Abstract
Studies have shown that albuminuria, obesity, and sarcopenia may share pathophysiological processes related to cardiovascular disease risk. Their direct relationships, however, have not been examined. This study investigated the association between albuminuria and sarcopenia in a representative fraction of the Korean population.
Of the 10,589 people who participated in the 2011 Korea National Health and Nutrition Examination Survey, 2158 participants aged over 19 years had been tested for albumin-to-creatinine ratio and for body composition data using dual-energy x-ray absorptiometry. Albuminuria was defined as an albumin-to-creatinine ratio ≥30 mg/g. Sarcopenia was defined as a skeletal muscle index (SMI) (SMI (%) = total appendicular skeletal muscle mass [kg]/weight [kg] × 100) of less than 1 standard deviation (SD) (grade 1) or 2 SD (grade 2) below the sex-specific mean for a younger reference group.
The prevalence of albuminuria was higher in those with grade 2 sarcopenia than in those with a normal SMI or grade 1 sarcopenia (33.3% versus 8.4% and 8.9%; P < 0.001). Conversely, grade 2 sarcopenia was also more prevalent in participants with albuminuria than in those with the upper tertile of normoalbuminuria. In addition, multiple logistic regression analysis showed the odds ratio for albuminuria risk in the grade 2 sarcopenia group was 2.93 (95% confidence interval [CI], 1.46–5.88), compared with normal SMI after adjusting for potential confounding factors, including the presence of obesity, diabetes, and hypertension. Moreover, individuals with albuminuria had an odds ratio of 3.39 (95% [confidence interval], 1.38–8.37) for grade 2 sarcopenia compared with those in the lowest tertile of normoalbuminuria.
This is the first study to demonstrate that individuals with sarcopenia exhibited increased risk of albuminuria and vice versa.
INTRODUCTION
Albuminuria is associated with an increased risk of all-cause mortality and cardiovascular morbidity and mortality in patients with type 2 diabetes or hypertension as well as in the general population.1,2 Even microalbuminuria is regarded as a risk factor for the progression of chronic kidney disease (CKD) and for cardiovascular disease (CVD).1 In addition, several clinical trials showed that interventions to reduce albuminuria are often accompanied by improvements in cardiovascular endpoints.3,4
Sarcopenia, the age-associated loss of muscle mass, is related to physical disability, metabolic impairments, and increased mortality.5–7 Although the etiology of sarcopenia or low muscle mass is still poorly understood, the cellular and molecular mechanisms responsible for sarcopenia, such as insulin resistance, inflammation, and oxidative stress, are associated with albuminuria. Moreover, sarcopenia is independently associated with type 2 diabetes, which is an important risk factor for CKD and CVD.8
Based on these findings, we hypothesized that sarcopenia and albuminuria share common pathophysiological processes and interact with each other to increase the risk of disease. Previous studies have found a significant association between albuminuria and either general or abdominal obesity or visceral fat.9–11 To the best of our knowledge, no previous studies, however, have explored whether individuals with albuminuria have a higher risk of sarcopenia than those without albuminuria and vice versa. Therefore, we designed this study to evaluate the association of albuminuria and sarcopenia or sarcopenic obesity.
METHODS
Study Population and Data Collection
We used data from the Korea National Health and Nutrition Examination Survey (KNHANES) V-2, a national program designed to assess the health and nutritional status of Koreans,12 which has been conducted in 1998, 2001, 2005, 2007 to 2009, and 2010 to 2012.13 Korea National Health and Nutrition Examination Survey has collected data on demographic, social, nutritional and health status via health interviews, and medical examinations. Urine albumin was measured starting in the 2011 study.12 Of a total of 10,589 people who participated in KNHANES V-2, 2011 2,158 participants aged over 19 years had the measurement of albumin-to-creatinine ratio (ACR) and complete data available on body composition using dual-energy x-ray absorptiometry (DXA) (QDR 4500A, Hologic Inc, Waltham, MA). Of these subjects, a younger subgroup (aged 19–39; 620 subjects; 226 men, 334 women) was used as a sex-specific young reference group. This study was approved by the institutional review board of Ilsan Paik Hospital, South Korea (IRB-2015–09–002).
Clinical and Laboratory Examinations
Systolic and diastolic blood pressure (BP) was measured by standard methods using a sphygmomanometer. Three BP measurements were made for all subjects at 5-minute intervals; the final BP value for study subjects was reported by average of the second and third measurements. Blood samples were obtained from each subject after fasting overnight for at least 8 hours. Total cholesterol, triglycerides, and glucose levels were measured in a central and certified laboratory using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). Diabetes was defined as fasting blood glucose ≥7.0 mmol/L that was first detected in this survey, use of an antidiabetes medication, or a previous diagnosis of diabetes by a doctor. Hypertension was defined as systolic BP ≥ 140 mm Hg, diastolic BP ≥ 90 mm Hg, or use of an antihypertensive medication.
Assessment and Definition of Microalbuminuria and Estimation of Glomerular Filtration Rate
Serum and urinary creatinine (Cr) concentrations were measured using a colorimetric method (Hitachi Automatic Analyzer 7600, Hitachi, Tokyo, Japan). Urinary albumin was measured in random urine samples using a turbidimetric immunoassay (Hitachi Automatic Analyzer 7600, Hitachi, Tokyo, Japan). A detailed explanation of these assays as well as the validity and reproducibility of them in KNHANES V-2 have been provided elsewhere.12,14 The ratio of urinary albumin to urinary Cr was reported as ACR in milligrams per gram of Cr.12,14 Normoalbuminuria was defined as ACR < 30 mg/g Cr and albuminuria as ACR ≥ 30 mg/g Cr.14 The eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration formula: eGFR (mL/min/1.73 m2) = 141 × min (SCr/k, 1)a × max (SCr/k, 1)−1.209 × 0.993Age [1.018 if woman] × [1.159 if black] (SCr: serum creatinine [mg/dL], k: 0.7 for women and 0.9 for men, a: −0.329 for women and −0.411 for men, min: minimum of SCr/k or 1, and max: maximum of SCr/k or 1).15 Chronic kidney disease was defined as <60 mL/min per 1.73 m2 in this study.
Definition of Obesity and Sarcopenia
Body mass index (BMI, [kg/m2]) was calculated as weight (kg) divided by the square of height (m2). Obesity was defined according to the criteria recommended by the Korean Society for the Study of Obesity, defining normal as BMI ≥ 18.5 and <25.0 kg/m2 and obese as BMI ≥ 25.0 kg/m2.16 As previous described,17 a whole-body DXA was performed for each individual to obtain total and regional lean mass (kg) and total body fat (kg). Appendicular skeletal muscle mass (ASM [kg]) was calculated as the sum of the lean soft-tissue masses of the arms and legs, following the method of Heymsfield et al.18 Sarcopenia was defined as a skeletal muscle index (SMI) (SMI (%) = total ASM [kg]/weight [kg] × 100) of between 1 and 2 standard deviations (SD) (grade 1) or more than 2 SDs (grade 2) below the sex-specific mean for a younger reference group aged 19 to 39 years.19 Participants were classified as having a normal muscle mass (men >29.6%, women >22.8%), grade 1 sarcopenia (men 26.9%–29.6%, women 20.0%–22.8%), or grade 2 sarcopenia (men <26.9%, women <20.0%).
Health-related Behaviors
Regular exercise was categorized as “yes” when participants performed moderate exercise on a regular basis (exercising ≥20 minutes at a time ≥5 times a week). Alcohol intake was assessed by questions about drinking behavior during the month before the interview. Heavy alcohol drinking was defined as drinking 4 or more times per week. Smoking status was defined based on self-reported cigarette use: current smoker, exsmoker, and nonsmoker.
Statistical Analyses
Clinical and biochemical characteristics were presented according to presence and degree of sarcopenia (Table 1). Data were expressed as mean ± standard error of the mean or number (percentages). Differences in continuous variables between the 3 groups were evaluated using analysis of covariance (Table 1, Tables 2–4). The Bonferroni method was also used to determine significant differences between the 2 groups as a post hoc test. Persons with normoalbuminuria were further divided into tertiles, giving us 4 ordered categories of albuminuria (Table 3 and Table 5). The normoalbuminuric population was not neatly divisible into tertiles because of the large numbers within each 0.01-mg/g Cr increment of ACR. Multiple logistic regression analysis for the presence of albuminuria (Table 6) or grade 2 sarcopenia (Table 5) was performed using age, sex, diabetes, hypertension, CKD, and obesity as covariates. Two-tailed analyses were conducted, and P < 0.05 was deemed to indicate statistical significance. All statistical analyses were conducted using SPSS (ver. 21.0 for Windows, SPSS, Chicago, IL).
TABLE 1.
Age, Sex, and Age- and Sex-adjusted Clinical Characteristics of Participants by Skeletal Muscle Index
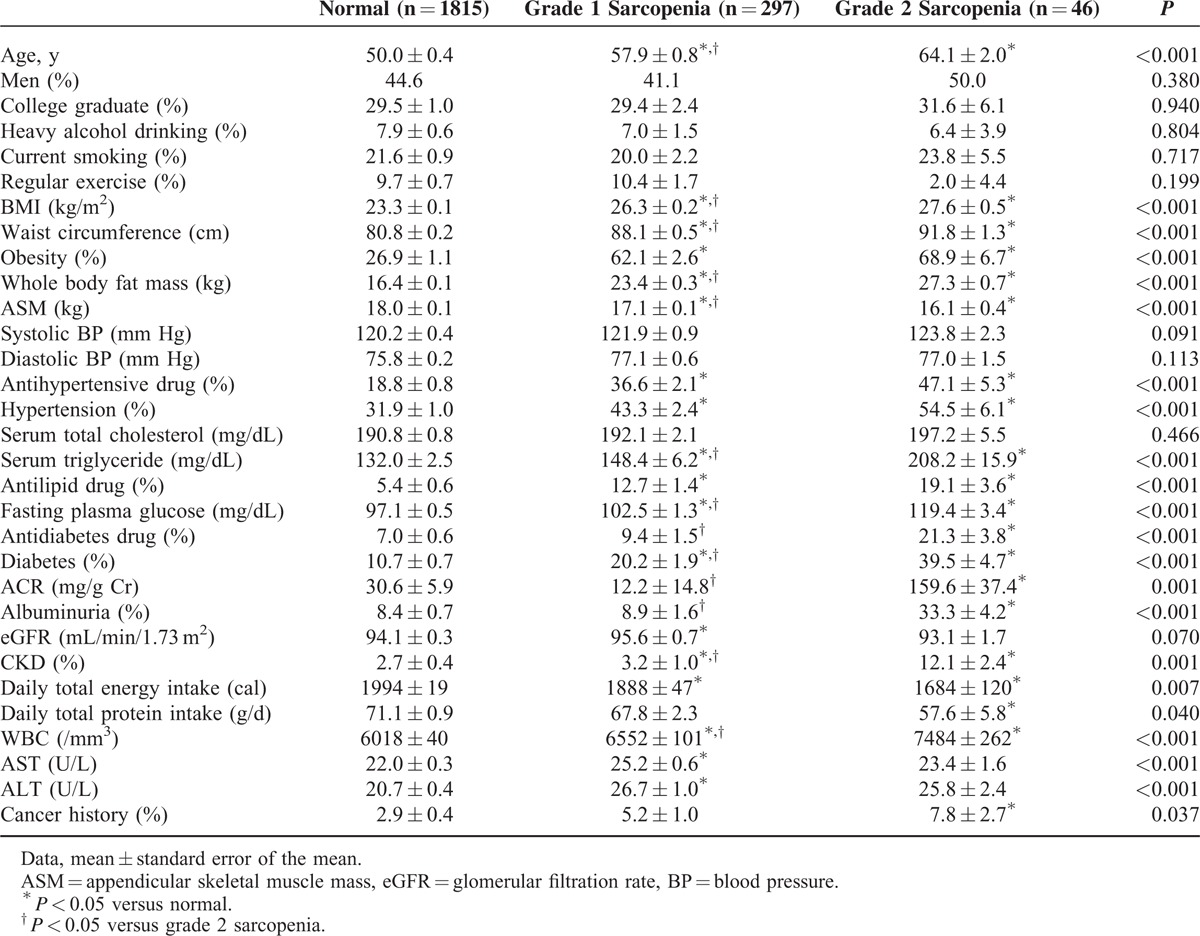
TABLE 2.
Prevalence of Albuminuria According to Skeletal Muscle Mass Index Classification
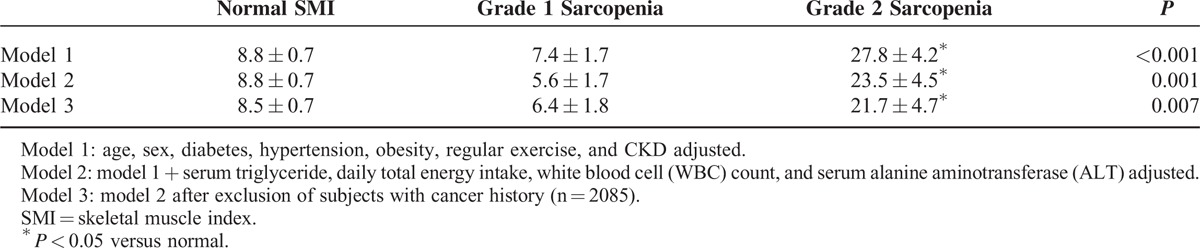
TABLE 4.
Prevalence of Albuminuria Among 4 Low Muscle Mass/Obesity Groups as Defined by Skeletal Muscle Index and Body Mass Index
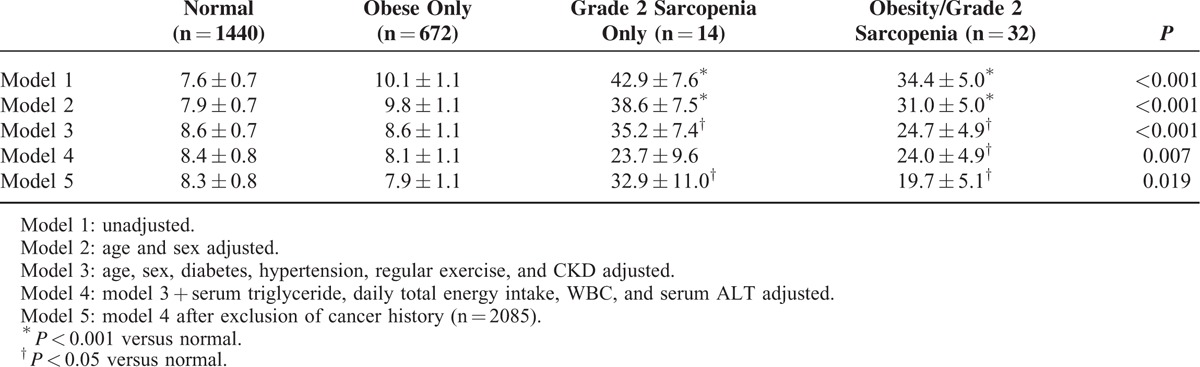
TABLE 3.
Prevalence of Grade 2 Low Muscle Mass by the Degree of Albuminuria
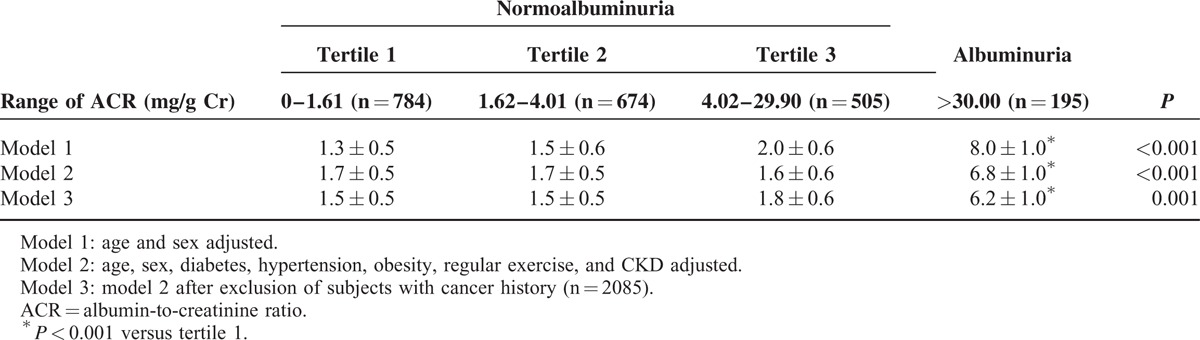
TABLE 5.
Logistic Regression Analysis for Grade 2 Sarcopenia
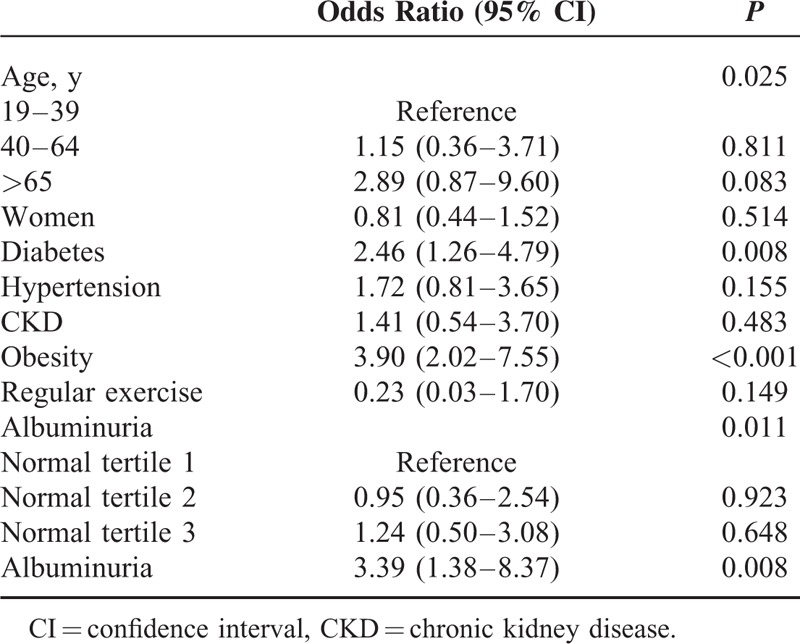
TABLE 6.
Logistic Regression Analysis for Albuminuria
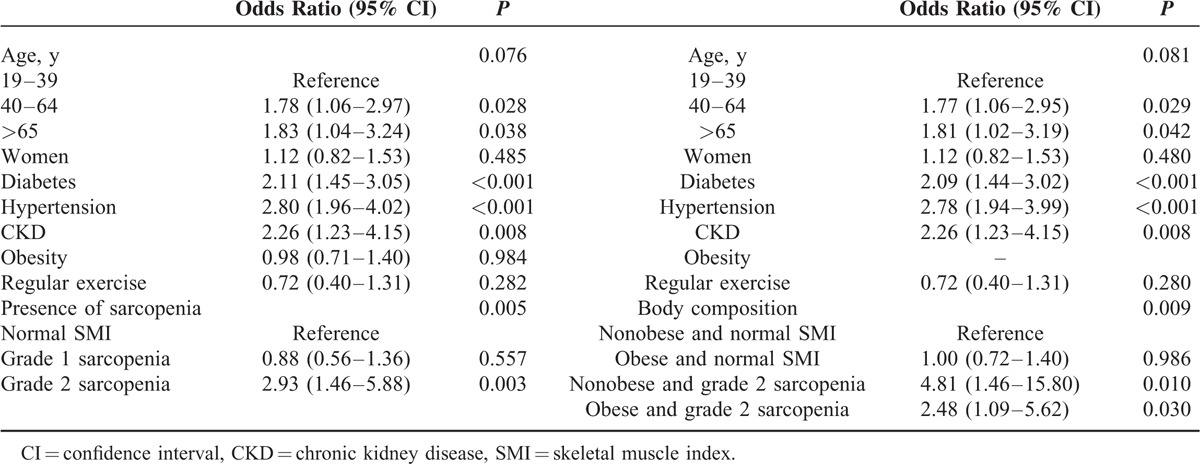
RESULTS
Demographic and Clinical Characteristics of the Study Population
The descriptive characteristics of the 2158 participants, categorized by SMI, are shown in Table 1. There were no significant differences across the 3 categories in sex, heavy alcohol drinking, current smoking, regular exercise, systolic and diastolic blood pressure, total cholesterol levels, and eGFR (Table 1). Subjects with normal SMI had lower BMI than those with grade 1 sarcopenia (P < 0.001), who in turn had lower BMI than those with grade 2 sarcopenia (P = 0.007). Subjects with normal SMI had higher ASM than those with grade 1 sarcopenia (P < 0.001), who in turn had slightly higher ASM than those with grade 2 sarcopenia (P = 0.013).
Prevalence of Albuminuria According to Skeletal Muscle Index Category
The prevalence of albuminuria was higher in those with grade 2 sarcopenia than in those with normal SMI and grade 1 sarcopenia (33.3% versus 8.4% and 8.9%; P < 0.001). Albuminuria was approximately 3 times as likely in the group with grade 2 sarcopenia as in the groups with normal and grade 1 sarcopenia even after adjusting for age, sex, diabetes, hypertension, obesity, serum triglyceride, daily total energy intake, white blood cell (WBC), and serum alanine aminotransferase (ALT) (Table 2).
Prevalence of Grade 2 Sarcopenia by the Degree of Albuminuria
Table 3 shows the prevalence of grade 2 sarcopenia according to the degree of albuminuria. Age- and sex-adjusted prevalences of grade 2 sarcopenia in the normoalbuminuric and albuminuric populations were 1.6% ± 0.6% and 8.0% ± 1.0%, respectively. There were no significant differences in the prevalence of grade 2 sarcopenia among the tertile groups in subjects with normoalbuminuria. Grade 2 sarcopenia was more prevalent in participants with albuminuria than those with the upper tertile of normoalbuminuria. Even with further adjustment for potential confounding factors, including age, sex, diabetes, hypertension, obesity, and CKD, the associations remained statistically significant (P < 0.001).
Prevalence of and Risk for Albuminuria According to Sarcopenia and/or Obesity Defined by Skeletal Muscle Index and Body Mass Index
The unadjusted prevalence of albuminuria according to the 4 sarcopenia/obesity groups defined by SMI and BMI were 7.6% in the normal group, 10.1% in the obesity-only group, 42.9% in the grade 2 sarcopenia-only group, and 34.4% in the obesity and grade 2 sarcopenia group. The prevalence of albuminuria was higher in both the grade 2 sarcopenia only and the combined obesity and grade 2 sarcopenia groups than in the normal group even after adjustment for age, sex, diabetes, hypertension, obesity, CKD, serum triglyceride, daily total energy intake, WBC, and ALT. The results remained significant after excluding data for subjects with cancer history (Table 4). The odds ratios (ORs) of albuminuria across the SMI tertile groups and the 4 groups based on obesity and sarcopenia were evaluated using multiple logistic regression analysis (Table 5). After adjusting for confounding variables, including age, sex, diabetes, hypertension, CKD, and obesity, the ORs for albuminuria in individuals with grade 2 sarcopenia were 2.93 (95% confidence interval [CI], 1.46–5.88) when compared with individuals with normal SMI. Albuminuria was more prevalent in the grade 2 sarcopenia group than the normal group regardless of the presence of obesity.
Multiple Logistic Regression Analysis for Grade 2 Low Muscle Mass Across the 4 Groups Based on Albuminuria Status
Multivariate logistic regression analysis to determine the predictors of grade 2 sarcopenia in the 4 groups (normoalbuminuric tertile groups and albuminuric group) is shown in Table 6. Data revealed that presence of diabetes (OR 2.46, 95% CI, 1.26−4.79), obesity (OR 3.89, 95% CI 2.01−7.53), and albuminuria (OR 3.41, 95% CI, 1.38−8.41) had independently significant associations with grade 2 sarcopenia. There, however, was no significant difference in risk of grade 2 sarcopenia among the normoalbuminuric tertile groups.
DISCUSSION
This nationally representative, population-based study demonstrates a higher risk of albuminuria in individuals with grade 2 sarcopenia compared with those with normal SMI. Moreover, individuals with combined obesity and grade 2 sarcopenia are more than 2 times more likely to have albuminuria than nonsarcopenic and nonobese subjects, whereas there is no significant difference in risk of albuminuria between the nonsarcopenic obese group and the nonobese group with normal mass. Furthermore, although association between low-grade albuminuria within the normal range and grade 2 sarcopenia was not significant, albuminuria is independently associated with grade 2 sarcopenia. Unlikely grade 2 sarcopenia, grade 1 sarcopenia was not independently associated with an increased likelihood of albuminuria in this study. These results suggest that modest reductions in skeletal muscle mass with aging do not cause albuminuria. If muscle loss, however, progresses to the point where the skeletal muscle mass relative to body weight is 2 SDs below the mean for young adults, there is an increased likelihood that albuminuria will occur.
The positive relationships of albuminuria with central obesity, metabolic syndrome and CVD have been well elucidated,10,20,21 but it was not until recently that the association of albuminuria with sarcopenia and sarcopenic obesity was paid much attention. Although studies showed that both low muscle mass and albuminuria are associated with CVD, most prior studies on the association between low muscle mass and albuminuria showed greater concern over the tendency of low muscle mass to bias urine ACR to higher levels than the investigation of interconnected relationships between the 2 disorders. These studies demonstrated that urine Cr excretion rate is highly and positively correlated with muscle mass,22,23 and that ACR tended to be underestimated in severely obese (BMI > 35 kg/m2) individuals as a consequence of the large creatininuria that is proportional to total body skeletal muscle mass.24 By contrast, among community-living individuals with 24-hour urinary albumin excretion (24-hour UAE) of less than 30 mg/d, ACR is higher in women and older people than could be explained by lower body weight alone, independently of 24-hour UAE.25 In the current study, sex and obesity, however, were not independently associated with albuminuria. Our study showed that ACR within the normal range is not associated with grade 2 sarcopenia. In addition, because there are very few severely obese individuals in this study, albuminuria may be a more important factor in the association of ACR with presence of grade 2 sarcopenia than urinary Cr. Furthermore, lower levels of timed urine Cr excretion rate and muscle mass are known to be related to CVD and total mortality.25,26 Therefore, ACR might be more strongly associated with sarcopenia-related metabolic disorders, such as metabolic syndrome, type 2 diabetes, and CVD than is 24-hour UAE.
The pathophysiological mechanisms that result in albuminuria and sarcopenia are considered multifactorial. In this context, the common underlying mechanisms, including endothelial dysfunction, inflammation, insulin resistance, and renin–angiotensin–aldosterone system (RAAS) activation between albuminuria and sarcopenia, could largely explain why albuminuria might be an independent indicator for sarcopenia. Endothelial dysfunction plays a crucial role in the development of albuminuria with a shift toward reduced vascular relaxation and inflammatory cell infiltration and slight inflammation in blood vessels.27 In addition, recent evidence has emerged suggesting that endothelial dysfunction and inflammation of muscle protein metabolism may considerably contribute to the initiation and progression of sarcopenia.28 The current study has reported that individuals with sarcopenia had increased white blood cell counts, a marker of inflammation, compared with those without sarcopenia. Moreover, both endothelial dysfunction and inflammation have been reported to increase with advancing age, which is a major risk factor for sarcopenia as well as CVD.29,30 Age-related increases in inflammatory cytokines can disrupt endothelial-dependent dilation by disturbing cell-to-cell communication through gap junctions. Although inflammation exacerbates endothelial dysfunction, elevated endothelin-1 and decreased nitric oxide, causes of impaired endothelial-dependent vasodilation, can lead to increased leukocyte–endothelium interaction, potentially increasing inflammation.28 Therefore, endothelial dysfunction and inflammation may be a promising mechanism linking CKD, sarcopenia, and CVD.
Skeletal muscle is the major tissue responsible for insulin-mediated glucose disposal in humans. Therefore, the loss of skeletal muscle as the largest insulin-sensitive tissue might cause insulin resistance, which promotes albuminuria and CVD. Various mechanisms may hypothetically mediate the association of insulin resistance and albuminuria. Podocytes of the glomerular filtration barrier are known insulin-sensitive cells. In animal studies, insulin resistance of podocytes increases their susceptibility to cell death and may contribute to albuminuria.31 Insulin resistance even in nondiabetic states is known to induce glomerular hyperfiltration, endothelial dysfunction, and increased vascular permeability, which all lead to albuminuria.32,33 Studies on patients with type 2 diabetes have also suggested that insulin resistance is independently associated with microalbuminuria.34 Although indices of insulin resistance were not measured in this study, the presences of hypertension and diabetes, as insulin-resistance clinical phenotypes, are independently associated with albuminuria. Moreover, presence of diabetes is also significantly associated with sarcopenia, which suggests that insulin resistance may be an important underlying factor associated with both sarcopenia and albuminuria.
In addition to endothelial dysfunction, inflammation and insulin resistance, the RAAS system is also believed to play a role in the link between sarcopenia and albuminuria. Renin–angiotensin–aldosterone system is locally expressed in tissue. From the view of sarcopenia, angiotensin-converting enzyme (ACE) is present on the membrane of vascular endothelial cells in muscle as well as in blood.35,36 Over half of the angiotensin II in the venous drainage of skeletal muscle is because of local synthesis and this angiotensin II might be more important for vasoconstriction than circulating angiotensin II.37 Furthermore, ACE inhibitors are known to ameliorate endothelial dysfunction, improve skeletal muscle blood flow, and enhance glucose uptake by skeletal muscle, suggesting that altering the RAAS may counter sarcopenia.36,38 On the contrary, although little is known about the mechanisms causing the reduced albuminuria by ACE inhibitor, the antialbuminuric effect of ACE inhibitors is relatively well established. In this context, ACE inhibitors might be mediating direct effects on both sarcopenia and albuminuria. Taken together, these mechanisms, such as endothelial dysfunction, inflammation, insulin resistance, and RAAS activation enhance our understanding of a close relationship between sarcopenia and albuminuria.
There are several limitations to this study. First, its cross-sectional study design did not allow us to identify causal relationships between albuminuria and sarcopenia. Our study, however, suggested that albuminuria could be the cause of sarcopenia or vice versa based on our results and the shared mechanisms of albuminuria and sarcopenia. Second, a consensus of standardized diagnostic criteria for sarcopenia has not been entirely established, and potentially relevant muscle quality measurements, such as voluntary muscle strength and muscle fiber size and number, were not performed as part of this study. Finally, only a single urine ACR result was used in the current study, which might result in misleading classifications of albuminuria. Repeated or direct measurement of these parameters, however, is costly in time and money and is therefore highly impracticable and almost impossible in nationwide studies.
On the contrary, the current study had several strengths. First, this was a large population-based analysis using well-examined national data, which strengthens the statistical reliability of the results and generalizability of the data. Furthermore, we assessed muscle mass using DXA, which enabled us to more precisely evaluate individuals with sarcopenia.
In conclusion, we showed that individuals with sarcopenia had an increased risk of albuminuria and vice versa after adjusting for confounding factors, including age, sex, and obesity-related metabolic disease, such as diabetes, hypertension, and CKD. The current study may be a stimulant to provoke further research about the novel relationship between sarcopenia and albuminuria.
.
Footnotes
Abbreviations: ACE = angiotensin-converting enzyme, ACR = albumin-to-creatinine ratio, ALT = serum alanine aminotransferase, ANCOVA = analysis of covariance, ASM = appendicular skeletal muscle mass, BMI = body mass index, BP = blood pressure, CI = confidence interval, CKD = chronic kidney disease, CVD = cardiovascular disease, DXA = dual-energy x-ray absorptiometry, eGFR = estimation of glomerular filtration rate, KNHANES = Korea National Health and Nutrition Examination Survey, KSSO = Korean Society for the Study of Obesity, OR = odds ratio, RAAS = renin–angiotensin–aldosterone system, SCr = serum creatinine, SD = standard deviation, SEM = standard error of the mean, SMI = skeletal muscle index, WBC = white blood cell.
This study was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF), and funded by the Ministry of Education, Science, and Technology (2010–0020224). The Inje Research and Scholarship Foundation also supported this work in 2014
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Yuyun MF, Khaw KT, Luben R, et al. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol 2004; 33:189–198. [DOI] [PubMed] [Google Scholar]
- 2.Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis 2013; 7:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med 1998; 338:645–652. [DOI] [PubMed] [Google Scholar]
- 4.Erdmann E. Microalbuminuria as a marker of cardiovascular risk in patients with type 2 diabetes. Int J Cardiol 2006; 107:147–153. [DOI] [PubMed] [Google Scholar]
- 5.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008; 9:213–228. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki H, Kasagi F, Yamada M, et al. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med 2007; 120:337–342. [DOI] [PubMed] [Google Scholar]
- 7.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem 2015; 116:1171–1178. [DOI] [PubMed] [Google Scholar]
- 8.Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010; 33:1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoenes M, Reil JC, Khan BV, et al. Abdominal obesity is associated with microalbuminuria and an elevated cardiovascular risk profile in patients with hypertension. Vasc Health Risk Manag 2009; 5:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandie Shaw PK, Berger SP, Mallat M, et al. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care 2007; 30:1840–1844. [DOI] [PubMed] [Google Scholar]
- 11.Tamba S, Nakatsuji H, Kishida K, et al. Relationship between visceral fat accumulation and urinary albumin-creatinine ratio in middle-aged Japanese men. Atherosclerosis 2010; 211:601–605. [DOI] [PubMed] [Google Scholar]
- 12.Won JC, Lee YJ, Kim JM, et al. Prevalence of and factors associated with albuminuria in the Korean adult population: the 2011 Korea National Health and Nutrition Examination Survey. PLoS One 2013; 8:e83273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y. The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiol Health 2014; 36:e2014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33:1004–1010. [DOI] [PubMed] [Google Scholar]
- 15.Liao LN, Li CI, Liu CS, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan Diabetes Study. PLoS One 2015; 10:e0130828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J 2011; 35:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TN, Park MS, Kim YJ, et al. Association of low muscle mass and combined low muscle mass and visceral obesity with low cardiorespiratory fitness. PLoS One 2014; 9:e100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 1990; 52:214–218. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002; 50:889–896. [DOI] [PubMed] [Google Scholar]
- 20.Hao Z, Konta T, Takasaki S, et al. The association between microalbuminuria and metabolic syndrome in the general population in Japan: the Takahata study. Intern Med 2007; 46:341–346. [DOI] [PubMed] [Google Scholar]
- 21.Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 2001; 249:519–526. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZM, Gallagher D, Nelson ME, et al. Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr 1996; 63:863–869. [DOI] [PubMed] [Google Scholar]
- 23.Welle S, Thornton C, Totterman S, et al. Utility of creatinine excretion in body-composition studies of healthy men and women older than 60 y. Am J Clin Nutr 1996; 63:151–156. [DOI] [PubMed] [Google Scholar]
- 24.Guidone C, Gniuli D, Castagneto-Gissey L, et al. Underestimation of urinary albumin to creatinine ratio in morbidly obese subjects due to high urinary creatinine excretion. Clin Nutr 2012; 31:212–216. [DOI] [PubMed] [Google Scholar]
- 25.Carter CE, Gansevoort RT, Scheven L, et al. Influence of urine creatinine on the relationship between the albumin-to-creatinine ratio and cardiovascular events. Clin J Am Soc Nephrol 2012; 7:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ix JH, de Boer IH, Wassel CL, et al. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation 2010; 121:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh M. Endothelial dysfunction as an underlying pathophysiological condition of chronic kidney disease. Clin Exp Nephrol 2012; 16:518–521. [DOI] [PubMed] [Google Scholar]
- 28.Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis 2013; 23:S44–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther 2012; 133:159–176. [DOI] [PubMed] [Google Scholar]
- 30.Bruunsgaard H, Pedersen AN, Schroll M, et al. Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol 2001; 37:127–136. [DOI] [PubMed] [Google Scholar]
- 31.Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int 2008; 73:1385–1393. [DOI] [PubMed] [Google Scholar]
- 32.De Cosmo S, Menzaghi C, Prudente S, et al. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 2013; 28:29–36. [DOI] [PubMed] [Google Scholar]
- 33.Groop PH, Forsblom C, Thomas MC. Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 2005; 1:100–110. [DOI] [PubMed] [Google Scholar]
- 34.Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 2006; 55:1456–1462. [DOI] [PubMed] [Google Scholar]
- 35.Schaufelberger M, Drexler H, Schieffer E, et al. Angiotensin-converting enzyme gene expression in skeletal muscle in patients with chronic heart failure. J Card Fail 1998; 4:185–191. [DOI] [PubMed] [Google Scholar]
- 36.Sumukadas D, Struthers AD, McMurdo ME. Sarcopenia: a potential target for angiotensin-converting enzyme inhibition? Gerontology 2006; 52:237–242. [DOI] [PubMed] [Google Scholar]
- 37.Saris JJ, van Dijk MA, Kroon I, et al. Functional importance of angiotensin-converting enzyme-dependent in situ angiotensin II generation in the human forearm. Hypertension 2000; 35:764–768. [DOI] [PubMed] [Google Scholar]
- 38.Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol 2003; 196:171–179. [DOI] [PubMed] [Google Scholar]


