Supplemental Digital Content is available in the text
Abstract
Human immunodeficiency virus (HIV)-associated tuberculosis is a major public health threat. We evaluated the safety and immunogenicity of the candidate tuberculosis vaccine M72/AS01 in HIV-positive and HIV-negative Indian adults.
Randomized, controlled observer-blind trial (NCT01262976).
We assigned 240 adults (1:1:1) to antiretroviral therapy (ART)-stable, ART-naive, or HIV-negative cohorts. Cohorts were randomized 1:1 to receive M72/AS01 or placebo following a 0, 1-month schedule and followed for 12 months (time-point M13). HIV-specific and laboratory safety parameters, adverse events (AEs), and M72-specific T-cell-mediated and humoral responses were evaluated.
Subjects were predominantly QuantiFERON-negative (60%) and Bacille Calmette–Guérin-vaccinated (73%). Seventy ART-stable, 73 ART-naive, and 60 HIV-negative subjects completed year 1. No vaccine-related serious AEs or ART-regimen adjustments, or clinically relevant effects on laboratory parameters, HIV-1 viral loads or CD4 counts were recorded. Two ART-naive vaccinees died of vaccine-unrelated diseases. M72/AS01 induced polyfunctional M72-specific CD4+ T-cell responses (median [interquartile range] at 7 days postdose 2: ART-stable, 0.9% [0.7–1.5]; ART-naive, 0.5% [0.2–1.0]; and HIV-negative, 0.6% [0.4–1.1]), persisting at M13 (0.4% [0.2–0.5], 0.09% [0.04–0.2], and 0.1% [0.09–0.2], respectively). Median responses were higher in the ART-stable cohort versus ART-naive cohort from day 30 onwards (P ≤ 0.015). Among HIV-positive subjects (irrespective of ART-status), median responses were higher in QuantiFERON-positive versus QuantiFERON-negative subjects up to day 30 (P ≤ 0.040), but comparable thereafter. Cytokine-expression profiles were comparable between cohorts after dose 2. At M13, M72-specific IgG responses were higher in ART-stable and HIV-negative vaccinees versus ART-naive vaccinees (P ≤ 0.001).
M72/AS01 was well-tolerated and immunogenic in this population of ART-stable and ART-naive HIV-positive adults and HIV-negative adults, supporting further clinical evaluation.
INTRODUCTION
Tuberculosis (TB) and acquired immunodeficiency syndrome (AIDS) are major global public health threats. One-third of the human immunodeficiency virus-1 (HIV-1)-infected population world-wide is estimated to be coinfected with Mycobacterium tuberculosis (Mtb).1 In 2013, there were 1.1 million incident cases and 0.36 million deaths of HIV-associated TB.2 India is a high-burden country for TB, with an estimated 2.1 million incident TB cases and 0.12 million incident TB cases in HIV-positive persons in 2013.2 HIV increases the risks of reactivating latent TB infection and of rapid TB progression after Mtb (re)infection,3–5 and, conversely, coinfection with Mtb leads to faster progression to AIDS.6
With early and strict implementation, antiretroviral therapy (ART) prevents TB disease, but the incidence in ART-stable populations remains higher than in HIV-negative populations.7–9 The only available TB vaccine, Bacille Calmette–Guérin (BCG), has variable efficacy against pulmonary TB10 and is contra-indicated in persons with impaired immunity. HIV-positive individuals would thus benefit from a safe and efficacious TB vaccine. Various candidate TB vaccines are in development and may be used in HIV-positive individuals.11,12 To date, the efficacy in these populations was either not identified13 or achieved by administration of a relatively high number of doses (5) over 12 months.14 M72/AS01, a candidate vaccine under development to prevent TB disease, has been tested in Mtb-naive, HIV-positive ART-stable adults in a Phase I/II study in Switzerland,15 and in HIV-negative infants, adolescents, and adults.16–20 In the study in Switzerland, M72/AS01 was well tolerated, immunogenic, and did not negatively affect the HIV-1 viral loads (VLs), CD4+ T-cell counts (CD4 counts), or ART regimens of the participants. The next step in the clinical development plan was to evaluate the vaccine in a TB-endemic setting, in order to confirm the above results,15 and to test M72/AS01 in an HIV-infected ART-naive population.
This study evaluated the safety and T-cell-mediated and humoral responses of M72/AS01 in HIV-negative and HIV-positive adults aged 18 to 59 years living in India, which were either Mtb-primed or Mtb-naive. Three cohorts were enrolled (ART-stable, ART-naive, and HIV-negative [control]), each comprising an M72/AS01 group and a placebo group. Long-term safety and immunogenicity will be evaluated in a 3-year follow-up, of which the 1st year is described here.
METHODS
Study Design and Objectives
The 1st period of this Phase II randomized, double-blind (observer-blind), and controlled trial (NCT01262976) was conducted from January 2011 to June 2013 at YRG CARE Medical Centre, VHS (“YRG CARE”) in Chennai (India), a tertiary HIV care and research center, after approval by the YRG CARE Institutional Review Board and the Drugs Controller General of India. Written or thumb-printed and witnessed informed consent was obtained from all participants before study entry. Overall, Good Clinical Practices and the principles of the Helsinki Declaration were followed, and corrective/preventive actions implemented whenever potential or actual issues regarding the study's conduct were identified or brought to the sponsor's attention.
HIV-positive participants were recruited from patients registered for follow-up at YRG CARE clinic, and eligible participants were assigned to the ART-stable or ART-naive cohorts. HIV-negative participants were recruited from the general population. In each cohort, eligible subjects were randomized 1:1 using an internet-based block randomization (SASv8.2; SAS Institute Inc.) to receive 2 doses of M72/AS01 or placebo, 1 month apart, and were followed until 1 year postdose 2 (month 13).
Since this was the 1st time that M72/AS01 was evaluated in ART-naive individuals, vaccination was staggered in the ART-naive cohort: a subset of 20 subjects received dose 1, and blinded safety data (biochemistry/hematology, VL, CD4 count, HIV clinical staging, and adverse events [AEs] collected until 21 days post-dose 1) were reviewed by an independent local safety monitor and a sponsor safety review team. If no safety issues were observed, progression to dose 2 and further vaccination of the rest of the cohort was conducted.
Study Population
Participants aged 18 to 59 years had no past or current TB disease, no evidence of pulmonary pathology (active and/or acute or chronic pulmonary disease as confirmed by chest X-ray), no history of extra-pulmonary TB or chemotherapy for TB based on medical history, and clinical laboratory values that were considered acceptable by the investigator.
At screening, participants were tested by QuantiFERON-TB (QFT) Gold assay (Cellestis Ltd., Australia). HIV-negative volunteers were tested for HIV. CD4 counts and VLs of HIV-positive participants were measured. Nadir CD4 counts prior to ART initiation, previous BCG vaccination, and/or the presence of a BCG scar were documented.
HIV-positive subjects had been under medical care for at least 6 months. Eligible ART-stable subjects were stable on ART for at least 6 months before screening, and had CD4 counts ≥250 cells/mm3 and plasma VLs <400 copies/mL at screening. Following the YRG CARE standard operating procedures, and in line with the national guidelines,21 subjects started with 1st-line ART regimens (tenofovir + lamivudineor emtricitabine + efavirenz or nevirapine; zidovudine + lamivudine + efavirenz or nevirapine; stavudine + lamivudine + efavirenz or nevirapine), while 2nd-line regimens (tenofovir + lamivudine or emtricitabine + atazanavir/r or lopinavir; zidovudine + lamivudine + atazanavir/r + lopinavir) were instituted in case of treatment failure. Eligible ART-naive subjects had never received ART after HIV diagnosis, were at screening not expected to commence ART within the subsequent year, had CD4 counts >350 cells/mm3 (following the then current WHO recommendations for ART initiation22) and VLs within the range of 5000–80,000 copies/mL. TB preventive therapy was not administered to HIV-positive (or HIV-negative) subjects, since this was not routinely used in India at the time of the study.
Key exclusion criteria were acute or chronic pulmonary, cardiovascular, hepatic or renal abnormalities (as determined by laboratory screening or physical examination), pregnancy, lactation, or any condition/illness (acute/chronic/historical) or medication potentially interfering with immunogenicity or safety evaluations. Additional exclusion criteria for the ART-stable cohort were ART-regimen changes within 12 weeks before screening, and chronic drug therapy to be continued during the study (other than ART or prophylaxis for opportunistic HIV-related infections/symptoms).
Study Vaccines
The M72 antigen is a recombinant fusion protein derived from the immunogenic Mtb proteins Mtb32A and Mtb39A. These 2 proteins, which are also present in BCG, were shown to stimulate T-cell responses in healthy purified protein derivative-positive persons in vitro.23,24 M72 (10 μg dose) was supplied as a lyophilized cake for reconstitution with the AS01E Adjuvant System,25,26 to a 0.5-mL dose volume. One dose of AS01E (henceforth in this article referred to as AS01) contains 25 μg MPL (3-O-desacyl-4′-monophosphoryl lipid A), 25 μg QS-21 (Quillaja saponaria Molina, fraction 21) (Licensed by GSK from Antigenics Inc, a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation), and liposome. Controls received 0.5 mL saline (0.9% NaCl). Administration was by intramuscular injection in the deltoid muscle of the arm.
Safety and Reactogenicity Evaluation
Solicited local (injection-site pain, swelling) and general (fever [temperature ≥37.5 °C], headache, fatigue, malaise, myalgia, and gastrointestinal symptoms) AEs were recorded for 7 days after each vaccination. Unsolicited AEs were recorded for 30 days after each vaccination. Serious AEs were recorded throughout the study. Biochemical/hematological parameters (complete blood count, creatinine, ALT, and AST) were recorded at screening, at vaccination, and 7 and 30 days postvaccination (days 0, 7, 30, 37, and 60). AE intensities were scored, with grade 3 (severe) AEs defined as preventing normal activity, swelling >50 mm, or fever >39.5 °C. ART-regimen adherence was monitored by the investigator throughout the trial.
CD4 counts were measured by Cytomics FC500 flow cytometer (Beckman Coulter), at screening, days 0, 30, 60 and months 7 and 13. HIV-1 RNA levels were measured at the same time-points and, for the ART-naive cohort only, also on days 7 and 37, by Abbott RealTime HIV-1 assay (Abbott Molecular; detection limit: 40 copies/mL).
Immunogenicity Evaluations
Blood samples for immunogenicity evaluations were collected at days 0, 30, and 60 and months 7 and 13, and, for cell-mediated immune (CMI) responses only, additionally on days 7 and 37. At YRG CARE, peripheral blood mononuclear cells (PBMCs) were isolated, frozen slowly to approximately −70 °C, then transferred to liquid nitrogen for storage, as previously described.18 Serum samples for humoral immunogenicity analyses were stored at −70 °C, until shipment to GSK's laboratories in Belgium for analyses. Transport conditions for the PBMC and serum samples were vapor-phase nitrogen (−196 °C) and dry ice, respectively. Upon arrival, PBMCs were stored at −196 °C and thawed in batches for analysis.
CMI Responses
M72-specific CD4+ and CD8+ T cells expressing interferon gamma (IFN-γ) and/or interleukin 2 (IL-2) and/or tumor necrosis factor alpha (TNF-α) and/or CD40L were detected by intracellular cytokine staining (ICS) upon short-term in vitro stimulation of PBMCs, using a pool of 15-mer peptides (1.25 μg/mL) overlapping by 11 amino acids (Eurogentec s.a.) and covering the M72 sequence, as described previously.15,17,18 Results are presented as background-subtracted frequencies of M72-specific CD4+ and CD8+ T cells, expressing any combination of the above immune markers, evaluated using FlowJo software (Tree Star Inc.).
Humoral Responses
M72-specific IgG antibodies were measured by enzyme-linked immunosorbent assay as described,17–19 with seropositive subjects having titers at or above the cut-off of 2.8 enzyme-linked immunosorbent assay units (EU)/mL. Seronegative subjects were assigned a value of 1.4 EU/mL. Anti-M72 seropositivity rates and geometric mean concentrations (GMCs) were calculated with 95% confidence interval (CI).
Statistical Methods
Statistical analyses were conducted using SAS v9. Safety analyses were performed on all subjects with at least 1 administration of vaccine/control documented (the Total Vaccinated Cohort). Immunogenicity was analyzed for all participants not meeting elimination criteria during the study and for whom immunogenicity data were available (the According-To-Protocol cohort; Table S1).
Percentages of doses followed by at least 1 solicited local or general AE (any or grade 3) and by vaccine-related AEs were assessed, and proportions of subjects reporting an unsolicited AE were tabulated with 95% CI. All solicited local AEs were considered to be vaccine-related. Biochemistry/hematology values outside of the predefined reference ranges were assessed for clinical significance. Vaccine-induced humoral responses were compared between cohorts by Satterthwaite test. ICS results were compared between cohorts or between QFT-positive and QFT-negative subgroups by Wilcoxon rank-sum test, and between time-points within a cohort by Wilcoxon signed-rank test. Statistical significance was set at P < 0.05.
RESULTS
Population Demographics
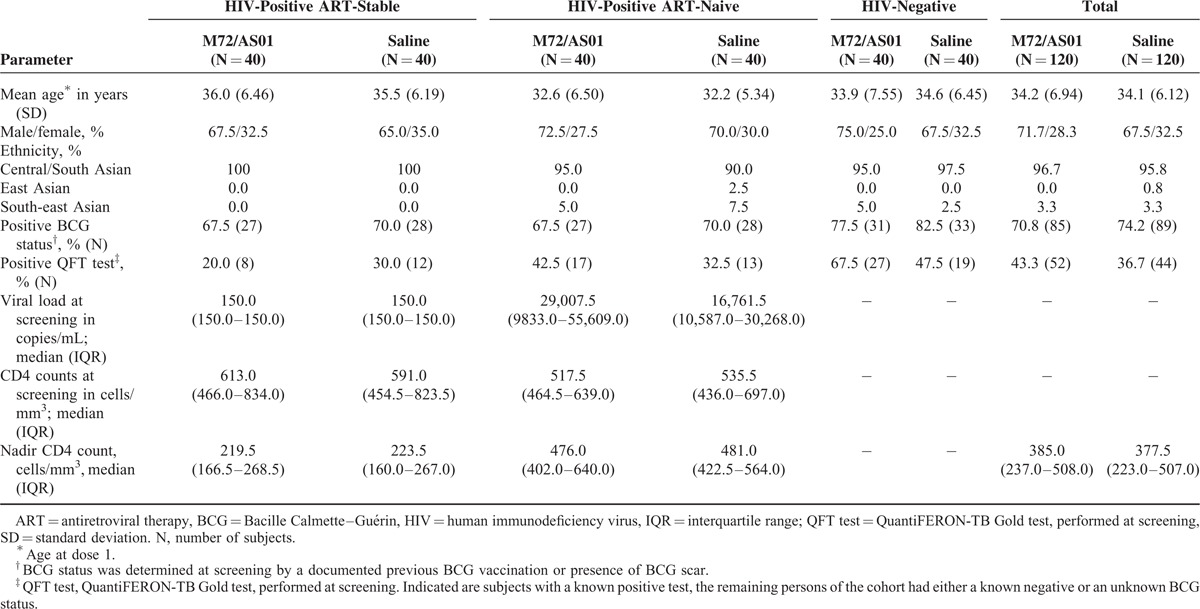
Of the 240 subjects enrolled, 203 (97 M72/AS01 and 106 placebo recipients) completed year 1 (Figure 1). In the ART-stable, ART-naive and HIV-negative cohorts, 9, 3, and 11 M72/AS01 recipients, and 1, 4, and 9 placebo recipients, respectively, withdrew, predominantly because these subjects had migrated and/or were unavailable for follow-up at month 13. Two ART-naive recipients died of vaccine-unrelated diseases (described below). The majority of the study population were BCG-vaccinated, and the majority of the HIV-positive subjects were QFT-negative (Table 1). Expectedly, nadir CD4 counts were higher in the ART-naive subjects than in ART-stable subjects. CD4 nadirs and counts at screening were comparable between the vaccine and placebo groups in both HIV-positive cohorts.
FIGURE 1.
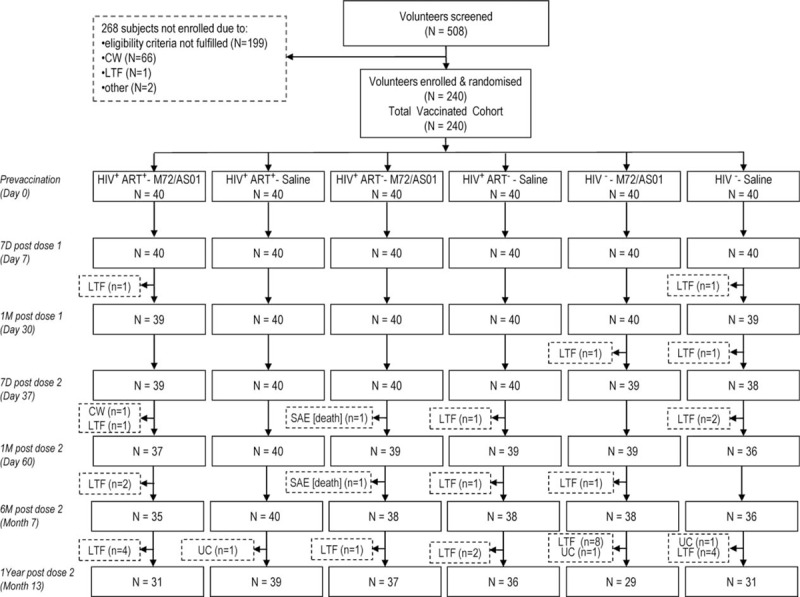
CONSORT diagram of study flow. CW = consent withdrawal, not due to an adverse event, LTF = subject lost to follow-up and/or migrated from the study area, N or n = number of subjects who received the vaccine, Other = other reasons, SAE [death] = serious adverse event; subject died of a cause not related to vaccination, UC = subject unable to come to the site for the visit at the time-point given.
TABLE 1.
Demographic Characteristics of the Total Vaccinated Cohort and Test Results Prior to Day 0
Safety and Reactogenicity
Injection-site pain was the most commonly reported solicited local AE in both the HIV-infected and the HIV-uninfected comparative cohorts (Figure 2A). Irrespective of cohorts, after all doses, grade 3 pain was reported infrequently and only in the M72/AS01 groups (≤7.6%). Across groups and cohorts, the frequency of swelling (any intensity) after all doses was low (≤8.9%) and no grade 3 swelling was reported.
FIGURE 2.
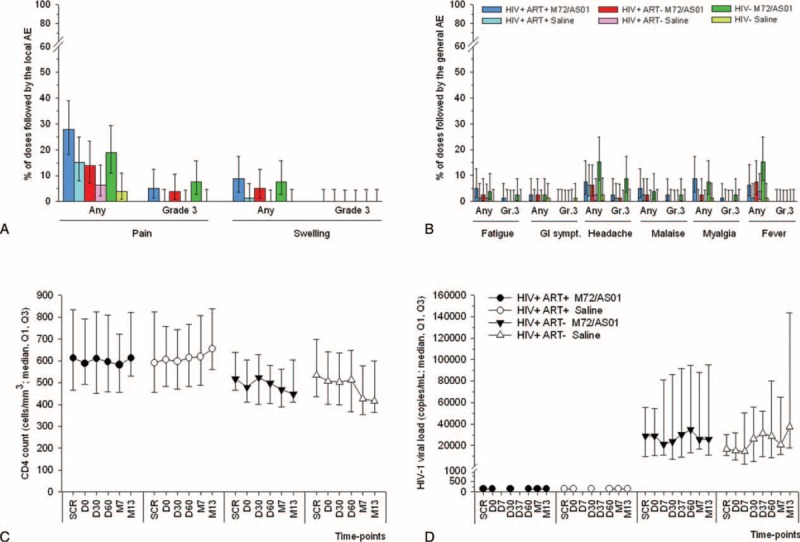
Safety and reactogenicity including HIV-specific parameters. Percentage of doses with 95% CI followed by solicited local (A) and general (B) AEs reported within 7 days postvaccination are shown for all subjects for whom at least 1 administration of vaccine or control was documented (the Total Vaccinated Cohort). GI sympt.: gastro-intestinal symptoms. CD4+ T-cell counts (C) and HIV-1 viral loads (D) obtained from the Total Vaccinated Cohort were measured at screening (SCR), prior to each vaccination (D0 and D30), and 1, 6, or 12 months after the 2nd dose (D60, M7, M13). In addition, HIV viral loads were measured at 7 days post each dose (D7 and D37). The detection limit of the HIV-1 viral load assay was 40 copies/mL. AE = adverse event, CI = confidence interval, HIV = human immunodeficiency virus.
Headache and fever were the most frequently reported solicited general AEs among the M72/AS01 vaccinees (with the highest frequencies in the HIV-negative cohort), and headache was also most frequently reported among the controls (Figure 2B). Grade 3 symptoms were infrequent or absent in the M72/AS01 groups, and in the placebo groups, the only grade-3 general AEs were headache and gastrointestinal symptoms, reported in the ART-stable and HIV-negative cohorts, respectively (1 subject each). There appeared to be no marked differences in the reporting rates of general AEs between the HIV-infected ART-naive and the ART-stable subjects, and incidences of the general AEs except fever and headache were similar between the 3 cohorts.
There were no clinically significant effects of vaccination on the median CD4 counts or VLs. Both parameters were comparable between the vaccine and placebo groups within each cohort, and remained relatively constant throughout the study (Figure 2C/D). Compared to the ART-stable cohort, lower median CD4 counts were reported in the ART-naive cohorts at all time-points, but VLs were higher in the ART-naive cohort.
The incidence of unsolicited AEs was generally comparable between the vaccine and placebo groups, and tended to be highest in the ART-naive cohort, followed by the ART-stable cohort and then the HIV-negative cohort (Table S2). Nasopharyngitis, back pain, headache, cough, decreased appetite, and pruritus were most frequently reported, without clear patterns between groups, and were all considered not clinically significant. No grade 3 unsolicited symptoms were reported. Vaccine-related unsolicited AEs (injection-site pain and arthralgia among HIV-negative subjects, and injection-site induration among ART-stable subjects; one subject per AE) were rare in the vaccine group and not reported in the placebo group.
Serious AEs were reported by 5 HIV-positive M72/AS01 recipients (of whom 3 were ART-naive) and none of these were vaccine-related (Table S3). Two ART-naive subjects died, one of a myocardial infarction due to underlying diabetes mellitus, and the other of postoperative complications following hemorrhoids surgery.
Although there were some fluctuations in the levels of hematological or biochemical parameters over time, no clinically relevant changes or vaccine-related trends were observed (data not shown).
Adherence to ART and Impact on HIV Status
No vaccine-related changes in the participants’ ART regimens were reported. Two vaccinees (1 at day 37 [planned] and 1 at month 13), and 3 placebo recipients (all at month 7) changed their regimen due to ART toxicity.
Self-reported ART adherence was assessed over the month preceding each visit. Full adherence was reported in both groups, with the exception of 4 vaccinees and 2 placebo recipients, who had missed at least 1 dose each. One ART-naive placebo recipient initiated ART at month 12.
T-Cell-Mediated Responses to M72/AS01
M72-specific CD4+ T cells expressing the immune markers CD40L, IL-2, TNF-α, and/or IFN-γ were assessed by ICS (Figure S1).
Several subjects in both groups exhibited preexisting M72-specific CD4+ T cells expressing at least 2 markers (Figure 3A). After administration of placebo, no increase in the median responses was observed until 1 year postvaccination. In contrast, 1 dose of M72/AS01 induced already at day 7 a modest increase in the median response in each cohort. After the 2nd dose, at day 37, these responses had increased further to levels exceeding those at day 7, and persisted in each cohort at month 13, albeit at lower levels (P < 0.001 vs prevaccination). Vaccine-induced responses in the ART-stable cohort were significantly higher compared with those in the ART-naive cohort from day 30 onwards, and compared with those in the HIV-negative cohort from day 60 onwards. Median responses were significantly higher in the HIV-negative cohort than in the ART-naive cohort at all postvaccination time-points except days 37 and 60.
FIGURE 3.
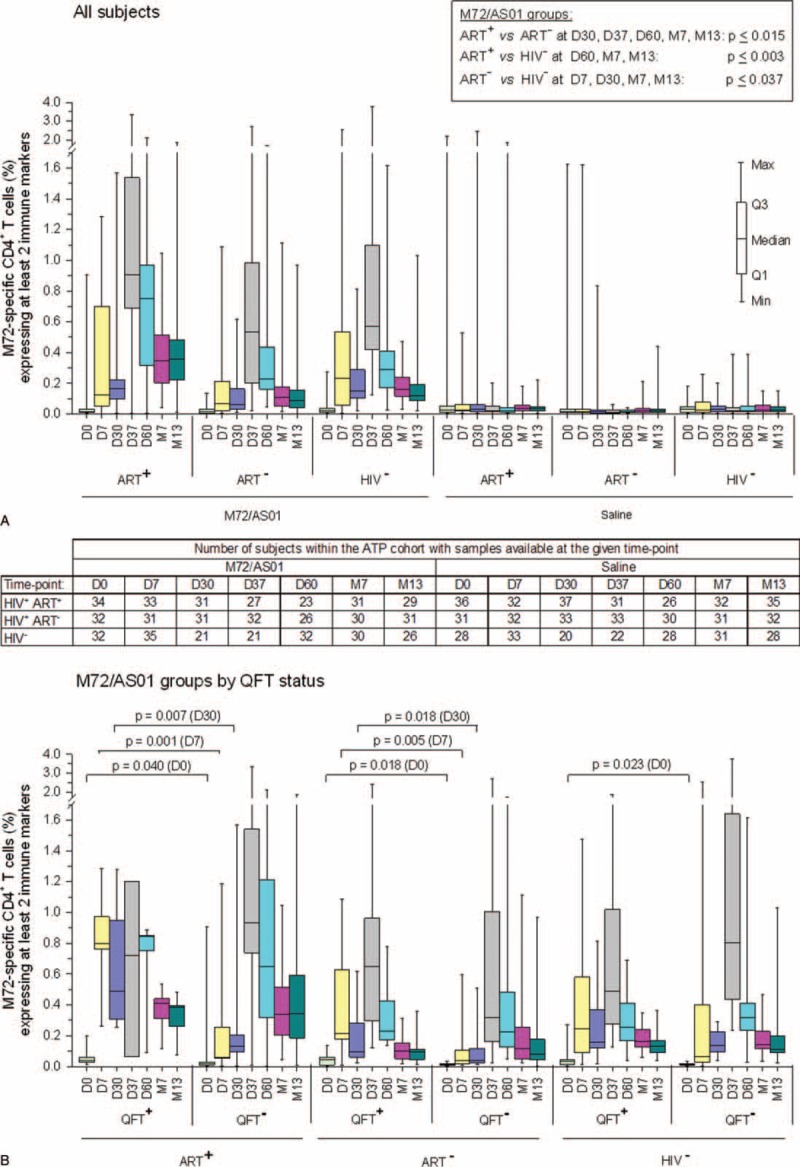
M72-specific CD4+ T-cell responses following vaccination with M72/AS01. Blood samples were obtained prior to each vaccination (D0 and D30), at 7 days post each dose (D7 and D37), and at 1, 6, or 12 months after the 2nd dose (D60, M7 and M13). Data from all subjects (A) and from all M72/AS01 vaccinees presented according to their QFT status (B) are reported as the percentages of M72-specific CD4+ T cells expressing (after in vitro stimulation) at least 2 immune markers among IFN-γ, IL-2, TNF-α, and CD40L of all CD4+ T cells, with 1st and 3rd quartiles, and the minimum/maximum values measured. Statistical analyses were performed by the Wilcoxon rank-sum test (level of significance P < 0.05). IFN-γ = interferon gamma, IL-2 = interleukin 2, QFT = QuantiFERON-TB, TNF-α = tumor necrosis factor alpha.
To assess whether previous Mtb infection had been a determining factor in the response magnitude, M72-specific CD4+ T-cell responses were also evaluated according to the subjects’ QFT status at screening (Figure 3B).
Among the HIV-positive subjects, regardless of their ART status, vaccine-induced median CD4+ T-cell frequencies were significantly higher in QFT-positive than in QFT-negative subjects after dose 1 (days 7 and 30), but comparable between the QFT subgroups at subsequent time-points. No significant difference in vaccine-induced responses between QFT subgroups was seen in the HIV-negative cohort. In all groups except the ART-stable QFT-positive group, there was a trend for higher median responses after dose 2 (at day 37) versus after dose 1 (at day 7), which most obvious among the QFT-negative groups.
Characterization of immune-marker expression showed that in each cohort, the vast majority of vaccine-induced CD4+ T cells were polyfunctional (coexpressing at least 2 immune markers) up to at least 1 year postvaccination (Figure 4). Mainly CD40L+ IL-2+, single-positive CD40L+, CD40L+ IL-2+ TNF-α+, and quadruple-positive T-cell subsets were induced. At prevaccination, the degree of CD4+ T-cell polyfunctionality was higher in HIV-negative versus HIV-positive subjects, however from day 7 onwards the polyfunctional profiles were comparable between cohorts.
FIGURE 4.
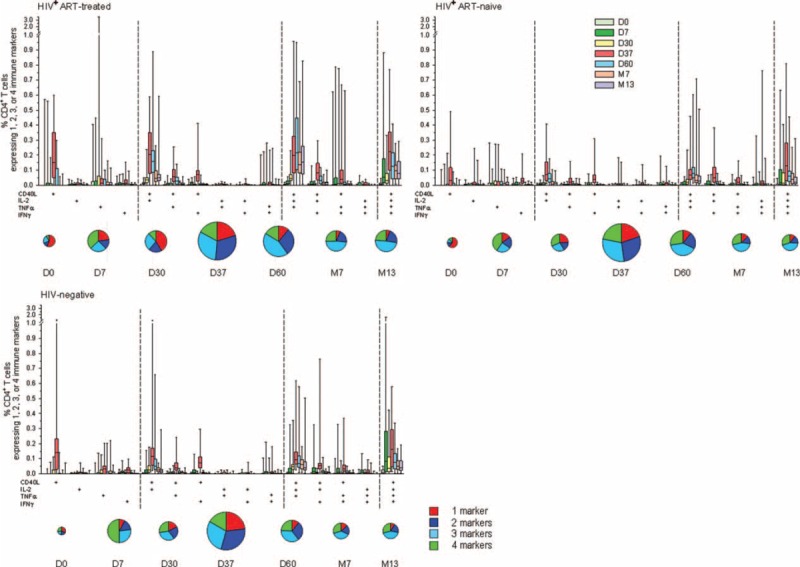
Immune-marker expression profiles following vaccination with M72/AS01. Phenotypes of M72-specific CD4+ T cells expressing (after in-vitro stimulation) single markers and any combination of TNF-α, IFN-γ, IL-2, and CD40L are shown. Box-and-whiskers plots represent the percentages of CD4+ T cells with 1st and 3rd quartiles, and the minimum/maximum values measured. Pie charts represent the mean proportions of cells expressing (after in-vitro stimulation) single markers and any combination of TNF-α, IFN-γ, IL-2, and CD40L marker-positive CD4+ T cells out of the total immune marker-expressing CD4+ T-cell response, at days 0, 7, 30, 37, 60 and months 7 and 13. Pie sizes reflect the mean frequencies (%) of total CD4+ T cells producing at least one marker, relative to the other time-points in the same cohort. IFN-γ = interferon gamma, IL-2 = interleukin 2, TNF-α = tumor necrosis factor alpha.
Cytokine expression profiles were also analyzed according to the QFT status of the subjects (Figure S2A/B). In both HIV-positive cohorts, the degree of polyfunctionality of the responding CD4+ T-cells tended to be higher in QFT-positive subjects after the 1st dose (days 7 and 30), but was comparable between the QFT subgroups at later time-points. No difference in profiles by QFT status was seen in the HIV-negative cohort.
No vaccine-induced CD8+ T-cell responses were observed (peak medians were 0.02%).
Due to problems during shipment by the courier, some samples had thawed upon arrival and were consequently lost. Therefore, for some subjects, samples were unavailable for CMI analysis for 1 or more time-points (Figure 3A). Post-hoc analyses showed that the T-cell-mediated responses for subjects with data available for each time-point were comparable to those for the According-To-Protocol cohort (data not shown).
Anti-M72 IgG Responses
No responses were observed with placebo (data not shown). At the time-points measured, vaccine-induced responses peaked at day 60 and persisted at month 13 in each cohort (Figure S3). GMCs in HIV-negative subjects were significantly higher compared with GMCs in ART-naive subjects at all postvaccination time-points, and with GMCs in ART-stable subjects at day 30. GMCs were also significantly higher in ART-stable subjects than in ART-naive subjects at months 7 and 13. In each cohort, at least 81% of the M72/AS01 vaccinees were seropositive for anti-M72 antibodies at month 13.
DISCUSSION
In HIV-infected subjects, even when they are on ART, cell-mediated and humoral responses to vaccination can often be impaired.27–29 We evaluated M72/AS01 for the 1st time in ART-naive subjects, and in HIV-positive participants in a TB-endemic region. As also reported for ART-stable subjects in Switzerland,15 M72/AS01 was clinically well tolerated and did not negatively affect the participants’ ART-regimens, VLs or CD4 counts. In each cohort, M72/AS01 induced persistent responses of M72-specific antibodies and polyfunctional CD4+ T cells. Thus, irrespective of their ART status, HIV-positive subjects can mount an immune response to M72/AS01.
Previously, we conducted a 3-year safety and immunogenicity follow-up in HIV-negative adults in a TB nonendemic region with a different M72/AS01 formulation.18 The current results and those of the next 2 years of follow-up will collectively comprise the 1st long-term data in HIV-negative and HIV-positive adults in a TB-endemic region, for the current M72/AS01 formulation. The 1st year postvaccination results show that M72/AS01 had an acceptable safety profile in ART-naive and ART-stable adults (similar to observations with another AS01-adjuvanted protein vaccine30), as well as in HIV-negative adults. In addition, we demonstrate that 2 doses can provide CD4+ T-cell responses persisting for up to 1 year in each of the study populations assessed. The next 2 years of follow-up will provide more definite results on the longevity of these responses. It is noteworthy that in a long-term study with the MVA85A vector-based TB candidate vaccine, antigen-specific T-cell responses (in IFN-γ ELISpot) persisted for 3 to 5 years after vaccination in ART-stable vaccinees, but were undetectable after this time-frame in ART-naive vaccinees.31
The magnitudes of vaccine-induced M72-specific CD4+ T-cell responses in the ART-stable cohort surpassed those in the ART-naive cohort up to 1 year postvaccination, and were overall comparable to those obtained with the same vaccine in ART-stable subjects in Switzerland.15 The latter is promising, given the potential exposure of the current subjects to environmental mycobacteria and helminths, which could interfere with the CMI responses to mycobacterial antigens.32–34 Given the high ART-adherence rates, and the relatively high CD4 counts of the ART-stable vaccinees (both nadir [Q1–Q3: 167–269 cells/mm3] and at screening [466–834 cells/mm3]), we assume that these subjects were not severely immunocompromised. This could explain the robust responses to M72/AS01 in this cohort. We have no clear explanation for the fact that after dose 2 (from day 60 onwards), responses in the ART-stable cohort were also higher than in the HIV-negative cohort, but we hypothesize that 2 possible nonexclusive effects may be underlying this result. First, assuming that the same number of CD4+ precursor memory T cells may be induced upon vaccination in HIV-negative and ART-stable subjects, and that the total number of CD4+ T cells was lower in the latter group, vaccination could result in a higher relative proportion of M72-specific CD4+ T cells per million CD4+ T cells in the ART-stable subjects. Alternatively or in addition, since ART can contribute to the specific replenishment of CD4+ T cells in the setting of HIV-induced lymphopenia,35 this can provide an environment for homeostatic proliferation with associated cytokine induction.36,37 After vaccination, such environment might have led to higher expansion rates of the newly induced M72-specific CD4+ T-cell clones in the ART-stable subjects.
On average, 31% of the HIV-positive participants were QFT-positive, and presumably latently TB-infected. In the HIV-positive cohorts, responses of M72-specific CD4+ T cells were highest in QFT-positive subjects after 1 dose, suggesting that in these individuals, these cells had already been primed by natural Mtb infection. However, this difference between QFT subgroups was not observed anymore after the 2nd dose (from day 37 onwards), and, consistent with PBMC-based results from an M72/AS01 trial in HIV-negative adolescents,20 there was also no significant difference between QFT subgroups after the 2nd vaccination in the HIV-negative cohort.
No vaccine-induced M72-specific CD8+ T-cell responses were observed. This may be due to either the used technology (PBMC-based ICS assay), since transient M72-specific CD8+ T-cell responses were previously observed in HIV-negative subjects at 7 days postvaccination using a whole-blood-based ICS assay,19,20 or to as-yet-unknown factors. The significance of Mtb-specific CD8+ T cells in protection against human TB remains unclear, but preclinical data suggest that these cells contribute to Mtb infection control (reviewed in ref.38). A protective immune response can only be defined from efficacy trial data.
Baseline CD4 counts in the ART-naive subjects (median ∼525 cells/mm3) were relatively high compared to the then current WHO-recommended cut-off for ART initiation of ≤350 cells/mm3, and approximated the presently recommended cut-off of ≤500 cells/mm3.39 The safety and immunogenicity of M72/AS01 in ART-naive subjects with CD4 counts below 350 cells/mm3 has therefore not been demonstrated, and, given the abovementioned currently recommended cut-off, may be difficult to demonstrate in an interventional trial.
Recent studies increasingly acknowledge a potential role of Mtb-specific antibody responses in the protection against TB.40,41 Our results suggest that ART had a positive influence on the anti-M72 IgG response persistence, since they were more persistent in the ART-stable than in the ART-naive cohort. Because preservation of naive T-cell populations can enhance antigen-specific antibody responses (as observed for influenza vaccines42), early commencement of ART is likely essential for humoral responses. Consistently, the CD4 nadirs recorded for the ART-stable vaccinees were relatively high (median 220 cells/mm3), suggesting that for these subjects, ART had not commenced at very low CD4 counts.
Finally, we note that this was a Phase II study, and results will need to be validated in a confirmatory trial in a larger population.
CONCLUSIONS
M72/AS01 was immunogenic in ART-stable, ART-naive, and HIV-negative adults in India, and well tolerated. We show that irrespective of their ART status, this population of HIV-positive subjects can mount cell-mediated and humoral responses to two M72/AS01 doses, which persist at 1 year post-vaccination.
Supplementary Material
Acknowledgments
The authors thank the participating volunteers, and acknowledge the contributions of the clinicians, nurses, QA/QC personnel, data management and cohort retention staff, pharmacists, and laboratory technicians of the clinical research site at YRG CARE Medical Centre. They are particularly grateful to the study nurses Uma Maheswari and Chenchulakshmi, as well as to Manohari S. and Sarala M. for QC and data management, Easter Thamburaj for cohort retention supervision, Dr. Pradeep and Dr. Chithra as study physicians, and Pearl Kosala Raman for site regulatory coordination. The authors also thank the following contributors from GSK Vaccines: Shailesh Mehta for study oversight in India, Dipti Phatarpekar for study monitoring, Evi de Ruymaeker for study management activities, An Ranquin, Domenica Majorino, and Jyothsna Krishnan for writing of the clinical report or protocol, and Paul Gillard and Didier Lapierre for helpful discussions. Finally, the authors thank Ellen Oe and Sophie Vanwetswinkel (both XPE Pharma&Science) for providing scientific writing services in the manuscript's development and publication management, respectively, on behalf of GSK Vaccines.
Footnotes
Abbreviations: AE = adverse event, ART = antiretroviral therapy, BCG = Bacille Calmette–Guérin, CMI = cell-mediated immune, CI = confidence interval, GMC = geometric mean concentration, HIV = human immunodeficiency virus, ICS = intracellular cytokine staining, Mtb = Mycobacterium tuberculosis, PBMC = peripheral blood mononuclear cell, QFT = QuantiFERON-TB, TB = tuberculosis, VL = viral load.
Current address: Leo Njock Ayuk, Department of Tuberculosis, Regional Hospital Bamenda, Box 818 Bamenda Cameroon, West Africa
GlaxoSmithKline Biologicals SA was the sponsor of the study, funded the study, was involved in all stages of the study conduct and analysis and also took responsibility for all costs associated with the development and publishing of the present manuscript. All authors had full access to the data and gave final approval before submission. The corresponding author was responsible for submission of the publication.
All authors participated in the design, implementation or analysis, the interpretation of the study, and the development of this manuscript. All authors had full access to the data and gave final approval before submission. The corresponding author was responsible for submission of the publication.
AB, EJ, M-AD, OO-A, and PM are employees of the GSK group of companies. EJ, OO-A, M-AD, and PM own GSK stocks/restricted shares. LA received a WHO/TDR Career Development Fellowship (CDF) grant from WHO-TDR in 2009 to follow a clinical R&D fellowship programme at GSK Vaccines. He also has received payment from the GSK group of companies for monitoring services in Africa since 2011. NK declares a grant support from the GSK group of companies to YRG CARE Medical Centre, VHS for the conduct of the clinical trial. FEB and SP have no conflict of interest to disclose.
REFERENCES
- 1.Getahun H, Gunneberg C, Granich R, et al. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 2010; 50 Suppl 3:S201–S207. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2014. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 3.Glynn JR, Murray J, Bester A, et al. High rates of recurrence in HIV-infected and HIV-uninfected patients with tuberculosis. J Infect Dis 2010; 201:704–711. [DOI] [PubMed] [Google Scholar]
- 4.Djoba Siawaya JF, Ruhwald M, Eugen-Olsen J, et al. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int J Infect Dis 2007; 11:289–299. [DOI] [PubMed] [Google Scholar]
- 5.Horsburgh CR, Jr, O’Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med 2010; 182:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlowski A, Jansson M, Sköld M, et al. Tuberculosis and HIV co-infection. PLoS Pathog 2012; 8:e1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suthar AB, Lawn SD, Del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Wood R, Kaplan R, et al. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One 2012; 7:e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Bekker L-G, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS 2005; 19:1113–1124. [DOI] [PubMed] [Google Scholar]
- 10.Kernodle DS. Decrease in the effectiveness of Bacille Calmette-Guérin vaccine against pulmonary tuberculosis: a consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin Infect Dis 2010; 51:177–184. [DOI] [PubMed] [Google Scholar]
- 11.Hawn TR, Day TA, Scriba TJ, et al. Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev 2014; 78:650–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Reyn CF, Bakari M, Arbeit RD, et al. New vaccines for the prevention of tuberculosis in human immunodeficiency virus infection. Int J Tuberc Lung Dis 2012; 16:718–723. [DOI] [PubMed] [Google Scholar]
- 13.Ndiaye BP, Thienemann F, Ota M, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2015; 3:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Reyn CF, Mtei L, Arbeit RD, et al. Prevention of tuberculosis in Bacille Calmette-Guérin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS 2010; 24:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thacher EG, Cavassini M, Audran R, et al. Safety and immunogenicity of the M72/AS01 candidate tuberculosis vaccine in HIV-infected adults on combination antiretroviral therapy: a phase I/II, randomized trial. AIDS 2014; 28:1769–1781. [DOI] [PubMed] [Google Scholar]
- 16.Idoko OT, Owolabi OA, Owiafe PK, et al. Safety and immunogenicity of the M72/AS01 candidate tuberculosis vaccine when given as a booster to BCG in Gambian infants: an open-label randomized controlled trial. Tuberculosis (Edinb) 2014; 94:564–578. [DOI] [PubMed] [Google Scholar]
- 17.Montoya J, Solon JA, Cunanan SR, et al. A randomized, controlled dose-finding Phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J Clin Immunol 2013; 33:1360–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leroux-Roels I, Forgus S, De Boever F, et al. Improved CD4+ T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine 2013; 31:2196–2206. [DOI] [PubMed] [Google Scholar]
- 19.Day CL, Tameris M, Mansoor N, et al. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med 2013; 188:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penn-Nicholson A, Geldenhuys H, Burny W, et al. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine 2015; 33:4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Aids Control Organisation (India). Antiretroviral therapy guidelines for HIV-infected adults and adolescents. Available at: http://www.naco.gov.in/upload/Policies%20&%20Guidelines/Antiretroviral%20Therapy%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents.pdf. National Aids Control Organisation, Government of India, 2013. [Accessed: September 27, 2015]. [Google Scholar]
- 22.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach-2010 Revision. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 23.Dillon DC, Alderson MR, Day CH, et al. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect Immun 1999; 67:2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skeiky YA, Lodes MJ, Guderian JA, et al. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect Immun 1999; 67:3998–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723–739. [DOI] [PubMed] [Google Scholar]
- 26.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10:471–486. [DOI] [PubMed] [Google Scholar]
- 27.Elrefaei M, McElroy MD, Preas CP, et al. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol 2004; 173:2184–2189. [DOI] [PubMed] [Google Scholar]
- 28.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS 2010; 24:2187–2192. [DOI] [PubMed] [Google Scholar]
- 29.Viganò A, Zuccotti GV, Pacei M, et al. Humoral and cellular response to influenza vaccine in HIV-infected children with full viroimmunologic response to antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 48:289–296. [DOI] [PubMed] [Google Scholar]
- 30.Harrer T, Plettenberg A, Arasteh K, et al. Safety and immunogenicity of an adjuvanted protein therapeutic HIV-1 vaccine in subjects with HIV-1 infection: a randomised placebo-controlled study. Vaccine 2014; 32:2657–2665. [DOI] [PubMed] [Google Scholar]
- 31.Tameris M, Geldenhuys H, Luabeya AK, et al. The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS One 2014; 9:e87340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rook GA, Dheda K, Zumla A. Immune systems in developed and developing countries; implications for the design of vaccines that will work where BCG does not. Tuberculosis (Edinb) 2006; 86:152–162. [DOI] [PubMed] [Google Scholar]
- 33.Elias D, Wolday D, Akuffo H, et al. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin Exp Immunol 2001; 123:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XX, Zhou XN. Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasit Vectors 2013; 6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCune JM, Hanley MB, Cesar D, et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest 2000; 105:R1–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol 2000; 165:1733–1737. [DOI] [PubMed] [Google Scholar]
- 37.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med 2000; 192:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin PL, Flynn JL. CD8 T cells and Mycobacterium tuberculosis infection. Semin Immunopathol 2015; 37:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. March 2014 Supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2014. [PubMed] [Google Scholar]
- 40.Kozakiewicz L, Phuah J, Flynn J, et al. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol 2013; 783:225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 2013; 13:250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez LA, Daniel A, Frank I, et al. Seroprotection of HIV-Infected subjects after influenza A(H1N1) vaccination is directly associated with baseline frequency of naive T cells. J Infect Dis 2014; 210:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


