Supplemental Digital Content is available in the text
Abstract
Recently, published studies have reported conflicting results regarding the association between efavirenz exposure and the risk of suicidality among patients with human immunodeficiency virus. The objective of this analysis was to compare the rate of suicidality among patients initiating efavirenz-containing versus efavirenz-free antiretroviral (ARV) regimens.
This retrospective cohort study used US administrative claims data for commercially and Medicaid-insured individuals for the years 2006 to 2013. ARV-naive patients aged ≥12 years initiating an efavirenz-containing or efavirenz-free ARV regimen with ≥6 months of continuous insurance enrollment prior to ARV initiation were selected. The primary outcome was suicidality, defined as the occurrence of any medical claim with a diagnosis code for suicidal ideation or an inpatient or emergency department medical claim for suicide attempt. Unadjusted incidence rates were calculated and propensity score-adjusted hazard ratios were estimated to account for differences in patient characteristics.
There were 19,983 patients (efavirenz-containing, n = 11,187; efavirenz-free, n = 8796) in the commercial database and 5154 patients (efavirenz-containing, n = 2224; efavirenz-free, n = 2930) in the Medicaid database. Unadjusted incidence rates (95% confidence interval [CI]) of suicidality per 1000 person-years were: commercial, efavirenz-containing (3.3 [2.4–4.4]), efavirenz-free (4.0 [2.7–5.8]); Medicaid, efavirenz-containing (25.7 [18.8–34.4]), efavirenz-free (40.6 [31.9–50.9]). In propensity score-adjusted analyses, efavirenz use was not associated with suicidality: adjusted hazard ratio (95% CI) of suicidality compared with efavirenz-free regimen, commercial, 1.029 (0.636–1.665); Medicaid, 0.902 (0.617–1.319).
This analysis found no conclusive evidence of an increased risk of suicidality among patients initiating an efavirenz-containing ARV regimen. However, channeling bias may exist even after adjusting for measured patient characteristics.
INTRODUCTION
Suicidality is highly prevalent among patients infected with human immunodeficiency virus (HIV), with an estimated 26% of patients reporting a history of lifetime suicidal ideation, planning, or attempt.1 The antiretroviral (ARV) medication efavirenz, commonly used to treat HIV, is a neuroactive substance, and as many as one-half of patients initiating efavirenz may experience neurologic symptoms such as dizziness, insomnia, and abnormal dreams.2–4 Typically, these symptoms are short-lived, peaking at 2 weeks and dissipating thereafter.2–4 The prescribing information for efavirenz indicates that patients experiencing such symptoms should be counseled that the presence of these symptoms is not predictive of subsequent onset of psychiatric symptoms5; however, some analyses have reported that a small number of patients have experienced depression or suicidality while receiving efavirenz therapy.6–9 Although no causal relationship between efavirenz treatment and suicidality has been established, these findings are included in the prescribing information for efavirenz-containing therapies.5,10
There are currently few published or presented studies that have directly attempted to examine the association between efavirenz exposure and suicidality. A recently published analysis of pooled data from 4 AIDS Clinical Trials Group (ACTG) clinical trials conducted from 2001 to 2010 reported that patients receiving efavirenz-containing regimens had more than double the rate of suicidality (suicidal ideation or attempted/completed suicide) compared with patients receiving nonefavirenz regimens, although the incidence rates of suicidality were low (<10 per 1000 person-years) in both groups.11 Another recently published disproportionality analysis of the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) reported no evident association between suicidality and ARVs, including efavirenz.12 Finally, a recently presented analysis based on the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study, which is a collaboration of 11 cohort studies in Europe, the United States, and Australia, reported that observed rates of death from suicide or psychiatric disease-related causes were similar for those receiving efavirenz-based ARV regimens compared with other ARV regimens.13
Given the conflicting nature of the existing literature reporting on the relationship between efavirenz and suicidality, additional evidence from alternative study settings may help to advance the current understanding of this subject. Therefore, this study used US administrative claims data collected from 2 different real-world cohorts, with different patient characteristics, receiving ARV medication in the current era. Specifically, this study examined commercially and Medicaid-insured HIV-infected patients who were treated with ARV medication in the setting of routine clinical practice to estimate incidence rates and compare the risk of suicidality among those initiating an efavirenz-containing regimen versus an efavirenz-free regimen, adjusting for suicidality risk factors.
METHODS
This study was registered with ClinicalTrials.gov (NCT02211807).
Data Source
This retrospective cohort study was conducted using Truven Health MarketScan Commercial Claims and Encounters (commercial) and Multi-State Medicaid (Medicaid) databases (Truven Health Analytics, Ann Arbor, MI). These databases comprise enrollment information, inpatient and outpatient medical, and outpatient pharmacy claims data for individuals with employer-sponsored primary (approximately 138 million individuals) or Medicaid (approximately 29 million individuals) health insurance. The commercial database includes claims collected from a convenience sample of over 300 large self-insured US employers and over 25 US health plans. The Medicaid database comprises claims from a convenience sample of 15 states that are heterogeneous with respect to size, geography, and industrial composition. Further identifying information about the included states is restricted by confidentiality agreements. These databases have been used in numerous published studies.14 The most recent data available at the time this study was conducted were used.
The databases satisfy the conditions set forth in Sections 164.514 (a)–(b)1ii of the Health Insurance Portability and Accountability Act of 1996 privacy rule regarding the determination and documentation of statistically deidentified data. Because this study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, institutional review board approval to conduct this study was not necessary.
Study variables were measured from the database using enrollment records, service dates, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, and National Drug Codes, as appropriate. The study dataset was constructed using SAS version 9.2 (SAS Institute, Cary, NC).
Patient Selection and Study Period
Patients were required to meet the following criteria: have ≥1 outpatient prescription claim for an anchor ARV (ie, nonnucleoside reverse transcriptase inhibitor, protease inhibitor, integrase inhibitor, fusion inhibitor, or CC chemokine receptor type 5 antagonist) between January 1, 2007 and June 30, 2013 in the commercial database or between January 1, 2007 and March 31, 2013 in the Medicaid database (an “index date” was defined as the date of the first ARV claim during these periods); be ≥12 years old as of the index date; have ≥6 months of continuous enrollment with medical and pharmacy benefits prior to the index date (this 6-month period was designated the “baseline period” and was used to establish a minimum period of naivety to ARV exposure); have no claims for HIV-related ARVs prior to the index date (searching all available data extending back to January 1, 2004); have ≥1 medical claim with an HIV diagnosis code (ICD-9-CM 042, 795.71, V08, 079.53) in any diagnosis position prior to (searching all available data extending back to January 1, 2004) or following the index date. Patients with Medicare eligibility were excluded because these patients’ outpatient pharmacy claims data are not available in the Medicaid database.
EXPOSURE
The exposure of interest was the presence of efavirenz as part of the initial ARV regimen, taken separately or as part of a coformulated fixed-dose combination. Initial ARV regimens were defined as all ARV medications filled within 14 days of the index date. Patients were categorized as initiating an efavirenz-containing regimen or initiating an efavirenz-free regimen containing nonefavirenz anchor agents.
Method of Follow-Up and Outcome Measurement
Outcomes were assessed during a follow-up period that began on the index date and ended on the date of the first occurrence of one of the following events: outcome of interest (defined later), end of exposure (defined later) to anchor agent(s) in the initial ARV regimen, disenrollment from insurance, or end of the study period. End of exposure to anchor agent(s) in the initial ARV regimen was measured using days supplied recorded on prescription claims. For efavirenz-containing regimens, end of exposure was triggered if the patient went ≥30 days without refilling a prescription for efavirenz after exhausting the days supplied of the prior prescription. For efavirenz-free regimens, end of exposure was triggered if the patient filled a prescription for efavirenz or went ≥30 days without refilling prescription(s) for the anchor agent(s) contained in the initial ARV regimen after exhausting the days supplied of the prior prescription(s). Patients who filled their prescriptions through mail order, which typically have ≥90 days’ supply, were allowed into the study.
The primary study outcome was suicidality, a composite of suicidal ideation and suicide attempt. Suicidal ideation was defined as the occurrence of a medical claim with an ICD-9-CM diagnosis code for suicidal ideation (V62.84) in any diagnosis position. Suicide attempt was defined as the occurrence of an inpatient or emergency department medical claim with an ICD-9-CM diagnosis code in the range for suicide and self-inflicted injury, excluding late effects of self-inflicted injury (E950–E958.9) in any diagnosis position. The accuracy of the ICD-9-CM code for suicidal ideation has not been validated against a gold standard; however, reported positive predictive values for the codes for suicide attempt are >85%.15–17
Secondary outcomes were suicide attempt (as defined above) and an expanded definition of suicide attempt based on a previously published algorithm.16 The expanded definition of suicide attempt was defined as the presence of an ICD-9-CM diagnosis code for suicide attempt on an inpatient or emergency department medical claim or the following combination: the presence of ICD-9-CM diagnosis codes for both injury (960.xx–979.xx, poisoning by psychotropic agents or by other drugs; 980.xx–989.xx, toxic effects; 881.xx, open wound to elbow, wrist, or forearm; 994.7x, Asphyxiation) and a psychiatric disorder (293.83, 296.2x, 296.3x, 296.90, 298.0x, 300.4x, 309.0x, 309.1x, 309.28, 311, depression; 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.6x, 296.7x, 296.8x, 296.99, mania; 301.xx, personality disorder; 309.2x, 309.3x, 309.4x, 309.5x, 309.6x, 309.7x, 309.8x, 309.9x, adjustment reaction; 300.9, unspecified nonpsychotic mental disorder) on an inpatient or emergency department medical claim.16
COVARIATES
Covariates included patient demographics and clinical characteristics thought to potentially confound the relationship between efavirenz and the risk of suicidality, based on literature review. Patient demographics such as age and sex were measured, using insurance eligibility files, at the time of the index date (Table 1). Clinical characteristics such as comorbidities and concomitant medications were measured from medical and prescription claims occurring during the baseline period (Table 2). Comorbid conditions and AIDS-defining illnesses were flagged as being present if a patient had ≥1 medical claim with an ICD-9-CM diagnosis code for the condition of interest in any diagnosis position.18 Medication use (antipsychotics, antidepressants, etc.) was considered concomitant if a patient had a claim in the baseline period for which the days’ supply continued after the index date or if a patient had ≥1 claim in the baseline period and ≥1 claim following the index date.
TABLE 1.
Patient Demographics
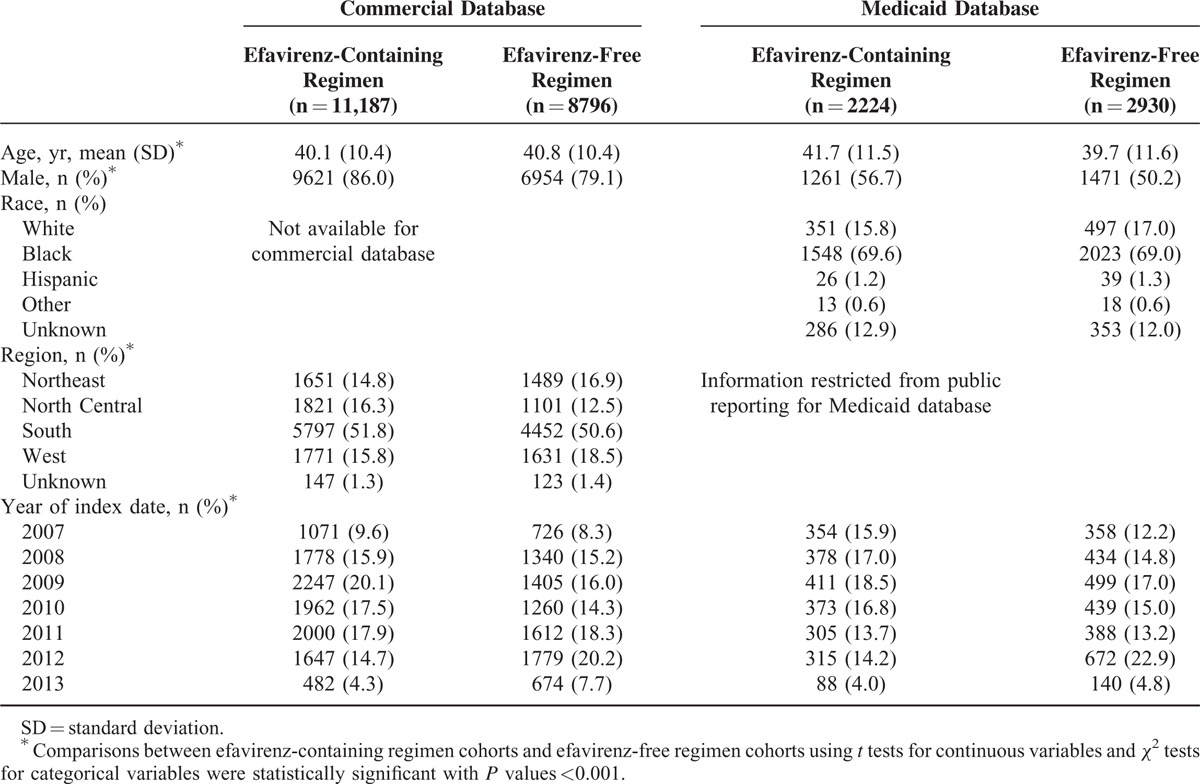
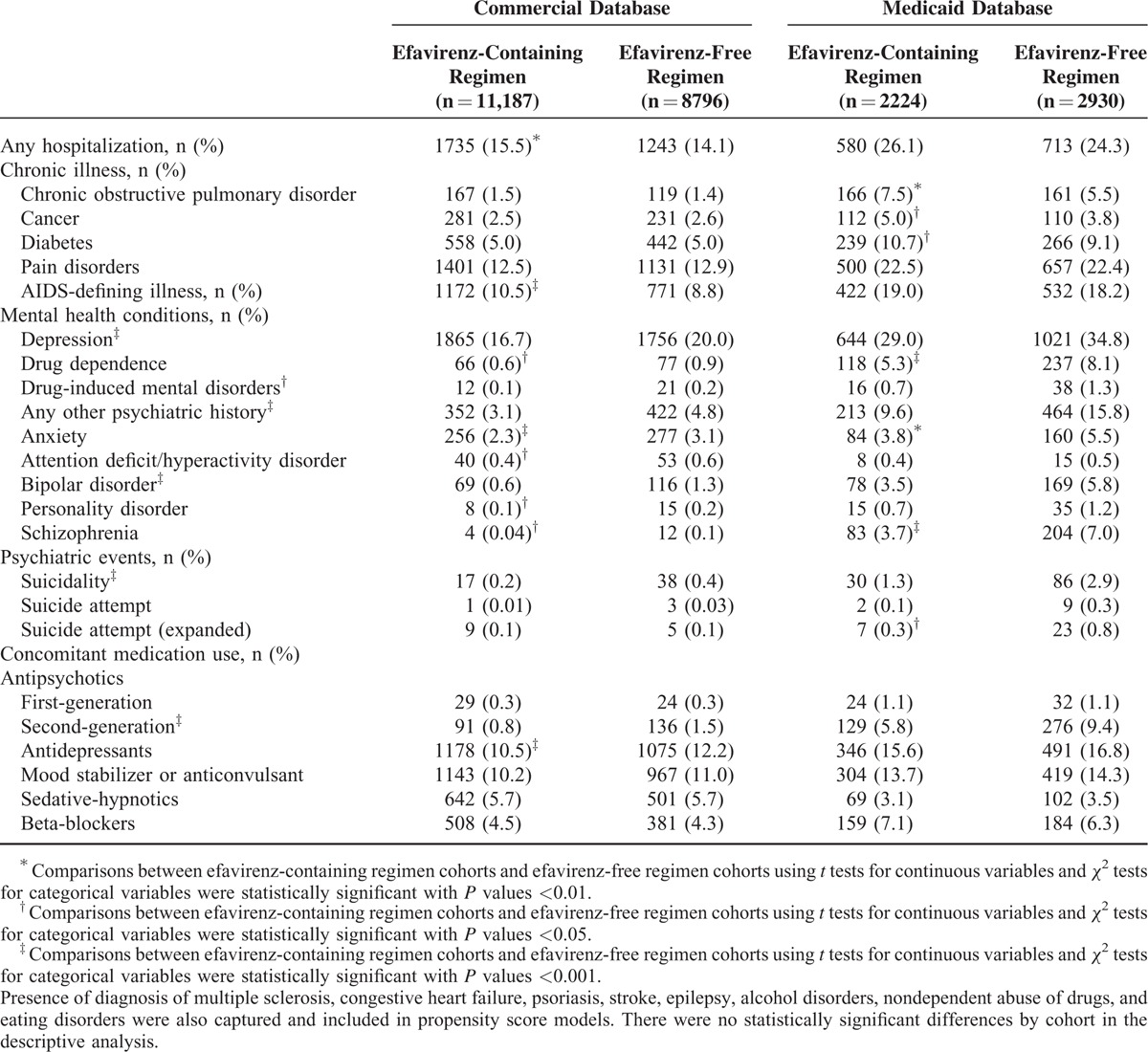
TABLE 2.
Baseline Clinical Characteristics
Statistical Analyses
All analyses were stratified by the commercial versus Medicaid databases because of the substantial heterogeneities in sociodemographic and clinical patient characteristics between these populations. Incidence rates of the outcomes were calculated by summing the number of events and dividing by the sum of person-time (time from index date to event or end of follow-up). The Kaplan–Meier method of survival analysis was used to depict the distribution of time to suicidality.19
Several statistical techniques were employed to adjust the comparisons of the study outcomes between the study cohorts. First, propensity scores of initiating an efavirenz-containing regimen were derived from logistic regression models using baseline demographic and clinical characteristics listed in Tables 1 and 2 as predictors. Although all variables were considered for use in the models regardless of their statistical significance, variables that were strongly related to exposures (odds ratio ≥2.0) but not outcomes were excluded, as inclusion would increase the variance of the estimated treatment effect without a concomitant reduction in bias.20 The hazards of suicidality and the expanded definition of suicide attempt were analyzed in the Cox proportional hazards regression models including propensity score octiles (commercial) or quintiles (Medicaid) as independent variables. Because of the low rate of the suicide attempt outcome, the propensity score was included directly as a continuous independent variable in Cox proportional hazards regressions corresponding to this outcome. Models were fit separately for each database. The Schoenfeld test was used to assess whether the models’ independent variables met the proportionality assumption.21
Additional analyses also used this method of propensity score adjustment. Cox proportional hazards models were conducted in which the hazards of the study outcomes were evaluated over 3 time periods (≤180 days after initiation, 181–365 days after initiation, and >365 days after initiation). Generalized linear models with a log link and Poisson error distribution were used to develop incidence rate ratios. Generalized linear models with an identity link and Poisson error distribution were used to compute incidence rate differences.
To address potential informative censoring in the absence of data on completed suicide, sensitivity analyses were conducted in which all of the aforementioned Cox proportional hazards models were weighed using inverse probability of censoring (IPC) weights. These weights were derived from logistic regression models with censoring status (patients who were censored due to end in continuous enrollment or study data, rather than an outcome) as the outcome and baseline demographic and clinical characteristics, including efavirenz exposure, as covariates.
Stata/MP 12 (StataCorp LP, College Station, TX) was used to fit all logistic regressions (using the “logit” procedure) and Cox proportional hazards regressions (using the “st cox” procedure) described above. SAS version 9.2 (SAS Institute, Cary, NC) was used to fit all generalized linear models described above (using the “GENMOD” procedure).
RESULTS
Study Cohorts
The final study cohorts comprised 19,983 patients (efavirenz-containing: n = 11,187, efavirenz-free: n = 8796) in the commercial database and 5154 patients (efavirenz-containing: n = 2224, efavirenz-free: n = 2930) in the Medicaid database (see Supplemental Table 1). Demographic characteristics are presented in Table 1. In the commercial database, patients initiating efavirenz-containing regimens were significantly younger, had a significantly higher proportion of males, and had a significantly higher proportion of patients who initiated ARV regimens in the earliest time period (2007–2009). In the Medicaid database, patients initiating efavirenz-containing regimens were significantly older and also had significantly higher proportions of males and patients initiating their regimens in earlier years of the study. Baseline clinical characteristics are presented in Table 2. Patients initiating efavirenz-containing regimens had significantly lower rates of depression diagnosis, other mental health conditions (schizophrenia, bipolar disease, anxiety disorders, etc.), or suicidality events.
Comparative Suicidality Outcomes
In the commercial database, patients initiating efavirenz-containing regimens were followed on average for 418 days (standard deviation [SD], 461), while patients initiating efavirenz-free regimens were followed for 300 days (SD, 375.8); in the Medicaid database, these figures were 292 days (SD, 392) and 234 days (SD, 332.1), respectively.
Suicidality was rare in both databases during the follow-up period, though more common in the Medicaid database (commercial: efavirenz-containing regimens, n = 42, efavirenz-free regimens, n = 29; Medicaid: efavirenz-containing regimens, n = 45, efavirenz-free regimens, n = 74). In the commercial database, the unadjusted incidence rate of suicidality per 1000 person-years was 3.3 (95% confidence interval [CI] = 2.4–4.4) among patients initiating efavirenz-containing regimens and 4.0 (95% CI = 2.7–5.8) among patients initiating efavirenz-free regimens. In the Medicaid database, the unadjusted incidence rate of suicidality per 1000 person-years was 25.7 (95% CI = 18.8–34.4) among patients initiating efavirenz-containing regimens and 40.6 (95% CI = 31.9–50.9) among patients initiating efavirenz-free regimens. Unadjusted cumulative hazard functions of suicidality are presented in Figure 1.
FIGURE 1.
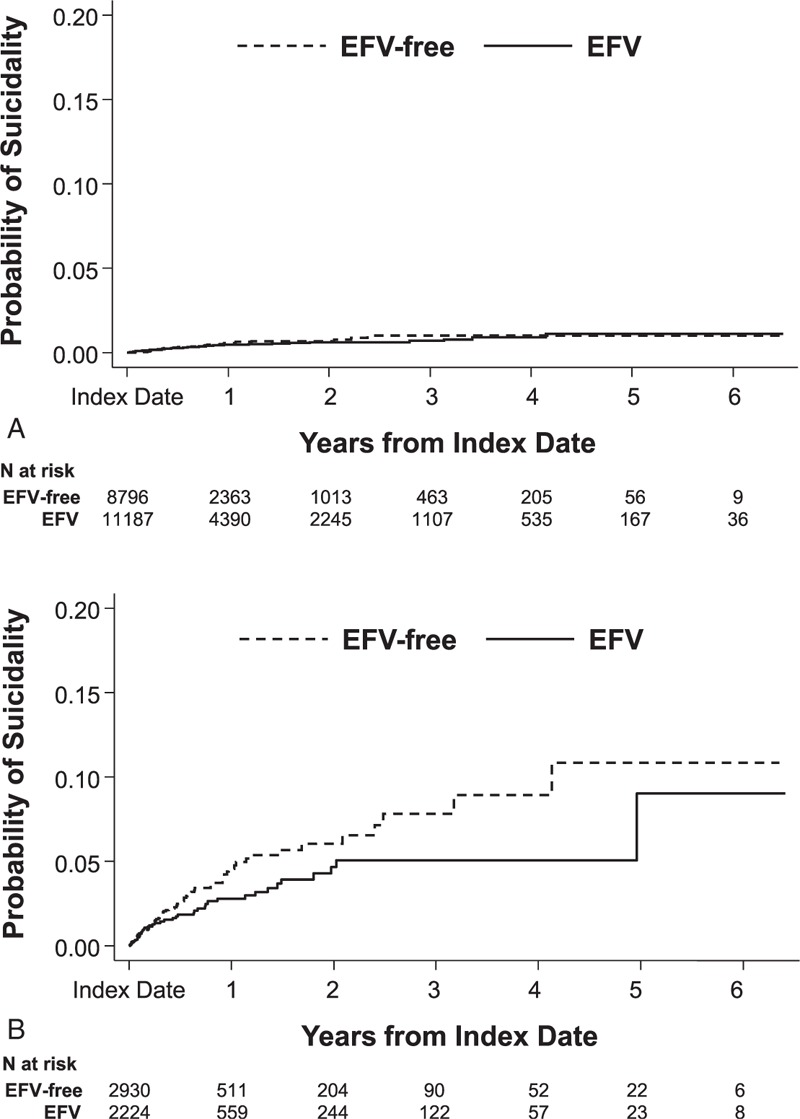
Cumulative hazards of suicidality in the (A) commercial and (B) Medicaid databases. EFV = efavirenz.
Unadjusted and adjusted effect estimates comparing hazards and incidence rates of the 3 study outcomes are presented in Table 3 and Supplemental Table 2. In both databases, the propensity score- and IPC-weighted hazard ratios for suicidality comparing patients initiating efavirenz-containing regimens to those initiating efavirenz-free regimens were not statistically significant. Additionally, the propensity score-adjusted incidence rate ratios or incidence rate differences for suicidality comparing patients initiating efavirenz-containing regimens to those initiating efavirenz-free regimens were not statistically significant.
TABLE 3.
Incidence Rates and Unadjusted and Adjusted Hazard Ratios for Study Outcomes
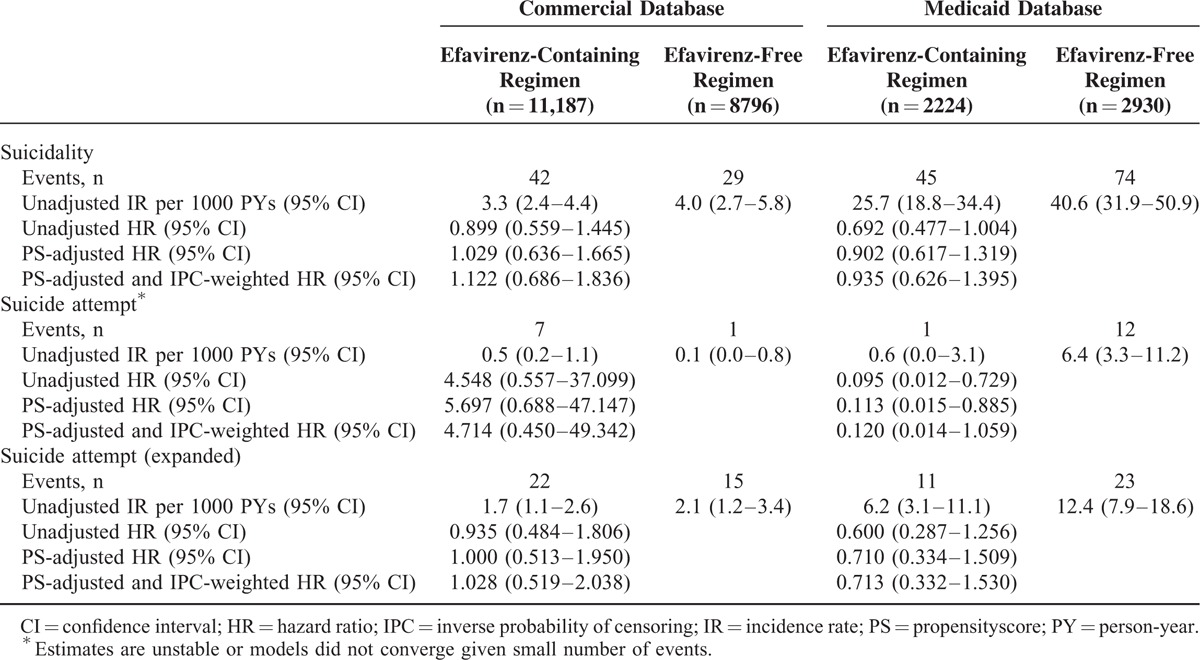
In the time-stratified Cox proportional hazards regressions, the propensity score-adjusted hazard ratios for suicidality comparing patients initiating efavirenz-containing regimens to those initiating efavirenz-free regimens were not statistically significant during any of the 3 time periods evaluated (Supplemental Table 2).
Given the small number of suicide attempts in the 2 study cohorts, the effect estimates produced by the models of suicide attempt were unstable, resulting in extremely wide CIs, or they did not converge. Using the expanded definition of suicide attempt, which had a larger number of events and for which the models did converge, there were no significant associations with exposure to efavirenz in either cohort.
Predictors of Suicidality
In regression analyses examining the bivariate association between each patient characteristic and the risk of suicidality, several characteristics had a statistically significant association with the risk of suicidality.
In the Commercial population, these baseline characteristics included: pain disorders (hazard ratio [HR] 2.18, 95% CI = 1.26–3.76), depression (HR = 4.18, 95% CI = 2.62–6.67), suicidality (HR = 9.75, 95% CI = 2.34–40.71), substance abuse (HR = 3.12, 95% CI = 1.71–5.72), other psychiatric history (HR = 5.64, 95% CI = 3.08–10.34), antipsychotic use (HR = 15.02, 95% CI = 7.98–28.27), antidepressant use (HR = 5.18, OR = 3.21–8.35), and mood stabilizer/anticonvulsant use (HR = 5.92, 95% CI = 3.68–9.52). In exploratory analyses of individuals with versus without any history of mental health conditions or medication use (as defined in Table 2), the unadjusted incidence rates of suicidality per 1000 person-years were: 1.8 (95% CI = 1.0–2.9) for patients initiating efavirenz-containing regimens with no mental health history; 6.4 (95% CI = 4.2–9.3) for patients initiating efavirenz-containing regimens with mental health history; 2.1 (95% CI = 1.0–3.9) for patients initiating efavirenz-free regimens with no mental health history; 7.5 (95% CI = 4.5–11.7) for patients initiating efavirenz-free regimens with mental health history.
In the Medicaid population, baseline characteristics that had a statistically significant association with the risk of suicidality included: white versus other race (HR = 2.16, 95% CI = 1.45–3.23), AIDS-defining illness (HR = 0.44, 95% CI = 0.24–0.83), epilepsy (HR = 3.17, 95% CI = 1.82–5.54), pain disorders (HR = 1.64, 95% CI = 1.11–2.43), depression (HR = 4.29, 95% CI = 2.92–6.31), suicidality (HR = 16.58, 95% CI = 10.42–26.38), substance abuse (HR = 3.85, 95% CI = 2.64–5.61), other psychiatric history (HR = 7.03, 95% CI = 4.86–10.17), inpatient admission (HR = 2.73, 95% CI = 1.89–3.93), antipsychotic use (HR = 4.92, 95% CI = 3.28–7.37), antidepressant use (HR = 5.02, 95% CI = 3.47–7.24), mood stabilizer/anticonvulsant use (HR = 2.98, 95% CI = 2.01–4.42), and sedative-hypnotic use (HR = 3.10, 95% CI = 1.64–5.89). In exploratory analyses of individuals with versus without any history of mental health conditions or medication use, the unadjusted incidence rates of suicidality per 1000 person-years were: 8.0 (95% CI = 3.2–16.5) for patients initiating efavirenz-containing regimens with no mental health history; 43.4 (95% CI = 30.7–59.6) for patients initiating efavirenz-containing regimens with mental health history; 17.0 (95% CI = 9.5–28.0) for patients initiating efavirenz-free regimens with no mental health history; 62.7 (95% CI = 47.7–80.8) for patients initiating efavirenz-free regimens with mental health history.
DISCUSSION
In this analysis of commercially and Medicaid-insured HIV patients initiating ARV regimens, suicidality events were uncommon, with a total of only 71 events among 19,983 commercially insured patients and 119 events among 5154 Medicaid-insured patients. After controlling for potential confounders such as previous psychiatric history, there was no conclusive evidence of increased suicidality risk for patients initiating efavirenz-containing regimens versus those initiating efavirenz-free regimens. These findings were robust to various different modeling methods. This analysis contributes to the body of work examining the association between efavirenz and suicidality which, to date, has given conflicting results.
A recently published post-hoc analysis by Mollan et al11 reported that initiation of efavirenz as part of an initial ARV regimen was associated with a doubling in the risk of suicidality, defined as suicidal ideation or attempted/completed suicide, compared with efavirenz-free regimens, during a median follow-up of 96 weeks. Additionally, the authors found that the association between efavirenz and increased risk of suicidality tended to be higher in the first 24 weeks following initiation, although the difference in risk by time period was not statistically significant.11 In comparison to the analysis presented here that utilized claims from a real-world setting, data from Mollan et al11 were derived from 4 ACTG randomized trials that may not be reflective of actual clinical practice. Additionally, as enrollment for the trials began as early as 2001, the comparator drugs in some trials are no longer commonly used regimens. Another important element of this analysis was that 3 of the 4 trials included in the analysis were open-label. Because the potential for experiencing neuropsychiatric events while receiving efavirenz was reported before the timeframe of the studies, it is possible that ascertainment bias may have affected the study results; however, the potential for such bias is also present in the current study and would tend to make the risk of the study outcomes greater in efavirenz.11 Finally, differences in patient characteristics and settings may help to explain some of the differences in results between the present study and the ACTG analyses. For example, patients in the ACTG analyses were not limited to U.S. residents, with approximately 28% of patients initiating efavirenz-containing regimens and 22% of patients initiating efavirenz-free regimens being of multinational (non-U.S.) residence. Within the U.S. Medicaid sample in the present study, approximately 70% of patients in both study groups identified as black, while the same was true for approximately 35% to 36% of U.S. patients in the ACTG analyses. The Medicaid population also comprised a larger proportion of female patients (57%–50%) when compared with patients in the ACTG analyses (26%–27%). With respect to history of antidepressant use, the present study's Commercial population and ACTG study population had similar baseline antidepressant use rates (11%–12% vs. 10%, respectively) but the Medicaid population had higher use rates (16%–17%). Several other differences exist across the studies with respect to patients’ characteristics, and future analyses to understand how such difference may have influenced the study results would be informative.
The results of the present analysis are consistent with 2 previous analyses. An analysis conducted by Napoli et al,12 which used FAERS data (a public dataset containing spontaneous reports of adverse events reported to the FDA), found that suicidality was not disproportionately reported for efavirenz. The authors utilized disproportionality analysis to identify increased reporting rates of adverse events for efavirenz and several other ARVs, as well as 2 antidepressants with known association with suicidality as positive controls.12 Suicidal ideation, suicide attempt, and completed suicide were not disproportionately reported for efavirenz or other ARVs but were disproportionately reported for the antidepressants, as expected.12 An analysis conducted by Smith et al, which used data from the D:A:D study, reported that observed rates of death from suicide or psychiatric disease-related causes were similar for those receiving efavirenz-based ARV regimens compared with other ARV regimens. For both of these studies and the present study, however, channeling bias may partially explain the results as providers may prescribe ARVs other than efavirenz for patients deemed to be at increased risk for suicide.12
The present analysis has limitations. As with any study conducted in administrative claims, there is a potential for misclassification of exposure or outcome. ARV exposure was based on the presence of outpatient pharmacy claims. It is not known whether patients took medication as instructed. Misclassification of exposure is likely nondifferential by outcome. Regarding suicidality, it is unclear how consistently suicidal ideation is captured or billed for in-routine medical care for patients with HIV. It has also been shown that the sensitivity of capturing suicide attempts in claims data is relatively low, despite high positive predictive value.14–16 A consequence of the low number of observed suicide attempts was instability and nonconvergence regression analyses related to this outcome. To mitigate the underreporting of suicide attempt, an expanded definition was employed that captured events indicative of self-harm. To account for censoring due to completed suicides, which may not result in a health insurance claim, we conducted IPC-weighted analyses. It is important to note that despite our efforts to address the suboptimal sensitivity of capturing suicide attempts in claims data, the true rate of suicidality and suicide attempt in this population is almost certainly higher than what is reported in the present analyses. Indeed, as noted above, an estimated 26% of HIV patients have reported a history of lifetime suicidal ideation, planning, or attempt.1 There are several factors that may influence the risk of suicidality but cannot be assessed in claims data and were therefore not accounted for within this analysis. Such factors may include coping self-efficacy, social or family support, and spirituality, among others.22–24
As this analysis was conducted in real-world databases, treatment exposure was not randomized. Therefore, the channeling bias exists, whereby the decision to initiate efavirenz treatment is influenced by patient characteristics that are also associated with suicidality. Indeed, we found that compared with patients initiating efavirenz-free regimens, those initiating efavirenz-containing regimens had lower baseline rates of depression, other psychiatric history, suicidality, and concomitant psychiatric medications. These findings indicate that real-world prescribing practices are consistent with the information provided in efavirenz package inserts.5,10 Propensity score adjustment for numerous patient characteristics, including specific factors that have been strongly associated with suicidality, was employed to minimize this bias.11 Nonetheless, residual confounding may be present as it was not possible to measure other relevant potential confounding factors, such as laboratory values, lifestyle factors, and socioeconomic status. Lastly, these findings may not be generalizable to patients with HIV insured through Medicare, those who are uninsured, or those who live outside the United States.
In this large claims analysis reflective of real-world practice, we observed no evidence that exposure to efavirenz is associated with increased risk of suicidal ideation or suicide attempt among patients with HIV initiating ARVs.
Supplementary Material
Acknowledgment
The authors acknowledge Boris Ivanov of Truven Health Analytics for completing the programming to build the dataset on which the analysis was based. Editorial assistance was provided by inScience Communications, Springer Healthcare, and funded by Bristol-Myers Squibb.
Footnotes
Abbreviations: ARV = antiretroviral, CI = confidence interval, FAERS = US Food and Drug Administration Adverse Event Reporting System, FDA = US Food and Drug Administration, HIV = human immunodeficiency virus, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IPC = inverse probability of censoring, SD = standard deviation.
This work was presented in a podium presentation entitled “Using Real World Data to Assess the Risk of Suicidality among Patients Initiating an Efavirenz versus an Efavirenz-Free Antiretroviral Regimen” at ID Week, Philadelphia, PA, October 8–12, 2014.
Our conflicts of interest are our employment: ETN, JC, LCR, DS, and AV-K are employees of Bristol-Myers Squibb. AMF, SSJ, and BCC are employees of Truven Health Analytics.
This study was sponsored by Bristol-Myers Squibb.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Badiee J, Moore DJ, Atkinson JH, et al. Lifetime suicidal ideation and attempt are common among HIV+ individuals. J Affect Disord 2012; 136:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Neuropsychiatric symptoms associated with efavirenz: prevalence, correlates, and management. A neurobehavioral review. AIDS Rev 2009; 11:103–109. [PubMed] [Google Scholar]
- 3.Gazzard B, Balkin A, Hill A. Analysis of neuropsychiatric adverse events during clinical trials of efavirenz in antiretroviral-naive patients: a systematic review. AIDS Rev 2010; 12:67–75. [PubMed] [Google Scholar]
- 4.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav 2011; 15:1803–1818. [DOI] [PubMed] [Google Scholar]
- 5.SUSTIVA [package insert]. Princeton, NJ: Bristol-Myers Squib; 2015. [Google Scholar]
- 6.Lochet P, Peyrière H, Lotthé A, et al. Long-term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Med 2003; 4:62–66. [DOI] [PubMed] [Google Scholar]
- 7.Arendt G, de Nocker D, von Giesen H, et al. Neuropsychiatric side effects of efavirenz therapy. Expert Opin Drug Saf 2007; 6:147–154. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez F, Navarro A, Padilla S, et al. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis 2005; 41:1648–1653. [DOI] [PubMed] [Google Scholar]
- 9.Catalan J, Harding R, Sibley E, et al. HIV infection and mental health: suicidal behaviour: systematic review. Psychol Health Med 2011; 16:588–611. [DOI] [PubMed] [Google Scholar]
- 10.ATRIPLA [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015. [Google Scholar]
- 11.Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 2014; 161:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoli AA, Wood JJ, Coumbis JJ, et al. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J Int AIDS Soc 2014; 17:19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith C, Ryom L, Monforte Ad, et al. Lack of association between use of efavirenz and death from suicide: evidence from the D:A:D study. J Int AIDS Soc 2014; 17 (4 Suppl 3):19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truven Health Analytics. MarketScan Studies Abbreviated Bibliography. 2014. http://sites.truvenhealth.com/bibliography/2014TruvenHealthMarketScanBibliography.pdf Accessed November 19, 2014. [Google Scholar]
- 15.Callahan ST, Fuchs DC, Shelton RC, et al. Identifying suicidal behavior among adolescents using administrative claims data. Pharmacoepidemiol Drug Saf 2013; 22:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iribarren C, Sidney S, Jacobs DR, Jr, et al. Hospitalization for suicide attempt and completed suicide: epidemiological features in a managed care population. Soc Psychiatry Psychiatr Epidemiol 2000; 35:288–296. [DOI] [PubMed] [Google Scholar]
- 17.Patrick AR, Miller M, Barber CW, et al. Identification of hospitalizations for intentional self-harm when E-codes are incompletely recorded. Pharmacoepidemiol Drug Saf 2010; 19:1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AIDS-defining conditions. Appendix 2a-4: ICD-9-CM codes for AIDS indicator diseases. http://www.portal.state.pa.us/portal/server.pt/community/hiv___aids/14241/attachment_archive/557348 Accessed September 9, 2015. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–481. [Google Scholar]
- 20.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosmer DW, Jr, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd ed.Hoboken: Wiley; 2008. [Google Scholar]
- 22.Heckman TG, Miller J, Kochman A, et al. Thoughts of suicide among HIV-infected rural persons enrolled in a telephone-delivered mental health intervention. Ann Behav Med 2002; 24:141–148. [DOI] [PubMed] [Google Scholar]
- 23.Cooperman NA, Simoni JM. Suicidal ideation and attempted suicide among women living with HIV/AIDS. J Behav Med 2005; 28:149–156. [DOI] [PubMed] [Google Scholar]
- 24.Gurm J, Samji H, Nophal A, et al. Suicide mortality among people accessing highly active antiretroviral therapy for HIV/AIDS in British Columbia: a retrospective analysis. CMAJ Open 2015; 3:E140–E148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


