Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of liver transplantation. In an attempt to predict their recurrence after liver transplantation, evaluation of tumor number and size, degree of histologic differentiation, and the presence of vascular invasion already have their importance established. In this context, the role of biologic markers such as alpha-fetoprotein (AFP) is still not clear. This retrospective cross-sectional study analyzed the AFP relationship with recurrence of HCC after orthotopic liver transplantation.
The current study retrospectively analyzed data from 206 patients with a histopathologic confirmed HCC between 1997 and 2010.
The overall survival rates at 1, 3, 5, and 14 years were 78.6%, 65.4%, 60.5%, and 38.7%, respectively. The frequency of recurrence was 15.5%, and recurrence was significantly associated with a lower survival rate (P < 0.001). No association was observed between survival and AFP level (P = 0.153). A correlation, however, was found between tumor recurrence and AFP level (P = 0.002). Univariate analysis of risk factors for recurrence revealed that an AFP level greater than 200 ng/mL, the number of tumors, the degree of cellular differentiation, and the presence of vascular invasion or satellite nodules were associated with relapse. By multivariate analysis, only an AFP level greater than 200 ng/mL remained as a risk factor.
Although an elevated AFP level did not correlate with survival in HCC patients undergoing orthotopic liver transplantation, a high AFP level was associated with a 3.32-folds increase in the probability of HCC recurrence.
INTRODUCTION
Liver cancer is currently one of the leading causes of cancer death worldwide. An estimated 782,500 new cases occurred in 2012, being the second malignancy more related to death in men in developing countries.1 Hepatocellular carcinoma (HCC) accounts for approximately 90% of all primary liver cancers and it is the fifth most common cancer worldwide.1,2 Cirrhosis is the main risk factor for developing HCC, with chronic hepatitis B virus and C virus (HCV) infection3,4 and, more recently, nonalcoholic fatty liver disease5 as the major causes of cirrhosis. In Brazil, liver disease is the eighth cause of death, being cirrhosis and liver cancer the most common of these.6
The American Association for the Study of Liver Diseases recommends that patients with cirrhosis undergo abdominal ultrasound every 6 months for surveillance of HCC.7 Currently, the observation of isolated alpha-fetoprotein (AFP) levels is considered unsuitable for HCC screening, surveillance, and diagnosis because of its low sensitivity and specificity.8
Orthotopic liver transplantation (OLT) is the best available treatment modality for HCC. Patients who meet the Milan criteria and undergo transplantation have an estimated 5-year recurrence-free survival rate of approximately 83%.9
The primary objective of patient selection criteria is to detect advanced disease, which causes early recurrence and treatment failure. The best predictors for tumor staging, however, can only be obtained after OLT by histologic examination of the explanted liver for vascular invasion, degree of differentiation, and the presence of satellite nodules.10
The value of an elevated pretransplantation AFP level as a predictor of poor prognosis has been increasingly recognized in recent studies.11–13 Therefore, although the significance of AFP levels in nontransplanted patients remains debatable, support is mounting for the utility of this marker in transplant candidates with HCC and for its impact on transplant outcomes. Recent studies have demonstrated that AFP level is an important predictor of posttransplantation tumor recurrence and survival in patients waitlisted for OLT.14,15
The objective of this study was to assess the prognostic value of pretransplantation AFP levels and their potential association with tumor recurrence.
METHODS
This was a retrospective, cross-sectional study of patients receiving follow-up at the Outpatient Adult Liver Transplantation Clinic of Complexo Hospitalar Santa Casa de Porto Alegre, in Brazil.
From December 1997 through September 2010, 768 OLTs were performed at the study. Of these, 213 patients had a confirmed diagnosis of HCC by histologic examination of the explanted liver. Seven patients were excluded from the study because of missing data in their medical records. Therefore, 206 patients remained for analysis of pre- and posttransplantation data. The data collection period ran until May 2012.
The following variables were assessed at baseline (pretransplantation): age, sex, etiology of cirrhosis, Child–Pugh score at the time of list placement, Model for End-Stage Liver Disease score at the time of transplantation, AFP level at the time of list placement (stratified into 3 levels: <50, 50–200, and >200 ng/mL), pretransplantation procedures (chemoembolization, percutaneous ethanol injection, radiofrequency ablation, and liver resection), and Milan criteria. After OLT, the following variables were assessed on the basis of explant examination: presence of incidental tumor, number of nodules, size of the largest nodule, degree of differentiation based on the Edmondson–Steiner classification,16,17 the presence of vascular invasion, and the presence of satellite nodules.
A preoperative diagnosis of HCC was established in accordance with the Barcelona Clinic Liver Cancer recommendations,18 as supported by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases guidelines published in 2001 and 2005,19,20 since the study population was assessed from 1997 to 2010. Patients transplanted before this period were diagnosed by liver biopsy.
Orthotopic liver transplantation was performed in patients with a tumor that was 5 cm or less in diameter (if a single tumor) or no more than 3 nodules, each 3 cm or less in diameter (if multiple tumors), and no evidence of vascular invasion or distant metastases.
When the predicted waiting time on the list exceeded 6 months, patients received locoregional therapy consistent with the number, size, and site of their lesions,21 considering the local availability.
The patients included in this study underwent ultrasound and dosing AFP every 6 months for HCC recurrence surveillance, as a routine service, and the suspicious lesions of HCC recurrence were biopsied.
Informed consent was obtained from each patient upon entering the transplant list, pointing out that the data of the subsequent research would always be expressed in numbers, preserving their identity. The project was submitted to the Santa Casa de Misericórdia de Porto Alegre Research Ethics Committee under protocol 378/11, approved under protocol No. 3615/11. Such study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Considering a 5-year average recurrence rate of 10%,22–26 we estimated that a sample size of 200 would be required to detect a hazard ratio (HR) ≥3 for an increased recurrence rate with a statistical power of 80% at the 5% significance level.
Continuous data were summarized as the means and standard deviations or as medians, interquartile ranges (P25–P75), and ranges when the normality of the data was in doubt. Categorical data were expressed as absolute and relative frequencies.
Quantitative variables were compared using Student t test or the appropriate nonparametric tests when needed. The χ2 test was used for bivariate comparisons of categorical data.
Patient survival and HCC recurrence rates were described using Kaplan–Meier curves, which were followed by log-rank tests for bivariate comparisons. A Cox regression multivariate model was used to simultaneously evaluate the impact of different AFP levels on recurrence and to adjust for potential confounders.
All the data were processed and analyzed using the statistical package for social science (SPSS - IBM, version 18.0, Armonk, NY, USA).
RESULTS
Our case series comprised 206 patients with cirrhosis and HCC who underwent liver transplantation. The demographic and clinical profiles of the patients are described in Table 1.
TABLE 1.
Demographic and Clinical Profile of Patients Who Underwent Orthotopic Liver Transplantation for Hepatocellular Carcinoma, 1997 to 2010
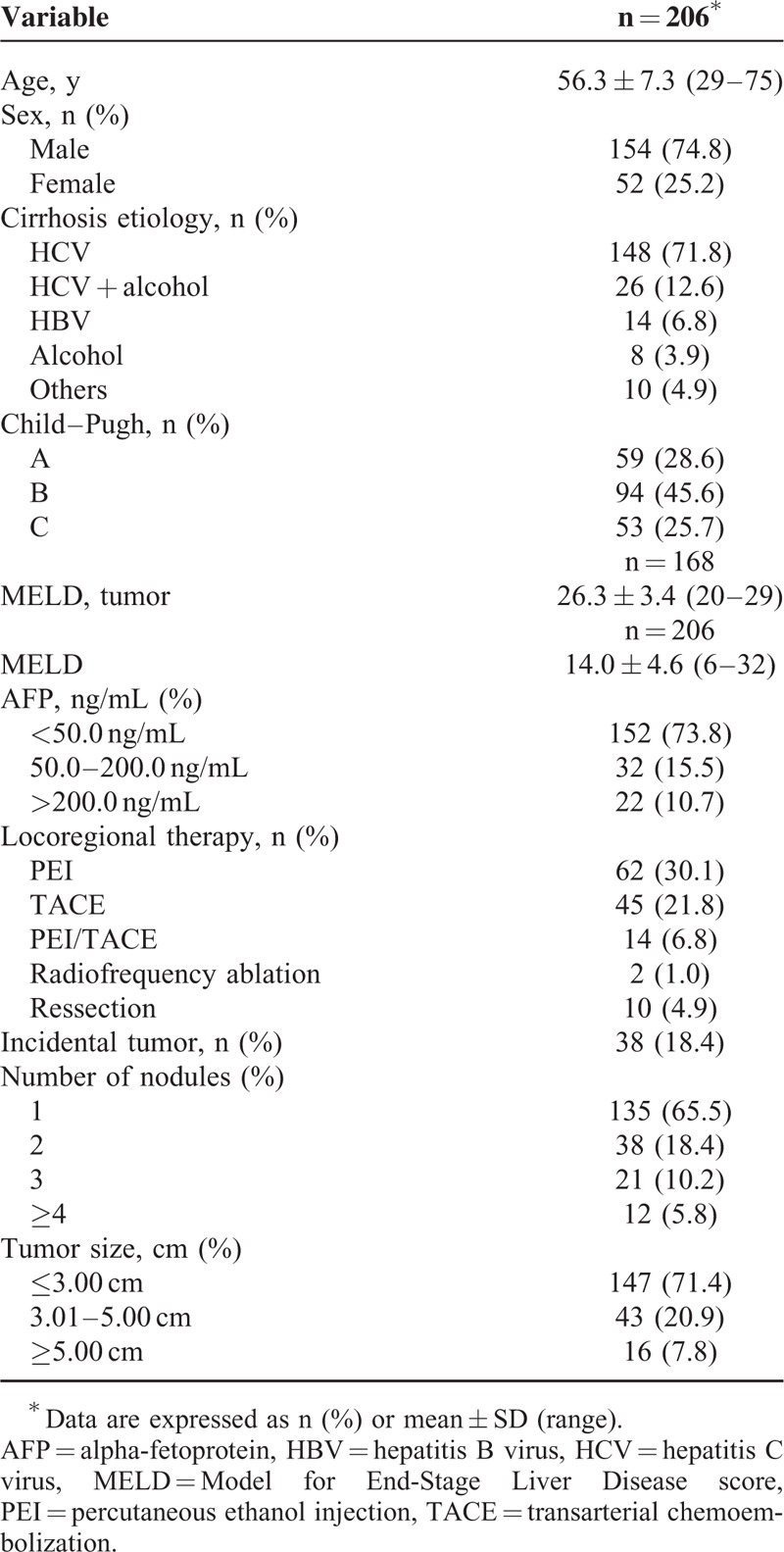
The leading causes of liver disease were chronic HCV infection (n = 136, 66%) followed by combined HCV infection and alcoholism (n = 23, 11.1%). Eleven patients (5.3%) had chronic hepatitis B virus, and 8 patients (3.9%) had an alcoholic etiology only. In other cases, the etiology was related to a combination of viruses or alcohol. In 6 patients, (2.9%), the etiology was considered cryptogenic cirrhosis.
Patients were followed for up to 173 months (mean, 49.8 months; median, 43.6 months), with an overall mortality rate of 44%. The survival rates for the OLT recipients in our study population at 1, 3, 5, and 14 years were 78.6%, 65.4%, 60.5%, and 38.7%, respectively.
Comparative analysis of the survival rates in patients with and without HCC recurrence revealed a higher survival rate in the recurrence-free group. The difference between groups was quite small in the first year but increased thereafter: the 1-year survival rate was 79.3% in recurrence-free patients compared with 75% in patients with recurrence, whereas at 3 and 5 years, these rates were 70.2% versus 39.8% and 67.5% versus 25.6%, respectively (P < 0.001) (Figure 1).
FIGURE 1.
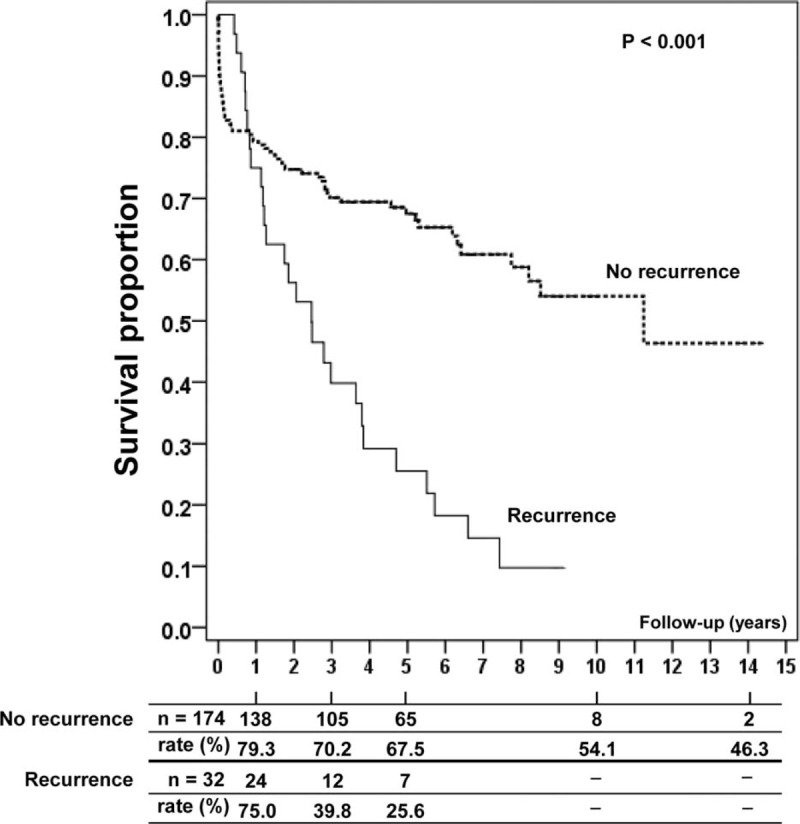
Survival of orthotopic liver transplant recipients stratified by hepatocellular carcinoma recurrence.
Analysis of survival rates stratified by AFP level revealed no significant correlation (P = 0.153).
As Figure 2 shows, the cumulative frequency of HCC recurrence was 10.5% within 1 year of OLT, 15.5% within 3 years, 17.9% within 5 years, and 25.1% at 10 years, after which time it reached a plateau.
FIGURE 2.
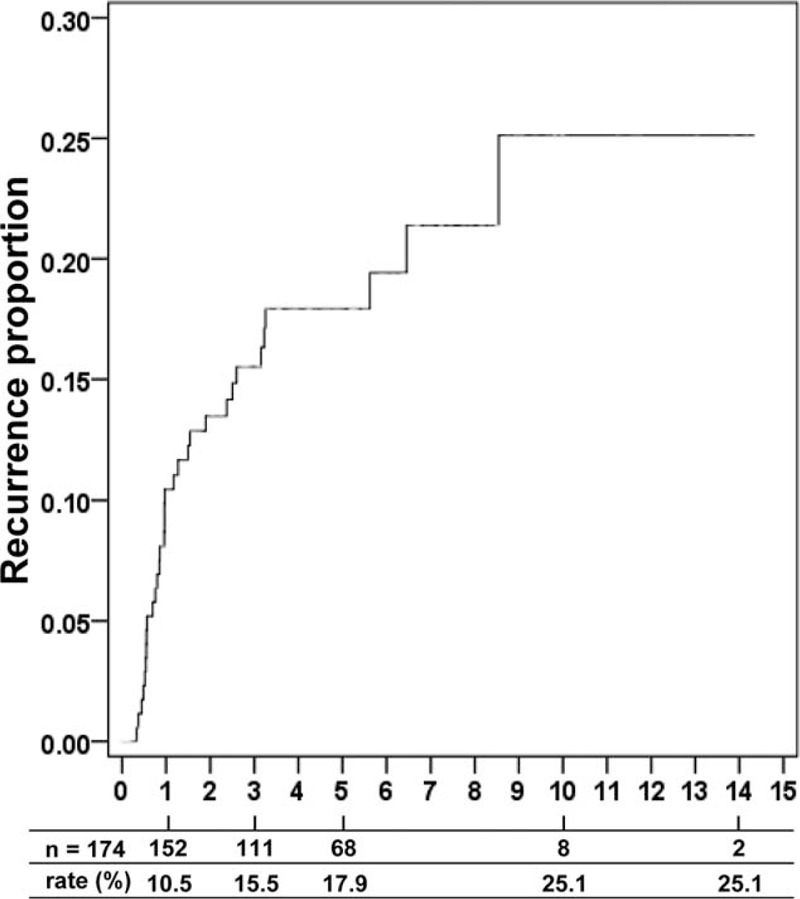
Cumulative frequency of tumor recurrence after orthotopic liver transplantation.
Analysis showed that the recurrence rate was progressively higher with increasing AFP levels. At 5 years after transplantation, the rate of HCC recurrence in patients with AFP levels <50 ng/mL was 13.1% compared with 29.4% in patients with AFP levels of 50 to 200 ng/mL and 36.8% in patients with AFP levels >200 ng/mL (P = 0.002) (Figure 3).
FIGURE 3.
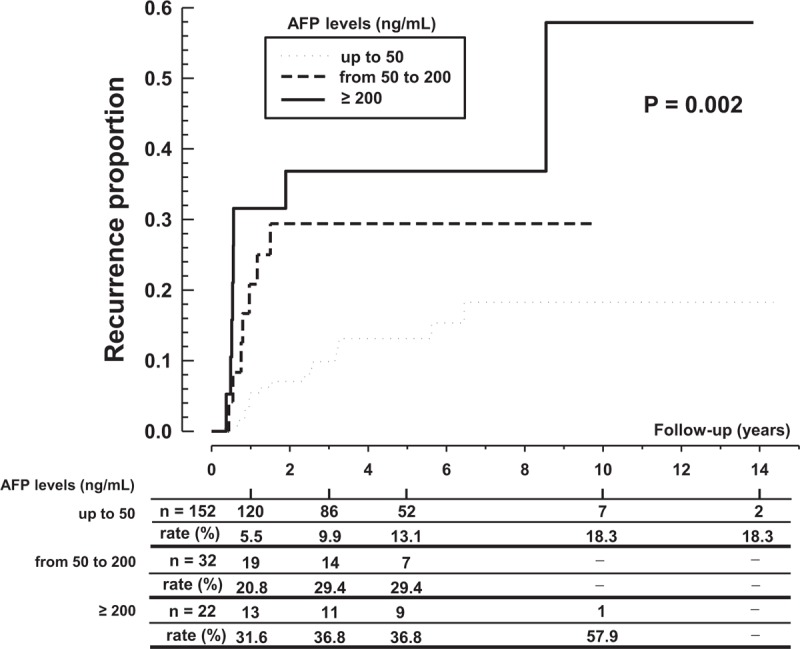
Rate of tumor recurrence by alpha-fetoprotein level.
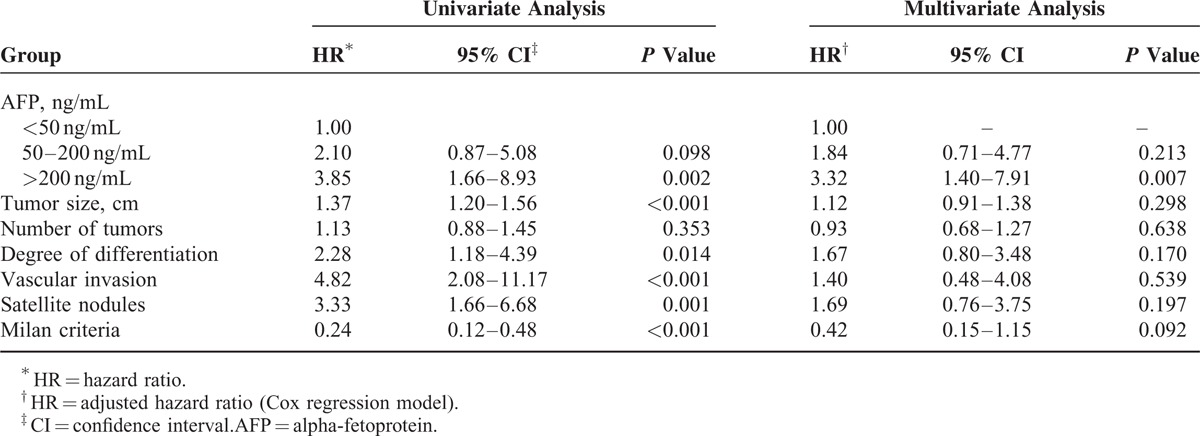
Univariate analysis of factors that were potentially associated with HCC recurrence revealed an HR of 3.85 (95% confidence interval [CI] of 1.66–8.93, P = 0.002) for AFP levels >200 ng/mL. The other risk factors for recurrence were the number of tumors (HR 1.37, 95% CI 1.20–1.56, P < 0.001), the degree of differentiation on the Edmondson–Steiner grading system (HR 2.28, 95% CI 1.18–4.39, P = 0.014), the presence of vascular invasion (HR 4.82, 95% CI 2.08–11.17, P < 0.001), and the presence of satellite nodules (HR 3.33, 95% CI 1.66–6.68, P = 0.001). In contrast, the presence of the Milan criteria was associated with a 76% reduction in the odds of recurrence (HR 0.24, 95% CI 0.12–0.88, P < 0.001). By multivariate analysis, only AFP levels >200 ng/mL remained a risk factor, with an HR of 3.32 (95% CI 1.40–7.91, P = 0.007). These data are shown in Table 2.
TABLE 2.
Potential Risk Factors for Hepatocellular Carcinoma Recurrence in Orthotopic Liver Transplant Recipients
The analysis of incidental tumors revealed a significant difference in size, with a median tumor size of 1.8 cm compared with 2.5 cm in nonincidental tumors (P < 0.001). The median AFP level was 11.7 ng/mL compared with 15.7 ng/mL in patients with a diagnosis of HCC established before OLT (P = 0.061). None of the other variables of interest (number of tumors, corrected Model for End-Stage Liver Disease score, and time on waiting list) showed any significant difference between groups.
The 5-year survival rates in patients with and without incidental tumors were 71.1% and 57.2%, respectively, with no significant difference between groups (P = 0.312).
Recurrence rates were lower in patients with incidental tumors than in patients without, with 5-year recurrence rates of 13.5% and 19.1%, respectively, and 10-year recurrence rates of 13.5% and 30.5%, respectively, although the difference was not statistically significant (P = 0.258).
Throughout the follow-up period, there were 32 cases of HCC recurrence (15.5%). The main sites of recurrence were the liver (n = 6), liver and lungs (n = 6), bones (n = 6), and lymph nodes (n = 5). Other sites included the liver and bones (n = 3), lungs (n = 2), multiple sites (n = 2), palate (n = 1), and adrenal gland (n = 1).
DISCUSSION
Until the 1990s, the cumulative experience of transplantation in HCC patients was extremely discouraging, with a 5-year disease-free survival rate below 40% and tumor recurrence in more than 50% of patients.27–29 This initial experience, however, enabled the discovery that a subgroup of patients with early-stage disease had a more favorable prognosis with OLT than with surgical resection.30,31 This finding, first highlighted by Bismuth et al32 and publicized further by the Milan group,9 led to a set of proposed selection criteria for OLT on the basis of a study in which the 4-year survival of OLT recipients with early-stage HCC was similar to that of recipients without cancer.
Although the use of elective criteria for transplantation in HCC has led to significant improvements in patient survival, the reported recurrence rates still range from 3% to 26%.22–24 Hence, there is a pressing need to identify prognostic markers that can be determined preoperatively to guide optimal therapy. Studies have increasingly proposed AFP level as 1 such marker.11–15
In the current study of 206 patients who underwent OLT for HCC, the overall survival rates at 1, 3, 5, and 14 years were 78.6%, 65.4%, 60.5%, and 38.7%, respectively. In a recent investigation, Vibert et al14 followed 153 patients who underwent transplantation for HCC and found survival rates of 89%, 77%, and 72% at 1, 3, and 5 years, respectively. Yaprak et al15 followed 102 patients and estimated the overall 5-year survival rate at 62.2%. According to a recent review33 in patients who meet the Milan criteria, 5-year survival rates after transplantation range from 50.9% to 89.9%. In 2 Brazilian studies,34,35 the overall 1-year survival rate in a group of 71 patients undergoing transplantation for HCC was 74.6%, and in a sample of 84 patients meeting the Milan criteria, the overall survival rates were 87.7%, 74.5%, and 65.3% at 1, 3, and 5 years, respectively.
The overall tumor recurrence rate in our sample was 15.5%. Although the use of more stringent criteria for transplantation in HCC has significantly improved patient survival, recurrence rates still range from 3% to 26%.9,22–26,36
The prognosis of patients with HCC recurrence remains poor. Comparative analysis of our sample population revealed significantly lower 3- and 5-year survival rates in patients with recurrence (39.8% and 25.6%, respectively) than in patients without recurrence (70.2% and 67.5%, respectively). In a recent study25 of 126 patients who underwent transplantation for HCC, survival curve analysis showed overall 3- and 5-year survival rates of 52.9% and 45.4%, respectively, in patients with recurrence compared with 87.5% and 87.5%, respectively, in patients without recurrence (P < 0.001). As a rule, studies have demonstrated lower survival rates after recurrence.25,37
Analysis of the potential role of AFP isolated as a prognostic marker for survival did not show a positive correlation. The rate of recurrence, however, was significantly higher with increasing AFP levels. Vibert et al14 stratified patients based on increasing or static AFP levels and found significantly lower overall survival rates among patients in whom levels of this oncoprotein increased over time compared with those whose levels were static (84%, 60%, and 54% in patients with increasing levels compared with 90%, 80%, and 77% in patients with static levels at 1, 3, and 5 years, respectively). It bears emphasizing that in contrast to the study by Vibert et al,14 we only analyzed 1 AFP measurement obtained before transplantation, which may in part explain the lack of association between this marker and survival in our sample. In addition, we found an overall mortality rate of 44% and only 15.5% of recurrence (n = 32). This small number of recurrences compared with the deaths may also explain this point. We, however, found a significant correlation between the presence of recurrence and AFP level. Such an association has been described elsewhere in the literature, especially when AFP levels were greater than 1000 ng/mL before OLT.12,13 Less significant values of AFP, however, have shown a positive association. Ho et al38 reported that pretransplantation AFP levels greater than 200 ng/mL led to increased HCC recurrence. Xu et al11 stratified patients into 3 categories by AFP level (<20, 20–400, and >400 ng/mL) and found tumor recurrence rates of 14.3%, 22.2%, and 52.3%, respectively. More recently, other authors obtained similar results.39,40 Thus, although the trend is to consider only AFP values above 1000 ng/mL significant for HCC recurrence, our study confirms the alert that lower values of AFP should also be appreciated, especially if used prognostic models, which incorporate AFP.40
The risk factors for HCC recurrence by univariate analysis of our sample population were an AFP level >200 ng/mL, number of tumors, degree of differentiation, vascular invasion, and the presence of satellite nodules. By multivariate analysis, only an AFP level >200 ng/mL remained a risk factor. In a 12-year follow-up of 289 patients who underwent transplantation for HCC, multivariate analysis also demonstrated the importance of measuring AFP levels before OLT.41
Several factors associated with tumor recurrence have been reported in the literature. Iwatsuki et al31 were among the first to highlight the importance of transplantation in patients with early-stage tumors and identified the presence of satellite nodules, vascular invasion, tumor size, number of nodules, and bilobar involvement as indicators of poor prognosis. Factors currently recognized as having prognostic utility include vascular invasion, degree of differentiation, tumor size, number of nodules, and AFP level.42–47 As mentioned before, a wide range of AFP levels have been associated with recurrence after transplantation.8,12,13,39,40,46,48–52
The prevalence of incidental HCC in cirrhotic patients undergoing liver transplantation ranges from 4.2% to 40%.53,54 In our study, there were 38 patients with incidental tumors (18.44%). As in previous investigations,55 we found that incidental tumors were smaller in diameter than those diagnosed before transplantation. Survival was higher and recurrence less frequent in this patient subgroup, although the difference was not significant compared with patients with nonincidental tumors. The relatively small number of patients with incidental HCC, however, precludes any definitive conclusions. The impact of an incidental diagnosis of HCC on survival and recurrence remains a matter of controversy in the literature. Molmenti et al56 found that patients with incidental tumors had a survival and recurrence advantage; similarly, other authors have reported a better prognosis for patients with incidental tumors.55,57
In our sample population, the main sites of recurrence were the liver, lungs, bones, and lymph nodes (72% of patients), which are locations that are consistent with the literature.11,23,25
Our study has some limitations—most notably the retrospective study design, only 1 AFP measurement obtained before transplantation and the small number of patients with an AFP level >200 ng/mL. This study, however, has shown that moderate increases in levels of AFP may be associated with an increase in HCC recurrence as with higher levels, with the advantage of being a presurgical marker. This result is in agreement with the findings worldwide but, to our knowledge, they are the first data regarding the Latin America. These findings should aid hepatologists in deciding when transplantation is indicated in this patient population.
Acknowledgments
The authors would like to let our deep gratitude to the statistician who contributed to this study, Mário Bernardes Wagner MD, PhD in Data Analysis Statistics in Clinical Research by the former King's College School of Medicine and Dentistry, University of London.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratio, MELD = Model for End-Stage Liver Disease, OLT = orthotopic liver transplantation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127:S35–S50. [DOI] [PubMed] [Google Scholar]
- 5.Alexander J, Torbenson M, Wu TT, et al. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol 2013; 28:848–854. [DOI] [PubMed] [Google Scholar]
- 6.Nader LA, de Mattos AA, Bastos GA. Burden of liver disease in Brazil. Liver Int 2014; 34:844–849. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biselli M, Conti F, Gramenzi A, et al. A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br J Cancer 2015; 112:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt JR, Marotta P, Alghamdi M, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl 2007; 13:543–551. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Ke QH, Shao ZX, et al. The value of serum alpha-fetoprotein in predicting tumor recurrence after liver transplantation for hepatocellular carcinoma. Dig Dis Sci 2009; 54:385–388. [DOI] [PubMed] [Google Scholar]
- 12.Hakeem AR, Young RS, Marangoni G, et al. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2012; 35:987–999. [DOI] [PubMed] [Google Scholar]
- 13.Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level >1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014; 20:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010; 10:129–137. [DOI] [PubMed] [Google Scholar]
- 15.Yaprak O, Akyildiz M, Dayangac M, et al. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2012; 11:256–261. [DOI] [PubMed] [Google Scholar]
- 16.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954; 7:462–503. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Rui JA, Ye DX, et al. Edmondson-Steiner grading increases the predictive efficiency of TNM staging for long-term survival of patients with hepatocellular carcinoma after curative resection. World J Surg 2008; 32:1748–1756. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19:329–338. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35:421–430. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236. [DOI] [PubMed] [Google Scholar]
- 21.Brasil Ministério da Saúde. Portaria n (1.160, de 29.5.2006: Critérios para distribuição de fígado de doadores cadáveres para transplante. Available at. http://bvsms.saude.gov.br/bvs/saudelegis/gm/2006/prt1160_29_05_2006_comp.html. Accessed May 2013. [Google Scholar]
- 22.Leung JY, Zhu AX, Gordon FD, et al. Liver transplantation outcomes for early-stage hepatocellular carcinoma: results of a multicenter study. Liver Transpl 2004; 11:1343–1354. [DOI] [PubMed] [Google Scholar]
- 23.Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004; 10:534–540. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Fan J, Wu ZQ, et al. Liver transplantation for patients with hepatocellular carcinoma at the Liver Cancer Institute of Fudan University, China. Chin Med J 2005; 118:654–659. [PubMed] [Google Scholar]
- 25.Chan KM, Chou HS, Wu TJ, et al. Characterization of hepatocellular carcinoma recurrence after liver transplantation: perioperative prognostic factors, patterns, and outcome. Asian J Surg 2011; 34:128–134. [DOI] [PubMed] [Google Scholar]
- 26.Felga G, Evangelista AS, Salvalaggio PR, et al. Hepatocellular carcinoma recurrence among liver transplant recipients within the Milan criteria. Transplant Proc 2012; 44:2459–2461. [DOI] [PubMed] [Google Scholar]
- 27.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma - an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 2006; 23:1535–1547. [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005; 40:225–235. [DOI] [PubMed] [Google Scholar]
- 29.Ishizaki Y, Kawasaki S. The evolution of liver transplantation for hepatocellular carcinoma (past, present, and future). J Gastroenterol 2008; 43:18–26. [DOI] [PubMed] [Google Scholar]
- 30.Selby R, Kadry Z, Carr B, et al. Liver transplantation for hepatocellular carcinoma. World J Surg 1995; 19:53–58. [DOI] [PubMed] [Google Scholar]
- 31.Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg 1991; 214:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993; 218:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortune BE, Umman V, Gilliland T, et al. Liver transplantation for hepatocellular carcinoma: a surgical perspective. J Clin Gastroenterol 2013; 47:S37–S42. [DOI] [PubMed] [Google Scholar]
- 34.Freitas AC, Parolin MB, Stadnik L, et al. Hepatocellular carcinoma: impact of waiting list and pre-operative treatment strategies on survival of cadaveric liver transplantation in pre-Model for End-stage Liver Disease era in one center in Brazil. Arq Gastroenterol 2007; 44:189–194. [DOI] [PubMed] [Google Scholar]
- 35.de Ataide EC, Garcia M, Mattosinho TJ, et al. Predicting survival after liver transplantation using up-to-seven criteria in patients with hepatocellular carcinoma. Transplant Proc 2012; 44:2438–2440. [DOI] [PubMed] [Google Scholar]
- 36.Hemming AW, Cattral MS, Reed AI, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg 2001; 233:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 2008; 143:182–188. [DOI] [PubMed] [Google Scholar]
- 38.Ho MC, Wu YM, Hu RH, et al. Liver transplantation for patients with hepatocellular carcinoma. Transplant Proc 2004; 36:2291–2292. [DOI] [PubMed] [Google Scholar]
- 39.Kashkoush S, El Moghazy W, Kawahara T, et al. Three-dimensional tumor volume and serum alpha-fetoprotein are predictors of hepatocellular carcinoma recurrence after liver transplantation: refined selection criteria. Clin Transplant 2014; 28:728–736. [DOI] [PubMed] [Google Scholar]
- 40.Varona MA, Soriano A, Aguirre-Jaime A, et al. Risk factors of hepatocellular carcinoma recurrence after liver transplantation: accuracy of the alpha-fetoprotein model in a single-center experience. Transplant Proc 2015; 47:84–89. [DOI] [PubMed] [Google Scholar]
- 41.Cescon M, Ravaioli M, Grazi GL, et al. Prognostic factors for tumor recurrence after a 12-year, single-center experience of liver transplantations in patients with hepatocellular carcinoma. J Transplant 2010; 2010:904152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrero JI, Sangro B, Quiroga J, et al. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl 2001; 7:631–636. [DOI] [PubMed] [Google Scholar]
- 43.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol 2002; 8:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol 2005; 100:2708–2716. [DOI] [PubMed] [Google Scholar]
- 45.Tamura S, Kato T, Berho M, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg 2001; 136:25–30. [PubMed] [Google Scholar]
- 46.Shetty K, Timmins K, Brensinger C, et al. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl 2004; 10:911–918. [DOI] [PubMed] [Google Scholar]
- 47.Shimoda M, Ghobrial RM, Carmody IC, et al. Predictors of survival after liver transplantation for hepatocellular carcinoma associated with hepatitis C. Liver Transpl 2004; 10:1478–1486. [DOI] [PubMed] [Google Scholar]
- 48.Figueras J, Ibañez L, Ramos E, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl 2001; 7:877–883. [DOI] [PubMed] [Google Scholar]
- 49.De Carlis L, Giacomoni A, Pirotta V, et al. Surgical treatment of hepatocellular cancer in the era of hepatic transplantation. J Am Coll Surg 2003; 196:887–897. [DOI] [PubMed] [Google Scholar]
- 50.Choi HJ, Kim DG, Na GH, et al. Clinical outcome in patients with hepatocellular carcinoma after living-donor liver transplantation. World J Gastroenterol 2013; 19:4737–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashiki N, Gaynor JJ, Kato T, et al. Competing risks analysis of predictors of delisting owing to tumor progression in liver transplant candidates with hepatocellular carcinoma. Am J Transplant 2004; 4:774–781. [DOI] [PubMed] [Google Scholar]
- 52.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440. [DOI] [PubMed] [Google Scholar]
- 53.Cho CS, Knechtle SJ, Heisey DM, et al. Analysis of tumor characteristics and survival in liver transplant recipients with incidentally diagnosed hepatocellular carcinoma. J Gastrointest Surg 2001; 5:594–601. [DOI] [PubMed] [Google Scholar]
- 54.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg 1998; 228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castillo E, Pelletier S, Kumer S, et al. Incidental hepatocellular carcinoma after liver transplantation: population characteristics and outcomes. Transplant Proc 2009; 41:219–221. [DOI] [PubMed] [Google Scholar]
- 56.Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: an update of the International Tumor Registry. Liver Transpl 2002; 8:736–748. [DOI] [PubMed] [Google Scholar]
- 57.Sotiropoulos GC, Malagó M, Molmenti EP, et al. Liver transplantation and incidentally found hepatocellular carcinoma in liver explants: need for a new definition? Transplantation 2006; 81:531–535. [DOI] [PubMed] [Google Scholar]


