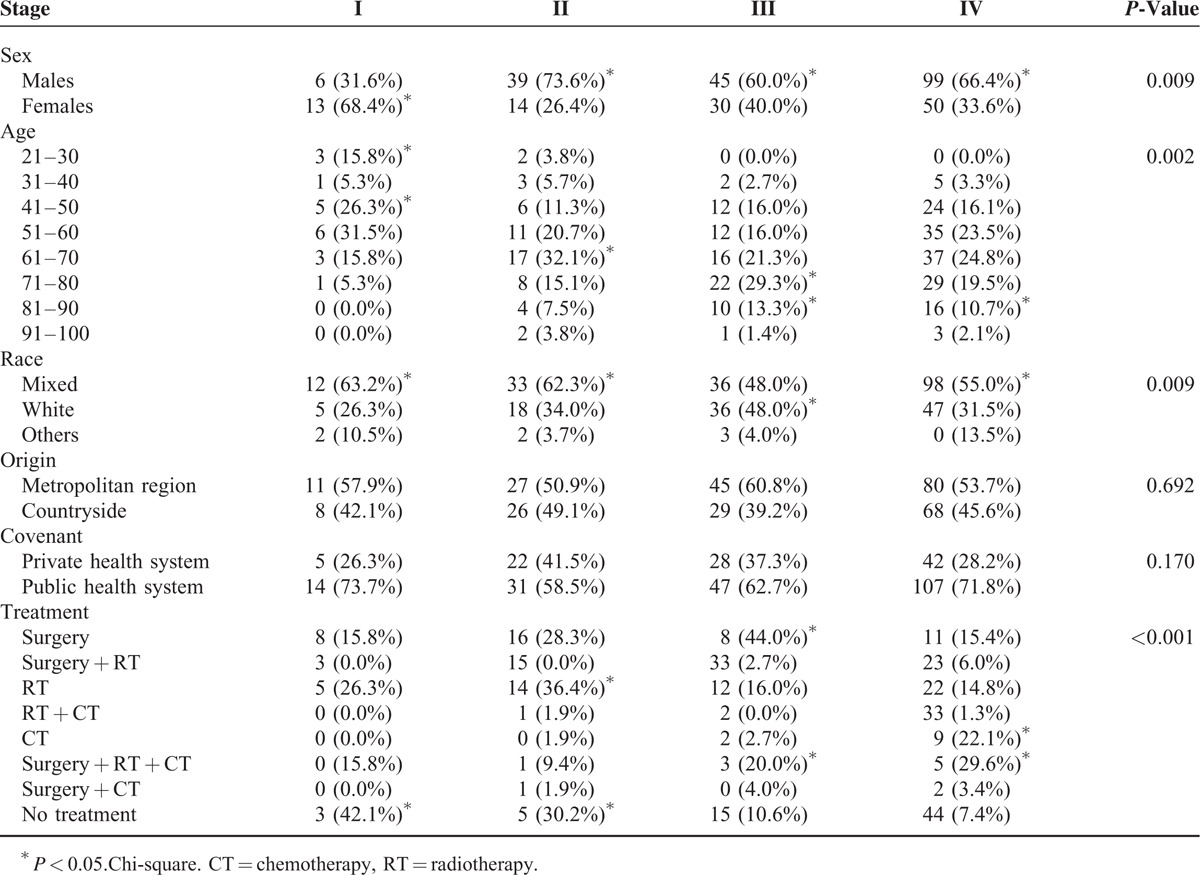
The patient's origin (P = 0.692) and the method of admission to the hospital (P = 0.170) did not influence the tumor staging. However, the therapy most frequently adopted was abstention from treatment for stage I (n = 3, 42.1%), RT alone (n = 14, 36.4%) and abstention from therapy (n = 5, 30.2%) for stage II, surgery alone (n = 8, 44.0%) for stage III, and chemotherapy alone for stage IV tumors (n = 9, 22.1%) (P < 0.001) (Table 5).
DISCUSSION
Oral cancer is a disease strongly influenced by social factors.14,15 In this sample, the majority of patients were male. This is in accordance with the epidemiological profile of our population where most men are smokers.16,17
In the present study, oral cancer was more common in individuals with 61 to 70 years of age. Ayaz et al18 in Pakistan noted an increasing involvement of oral malignant lesions in patients less than 40 years of age, diverging from our data. Despite this contradiction, Wunsch-Filho16 reported that cigarette smoking is a principle risk factor for oral cancer and combined with an increase in age magnifies the development of these lesions.
The primary locations of the tumors with the highest prevalence were the floor of the mouth and tongue. Chen et al11 and Bhurgri et al19 (Pakistan and Taiwan, respectively) discovered that the floor of the mouth is the most common site of cancer involvement and is associated with the habit of chewing tobacco and betel. Gellrich et al20 (Europe) also found that the floor of the mouth was a prevalent site of cancer development, but this site was not related to the habit of chewing. Other epidemiological studies showed that the tongue is the most affected site.21–23
In the present study, the racial distribution of the sample showed a higher prevalence of lesions in patients of mixed race. This increased incidence was related to the large number of people in our country who are considered multiracial.25 Gervásio et al24 stated that significant mixing of the population has made it difficult to perform race analyses, as multiracial people are considered the majority.
Regarding education, the majority of the patients had an incomplete primary school or was illiterate. Oral cancer is related to a low education level, which may be due in part to a reduced access to information about the disease in general, including the diagnosis and treatment.8,16,26 Due to the economic status of these patients, many were admitted to the hospital through the public health system.22
Studies in developing countries have found that oral cancer is diagnosed at advanced stages, unlike in developed countries in which the most prevalent stages are I and II.12,27–30,31,32 This situation revealed a strong influence of socioeconomic factors on the delayed diagnosis.
The SCC was the main histological type detected in the present study, weighing in strongly in survival rate of 10 years, observed in 56.5%.19,22,27,33 Few studies have investigated long periods of survival and the majority of these have been restricted to 5 years. During this evaluation period, survival varies from 30% to 80% and changes according to the study site, socioeconomic, and cultural factors.13,26,34
The patient age is a major factor in the survival of oral cancer. In this study, and in another by Razak et al,12 an inverse association between age and stage of disease was observed. Individuals older than 60 years were associated with stages II, III, and IV, and a worse survival compared to younger patients.
Consistent with a study by Razak et al12 in which size T1 tumors were associated with a significantly better survival, Iype et al27 showed that after 5 years, no groups of patients diagnosed with T4 tumors in India remained alive.
In the present study, only patients with stage I disease had good survival rates; women comprised a high percentage of patients in this group because they sought earlier and more health services compared with men, thus affecting the survival rate.35 Thus, men tended to survive for shorter periods of time, as a result of the delayed diagnosis.13
In the current study, illiterate patients showed an increased frequency of therapeutic abstention, revealing a close relationship between a low education level, late diagnosis, and poor prognosis. Despite this data, Wong et al10 reported that there was no correlation between patient survival and their schooling.
Consequently, associated surgery or omitting RT treatments normally used for tumors diagnosed early were associated with a survival rate significantly higher than the other treatment options. Al-Rawi and Talabani36 (Iraq) stated that earlier stages lead to less invasive treatments, which are associated with a better prognosis.
Patients with SCC had a worse survival rate compared with those with non-SCC. The SCC patients consisted mostly of men with an advanced age, whereas females with younger age ranges had a higher prevalence of non-SCC. Additionally, patients with SCC were more frequently in illiterate and incomplete primary school groups and in association with disease stages III and IV. These data were in agreement with previous studies showing that cancers such as non-SCC are more prevalent in women. In men SCC is more common and often diagnosed much later, leading to a worse prognosis.24,36,37
Thus, the SCC malignancy accounts for more than 90% of the oral cancers analyzed in the present study. Previous studies have demonstrated that the illness process is treated differently in the population with a lower level of education. This has led some authors to consider oral cancer, particularly when speaking of SCC, as a disease that is characteristic of people with a low economic and educational level.14 Oji and Chukwuneke29 (Nigeria) concluded that one of the factors closely related to an advanced stage of oral cancer is the lack of education of the population. In Brazil, patients with a lower income and education level had a higher mortality rate due to oral cancer.10
Although studies14,15 showed that oral cancer survival is closely related to social factors, the present study demonstrated that the educational level of the patients influenced the survival significantly. The majority of the studies evaluated 5-year survival, but our results exhibit 10 years of follow-up. In addition, we observed a significantly lower survival rate in patients with SCC compared to patients with malignancies of other histological types.
The incidence of oral cancer in a population of Northeast Brazilians contained a large proportion of SCC and that the survival of the patients was inversely related to their level of education, which could lead to a diagnosis at advanced stages of disease, abstention from treatment, and poor prognosis.
In the present study, the influence of socioeconomic factors on the prognosis of oral cancer suggests that the association between poverty and mortality due to oral cancer requires intervention by public health policies in populations with low social status and income levels to improve life expectancy and quality of life. It is important to emphasize that the Haroldo Juaçaba Hospital serves as a reference treatment center for lower income individuals fighting all types of cancer in the state of Ceará (northeastern Brazil). Large multicenter studies are needed to know the closest oral cancer profile of this population.
Acknowledgments
The authors would like to thank FUNCAP (Cearense Fund Research Support) for financial support of the study (Scholarship).
Footnotes
Abbreviations: RT = radiotherapy, SCC = squamous cell carcinoma, TNM = tumor size lymph node metastasis, WHO = World Health Organization.
This study is financially supported by FUNCAP (Cearense Fund Research Support) Scholarship.
The authors have no conflicts of interests to disclose.
REFERENCES
- 1.Brasil: Ministério da Saúde. Estimativa 2014, Incidência de câncer no Brasil. 1st ed.Rio de Janeiro: Instituto Nacional do Câncer; 2014. [Google Scholar]
- 2.De Sousa DLB, Pérez MMB, Cuardo MP. Predicted incidence of oral cavity, oropharyngeal, laryngeal, and hypopharyngeal cancer in Spain and/implications for cancer control. Cancer Epidemiol 2001; 35:510–514. [DOI] [PubMed] [Google Scholar]
- 3.Ganesh R, John J, Saravanan S. Socio demographic profile of oral cancer patients residing in Tamil Nadu – a hospital based study. India J Cancer 2013; 50:9–13. [DOI] [PubMed] [Google Scholar]
- 4.McDonald JT, Johnson-Obaseki S, Hwang E, et al. The relationship between survival and socio-economic status for head and neck cancer in Canada. J Otolaryngol Head Neck Surg 2014; 43:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez M, Carrard VC, Haas AN, et al. A 10-year study of specimens submitted to oral pathology laboratory analysis: lesion occurrence and demographic features. Bras Oral Res 2012; 26:235–241. [DOI] [PubMed] [Google Scholar]
- 6.Alvarenga LM, Ruiz MT, Pavarino-Bertelli E, et al. Epidemiologic evaluation of head and neck patients in a university hospital of Northwestern São Paulo State. Rev Bras Otorrinolaringol 2008; 74:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos LCO, Batista OM, Cangussu MT. Characterization of oral cancer diagnostic delay in the state of Alagoas. Braz J Otorhinolaryngol 2010; 76:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway DI, Petticrew M, Marlborough H, et al. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int J Cancer 2008; 122:2811–2819. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira MA, Gomes MN, Michels FA, et al. Social inequality in morbidity and mortality from oral and oropharyngeal cancer in the city of São Paulo, Brazil: 1997–2008. Cad Saúde Pública 2012; 28:1663–1673. [DOI] [PubMed] [Google Scholar]
- 10.Wong YK, Tsai WC, Lin JC, et al. Socio-demographic factors in the prognosis of oral cancer patients. Oral Oncol 2006; 46:893–906. [DOI] [PubMed] [Google Scholar]
- 11.Chen YK, Huang HC, Lin LM, et al. Primary oral SCC: an analysis of 703 cases in southern Taiwan. Oral Oncol 1999; 35:173–179. [DOI] [PubMed] [Google Scholar]
- 12.Razak AA1, Saddki N, Naing NN, et al. Oral cancer survival among Malay Patients in Hospital Universiti Sains Malaysia, Kelantan. Asian Pacific J Cancer Prev 2010; 11:187–191. [PubMed] [Google Scholar]
- 13.World Health Organization. CID-0: Classificação Internacional de Doenças para Oncologia. 3th edSão Paulo: Edusp; 2005. [Google Scholar]
- 14.Madani AH, Dikshit M, Bhaduri D, et al. Relationship between Selected Socio-demographic factors an cancer of oral cavity – a case control study. Cancer Inform 2010; 9:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal AK, Sethi A, Sareen D, et al. Treatment delay in oral and oropharyngeal cancer in our population: the role of socio-economic factors and health-seeking behaviour. Indian J Otolaryngol Head Neck Surg 2011; 63:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wunsch-Filho V. The epidemiology of oral and pharynx cancer in Brazil. Oral Oncol 2002; 38:737–746. [DOI] [PubMed] [Google Scholar]
- 17.Sales MPU, Fogueiredo MRF, Oliveira MI, et al. Ambulatório de apoio ao tabagista no Ceará: perfil dos pacientes e fatores associados ao sucesso terapêutico. J Bras Pneumol 2006; 32:410–417.17268744 [Google Scholar]
- 18.Ayaz B, Saleem K, Azim W, et al. A clinico-pathological study of oral cancers. Biomedica 2011; 27:29–32. [Google Scholar]
- 19.Bhurgri Y, Bhurgri A, Hussainy AS, et al. Cancer of the oral cavity and pharynx in Karachi – identification of potential risk factors. Asian Pacific J Cancer Prev 2003; 4:125–130. [PubMed] [Google Scholar]
- 20.Gellrich NC, Suarez-Cunqueiro MM, Bremerich A, et al. Characteristics of oral cancer in a central European population: defining the dentist's role. JADA 2003; 134:307–314. [DOI] [PubMed] [Google Scholar]
- 21.Ariyoshi Y, Shimahara M, Yamamoto KOE, et al. Epidemiological study of malignant tumors in the oral and maxillofacial region: survey of member institutions of the Japanese Society of Oral and Maxillofacial Surgeons, 2002. Int J Clin Oncol 2007; 13:220–228. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro AC, Silva AR, Simonato LE, et al. Clinical and histopathological analysis of oral SCC in young people a descriptive study in Brazilians. Br J Oral Maxillofac Surg 2009; 47:95–98. [DOI] [PubMed] [Google Scholar]
- 23.Udeabor SE, Rana M, Wegener G, et al. SCC of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol 2012; 4:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gervásio OL, Dutra RA, Tartaglia SM, et al. Oral SCC: a retrospective study of 740 cases in a Brazilian population. Braz Dent J 2001; 12:57–61. [PubMed] [Google Scholar]
- 25.Instituto Brasileiro de Geografia e Estatística (IBGE). Censo 2010. Disponível em: http://censo2010.ibge.gov.br/ Accessed Nov 5, 2014. [Google Scholar]
- 26.Antunes JL, Biazevic MG, de Araujo ME, et al. Trends and spatial distribution of oral cancer mortality in São Paulo, Brazil 1980–1998. Oral Oncol 2001; 37:345–350. [DOI] [PubMed] [Google Scholar]
- 27.Iype EM, Pandey M, Mathew A, et al. Oral cancer among patients under the age of 35 years. J Postgrad Med 2001; 47:171–176. [PubMed] [Google Scholar]
- 28.Iamaroon A, Pattanaporn K, Pongsiriwet S, et al. Analysis of 587 cases of oral SCC in northern Thailand with a focus on young people. Int J Maxillofac Surg 2004; 33:84–88. [DOI] [PubMed] [Google Scholar]
- 29.Oji C, Chukwuneke FN. Oral cancer in Enugu, Nigeria, 1998–2003. Br J Oral Maxillofac Surg 2007; 45:298–301. [DOI] [PubMed] [Google Scholar]
- 30.Rivera H, Nikitakis NG, Correnti M, et al. Oral and oropharyngeal cancer in a venezuelan population. Acta Odontol Latinoam 2008; 21:175–180. [PubMed] [Google Scholar]
- 31.Kovács AF, Megahed W, Scholz M, et al. Überlebensverbesserung eines unizentrischen Gesamtkollektivs aus 20 Jahren. Mund Kiefer Gesichtschir 2007; 11:267–283. [DOI] [PubMed] [Google Scholar]
- 32.Bascones-Martínez A, Rodríguez-Gutiérrez C, Rodríguez-Gómez E, et al. Epidemiological study of oral cancer patients in Alava province, Spain. Exp Ther Med 2011; 2:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadbir AA, Mehrabani D, Heydari ST. Epidemiology of SCC of the oral cavity in Iran. J Craniofac Surg 2008; 19:1699–1702. [DOI] [PubMed] [Google Scholar]
- 34.Chandu A, Adams G, Smith ACH. Factors affecting survival in patients with oral cancer: an Australian perspective. Int J Oral Maxillofac Surg 2005; 34:514–520. [DOI] [PubMed] [Google Scholar]
- 35.Gomes R, Nascimento EF, Araujo FC. Why do men use health services less than women? Explanations by men with low versus higher education. Cad Saúde Pública 2007; 23:565–574. [DOI] [PubMed] [Google Scholar]
- 36.Al-Rawi NH, Talabani N. SCC of the oral cavity: a case series analysis of clinical presentation and histological grading of 1,425 cases from Iraq. Clin Oral Invest 2008; 12:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadbir AAA, Mehrabani D, Heydani ST. Primary malignant tumors of orofacial origin in Iran. Craniofac Surg 2008; 19:1538–1541. [DOI] [PubMed] [Google Scholar]


