Abstract
We evaluated visual outcomes, changes of maximum macular thickness (MMT) and subretinal fluid (SRF), and safety in patients with chronic central serous chorioretinopathy (CSC) after treatment with selective retina therapy (SRT). Retrospective cohort study of patients with chronic CSC presenting to a university-based hospital from January 2014 through January 2015 was conducted. A total of 12 eyes of 12 patients with chronic CSC lasting for at least 3 months was recruited. The follow-up period ranged from 3 to 12 months. Following evaluation of test spots at temporal arcades, SRT (Q-switched neodymium-doped yttrium lithium fluoride [Nd:YLF] laser; wavelength, 527 nm, pulse duration, 1.7 microsececond) was applied to the surrounding areas of leakage observed on fluorescein angiogram and/or pigment epithelial detachment (PED). Changes in best-correct visual acuity (BCVA), MMT, and SRF and macular sensitivity (MS) by microperimetry (MP) were evaluated. Eyes received treatment in a mean of 3.83 spots at the pulse energy of 65 to 90 μJ. Mean BCVA (logMAR) improved from 0.23 ± 0.12 at baseline to 0.14 ± 0.13 at 3 months. MMT decreased from 341.4 ± 85.5 μm at baseline to 236.0 ± 57.9 μm at 3 months. SRF completely resolved in 75% (9 eyes) at 3 months. Large PEDs (2 eyes) were flattened at 3 months. Retreatment was performed in 4 eyes. MP showed no evidence of scotoma around SRT-treated lesions. SRT treatment targeting the surrounding area of leakage point showed favorable visual and structural outcomes in chronic CSC patients without the risk of scotoma.
INTRODUCTION
Central serous chorioretinopathy (CSC) is characterized by an idiopathic serous detachment of the neurosensory retina with leakage of fluid through the retinal pigment epithelium (RPE) into the subretinal space, with or without concomitant pigment epithelial detachment (PED).1 Dysfunction of RPE cells plays a significant role in the development of subretinal fluid (SRF) and PED.2,3 The etiology and pathophysiologic mechanisms of CSC remain poorly understood, although increased tissue hydrostatic pressure in the choroid overwhelms the barrier function of the RPE and leads to SRF accumulation.4
Common treatment modalities include conventional laser, photodynamic therapy (PDT), and intravitreal bevacizumab injection. Application of thermal burns by conventional laser can promote the resolution of SRF, but put the patient at risk for central or paracentral scotoma, loss of contrast sensitivity, inadvertent foveal damage, and choroidal neovascularization.1 PDT has been reported to be an effective treatment option for chronic CSC. Although good anatomic and visual results were reported, it is costly and can cause complications, such as progressive RPE atrophy and choroidal ischemia.5 Small case series have reported potential benefits of intravitreal injection of bevacizumab.6,7 However, the role of bevacizumab is still controversial.
Retina-sparing photocoagulation such as subthreshold micropulse laser (SDM)8,9 and selective retina therapy (SRT)10,11 showed a favorable effect on CSC in several clinical studies. SRT was developed to minimize damage to surrounding tissues by laser therapy and retain the therapeutic effect of it. SRT selectively damages RPE cells without thermally damaging the neural retina.10–16 Thus SRT can target focal RPE leaks. This selective damage is achieved by the application of microsecond laser pulses. The delivered pulse energy is primarily absorbed by intracellular melanosomes of RPE cells. At appropriate energy levels and pulse duration, SRT can selectively damage these cells by microvaporization, which triggers mechanical disruption of the RPE cells. Then, SRT showed therapeutic effect by stimulating RPE cell migration and proliferation into irradiated areas to improve metabolism at disease affected locations.17
Here we report our experience with application of SRT adjacent to points of leakage and surrounding areas of PED rather than direct targeting method in previous studies.11,12 Our aim was to determine if this application pattern of SRT is a safe and effective treatment for chronic CSC.
METHODS
Study Population
We retrospectively reviewed the medical records of 21 symptomatic chronic CSC patients treated with SRT from January 2014 through January 2015. Data collection was approved by the local institutional review board. Patient's eyes were included if they had a diagnosis of chronic CSC. Inclusion criteria were as follows: presence of SRF involving the fovea for a period of 3 months or longer in optical coherence tomography (OCT) images; presence of CSC-induced leakage on fundus fluorescein angiography (FFA); and availability of 3 months of ophthalmic data following SRT treatment. Eyes with serous retinal detachment unrelated to CSC were excluded. Eyes with previous PDT or conventional laser photocoagulation for CSC were excluded. However, eyes that had received prior treatment with intravitreal injection of bevacizumab were included, if prior treatment had occurred 10 weeks before SRT. Twelve patients of 21 patients were included for the study based on the suggested inclusion and exclusion criteria.
Outcome Measures
At baseline, all patients had undergone a complete ophthalmologic examination, including best-corrected visual acuity (BCVA), intraocular pressure, slit-lamp biomicroscopy, color fundus photography (CF-60Uvi; Canon Inc., Utsunomiya, Japan) fundus autofluorescence (FAF) (Heidelberg Retina Angiograph 2 [HRA2]; Heidelberg Engineering, Heidelberg, Germany), fundus fluorescein angiography (FFA) (HRA2), and spectral-domain OCT (Cirrus, Carl Zeiss Meditec, Dublin, CA). Every patient was followed-up monthly after SRT treatment. At each visit, patients were asked to report any suspected adverse effects associated with SRT. Visual acuity was measured with a Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR). Maximum macular thickness (MMT) and the presence of SRF were evaluated by macular cube scan protocol (an area of 6 × 6 mm2), and subfoveal choroidal thickness (maximum distance between the outer neurosensory retina and RPE) was measured by enhanced depth imaging (EDI) mode. Fundus photography and FAF were conducted in all patients at each visit. FFA was repeatedly performed for treatment when SRF was maintained or increased during follow-up. Microperimetry (MP) (MAIA; CenterVue, Padova, Italy) was performed after a resolution of SRF to assess whether SRT could cause scotoma because the markedly decreased macular sensitivity (MS) due to SRF lessens the discrimination of scotoma. MP measured the MS within a central point, and within three concentric rings representing 2°, 6° and 10°, with 12 test points each, using a standard grid (37 or 68 stimuli) as previously described.17 Main outcome measures included changes in BCVA and MMT, and MS over the SRT-treated lesions.
SRT Procedure
Laser treatments were performed with a Q-switched neodymium-doped yttrium lithium fluoride [Nd:YLF] laser system (Medical Laser Center Lübeck GmbH, Lübeck, Germany) emitting at a wavelength of 527 nm. The SRT laser uses multiple short laser pulses (30 pulses per spot; pulse duration, 1.7 microsececond; pulse repetition rate, 100 Hz). While SDM lesions are invisible on FFA, SRT spots can be visualized by FFA. The threshold for selective RPE damage can vary between patients and even within regions of the same eye. Therefore, before each SRT treatment, 3 to 10 preliminary test spots were applied away from the macular region and planned treatment area, at the superior or inferior temporal arcade vessels (from 65 to 120 μJ), in order to determine the individually optimal pulse energy for selective RPE damage (Figure 1). Following the test spots, FFA was performed to determine the correlation between the pulse energy and the visibility of the laser spot, determining the required pulse energy for treatment. The minimum pulse energy that allows visible spots on FFA was chosen as the desired treatment energy because of invisibility of SRT spots on ophthalmoscopy. SRT spots were applied immediately adjacent to the sites of foveal leakage or circumferentially around areas of PED, rather than directly targeting the leaking point, or confluent treatment over the area of PED. For treatment of leaks that were present very close to the foveal avascular zone, SRT spots were irradiated in a location up to 300 μm from the center of the fovea.
FIGURE 1.
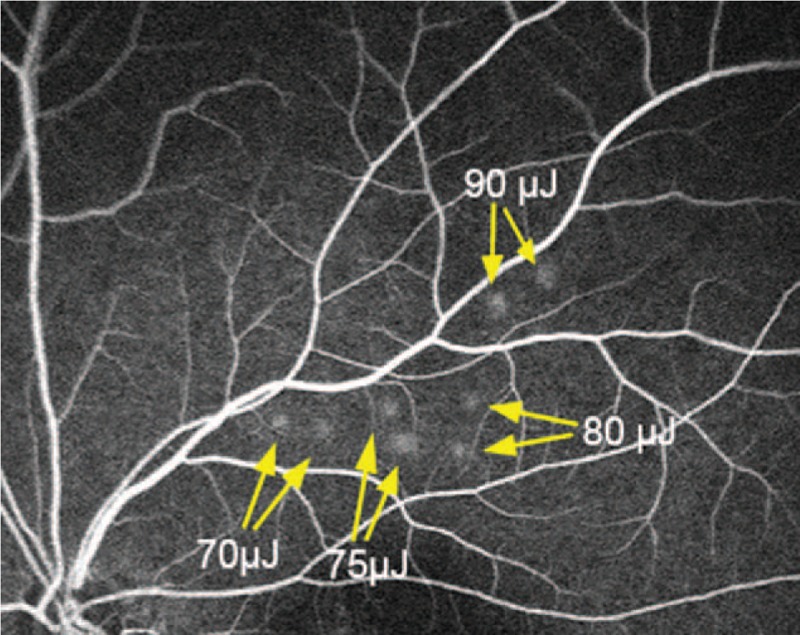
Representative image of fundus fluorescein angiography showing 8 test spots. Each pulse energy with 70, 75, 80, and 90 μJ was tested in duplicate.
Dosimetry
Since SRT spots are not discernable as grey or white lesions during irradiation, both optoacoustic and reflectometric dosimetry could be used as SRT endpoint references. Briefly, optoacoustic method detects microbubble-related pressure transients from the damaged RPE.18–20 On the other hand, reflectometry detects signals of the back-scattered light, providing an optical feedback value (OFV). The endpoint of SRT is selective RPE cell damage, produced by laser-induced intracellular microbubble formation. Overtreatment can be indicated by high OFV with large bubbles, while the absence of microbubbles demonstrates undertreatment.20,21 Although the dosimetry values were not used as treatment endpoints in this study, 2 dosimetry values of test spots were analyzed in association with therapeutic endpoints including ophthalmoscopically invisible and FFA visible lesions. We evaluated 73 test spots (from 65 to 120 μJ) of 12 patients to optimize the dosimetry algorithm. We did not include treatment spots (from 65 to 90 μJ) in the analysis because the visibility on FFA could be affected by SRF. In this study, while 68 of 73 test spots showed hyperfluorescence on FFA, no overtreatment was observed in any of the SRT spots. Since 5 of 73 test spots were negative on FFA, we did not consider its energies for treatments (Figure 2).
FIGURE 2.
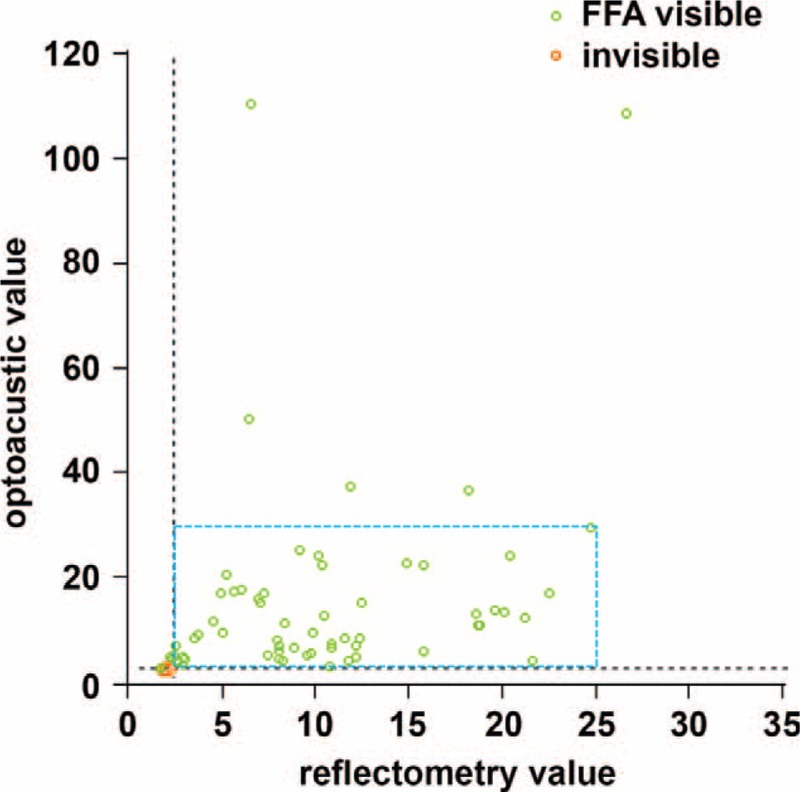
Scatter plot of reflectometry and optoacustic dosimetry demonstrating the value of each test spot. In this study, 68 of 73 test spots showed hyperfluorescence on fundus fluorescein angiography (FFA) and no overtreatment was observed in any of the selective retina therapy (SRT) spots. Spots without cell damage (FFA invisible; red circles) and selective damaged retinal pigment epithelium cells (FFA visible; green circles) are shown. The energy of treatment spots was chosen among FFA-positive spots within the range of the blue rectangle.
Statistical Methods
A probability value of 0.05 was considered to be statistically significant in the statistical analysis of the outcome measures. All values are presented as mean ± standard deviation (SD). The Wilcoxon signed rank test was performed to test the differences of mean BCVA, MMT, SRF, and subfoveal choroidal thickness. Statistical software (SPSS 18.0 software; SPSS, Inc., Chicago, IL) was used for statistical analyses.
RESULTS
Patient Demographics
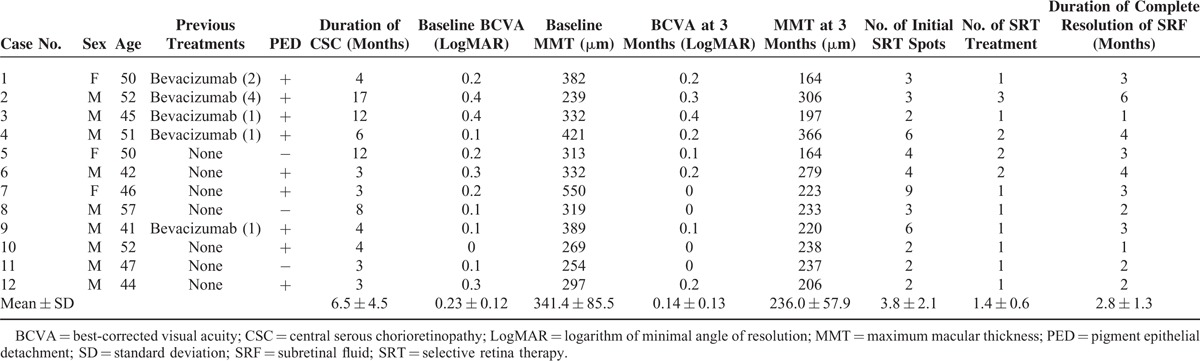
The medical records of 12 patients (12 eyes) with symptomatic chronic CSC were reviewed. The mean age was 48.9 ± 5.4 years (range, 41–59 years). Nine of 12 patients were male, and 3 were female. All patients had experienced symptoms of CSC due to SRF for at least 3 months. The average duration of CSC was 7.5 ± 5.7 months (range, 3–19 months). Five eyes had received prior intravitreal injection of bevacizumab. Nine eyes (75%) had PED, of which 7 eyes had small PED. The location of leaks was within 500 μm from the center of the fovea in 11 eyes (91.6%). The follow-up period of 12 eyes ranged from 3 to 12 months. The baseline characteristics of the study subjects are shown in Table 1.
TABLE 1.
Characteristics of 12 Patients With Chronic Central Serous Chorioretinopathy
Visual Acuity
The mean BCVA (logMAR) of 12 eyes was improved from 0.23 ± 0.12 (range, 0.1–0.4) at baseline to 0.15 ± 0.11 at 1 month (P = 0.058), 0.14 ± 0.09 at 2 months (P = 0.035), and 0.14 ± 0.13 at 3 months (P = 0.035) (Figure 3A). Overall, 11 eyes (91.6%) showed either improvement (7 eyes) or stationary (4 eyes) in BCVA at 3 months. One of 12 eyes showed decreased BCVA at 3 months compared to baseline. However, after retreatment at month 3, BCVA recovered to baseline level at 4 months. Changes in BCVA from baseline to month 3 are shown in Figure 3B.
FIGURE 3.
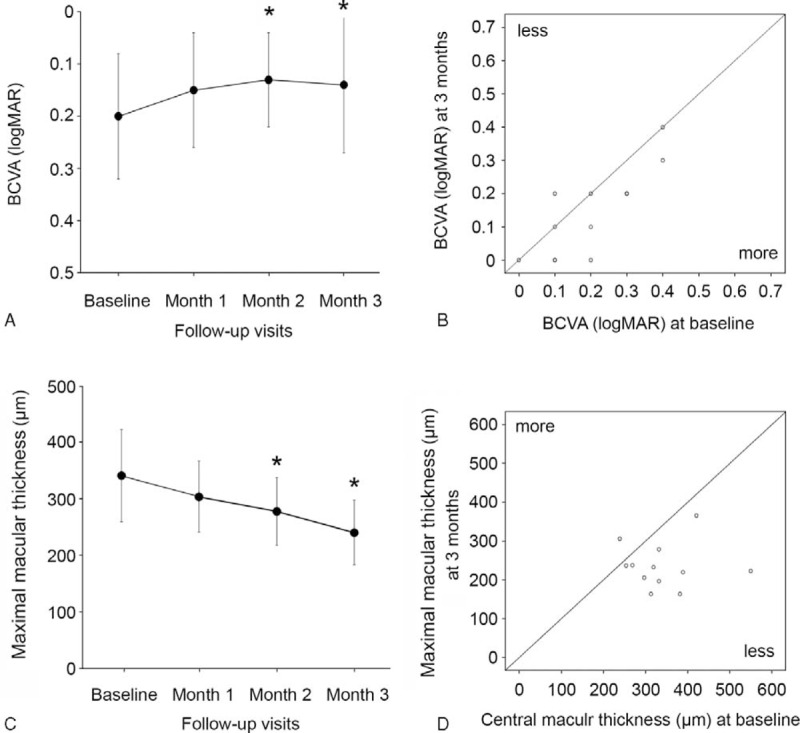
(A) Change of best-correct visual acuity (BCVA) (logMAR) in selective retina therapy (SRT)-treated patients with chronic central serous chorioretinopathy (CSC). Error bars represent standard deviation (SD). The difference of BCVA (logMAR) was statistically significant at 2 and 3 months after SRT. ∗P < 0.05. (B) Scatter plot of BCVA (logMAR) of patients at baseline and at 3 months. (C) Change of maximum macular thickness (MMT) measured by optical coherence tomography (OCT) in SRT-treated patients with chronic CSC. Error bars represent SD. The difference of MMT was statistically significant at 2 and 3 months after SRT. ∗P < 0.05. (D) Scatter plot of MMT of patients at baseline and at 3 months.
MMT and Resolution of SRF/PED
The mean MMT improved from 341.4 ± 85.5 μm before SRT treatment to 298.5 ± 69.2 μm at 1 month (P = 0.192), 277.7 ± 59.7 μm at 2 months (P = 0.048), and 236.0 ± 57.9 μm at 3 months after SRT (P = 0.008) (Figure 3C). Changes in MMT from baseline to month 3 are shown in Figure 3D. SRF was completely resolved in all 12 eyes. Among 12 eyes, the complete resolution of SRF periodically occurred in 2 eyes by month 1, 5 eyes by month 2, 9 eyes by month 3, 11 eyes by month 4, and 12 eyes by month 6 after SRT treatment (Figure 4). The mean SRF thickness decreased from 205.7 ± 145.5 μm before SRT treatment to 115.1 ± 78.9 μm at 1 month (P = 0.071), 73.0 ± 76.5 μm at 2 months (P = 0.011), and 30.0 ± 54.8 μm at 3 months after SRT (P = 0.001) (Figure 4). Of the 9 eyes with PED, 4 eyes had PED as the cause of leakage in FFA. After PED was treated circumferentially with SRT, PED disappeared in 2 eyes (Figures 5 and 6) and decreased in size in the other 2 eyes.
FIGURE 4.
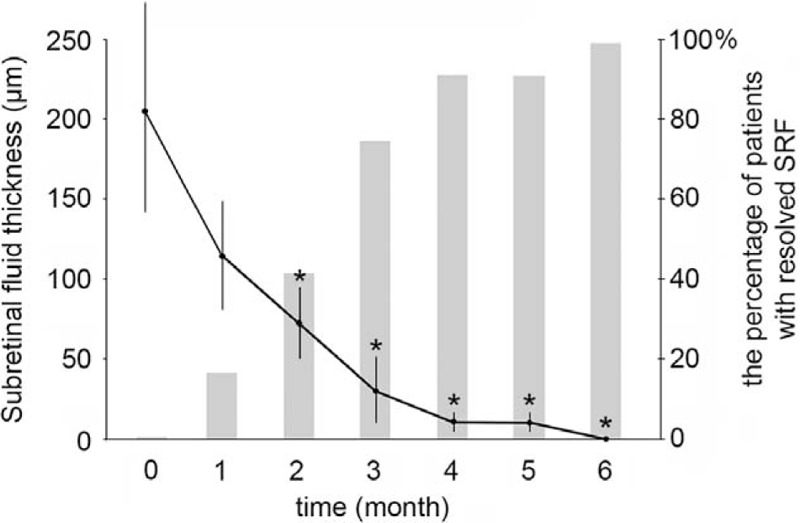
Changes of subretinal fluid (SRF) thickness (left axis) and the percentage of patient with completely resolved SRF (right axis) in SRT-treated patients with chronic central serous chorioretinopathy (CSC). Error bars represent SD. ∗P < 0.05.
FIGURE 5.
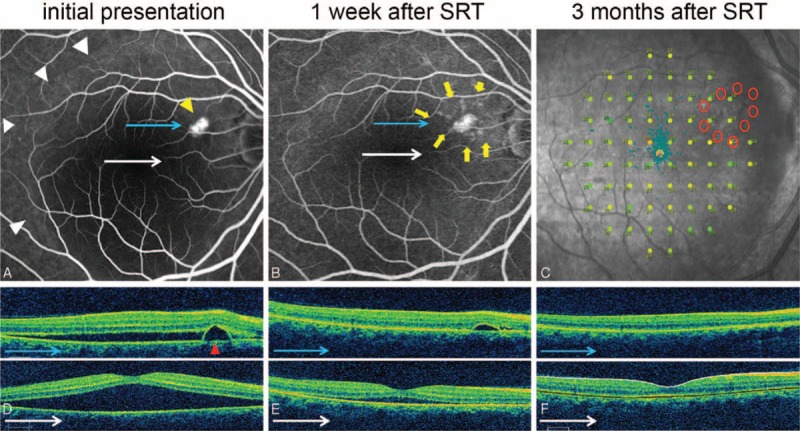
Case 7: A 46-year-old woman presented with a 3-month history of distortion of central vision in the right eye. Her right best-correct visual acuity (BCVA) was 20/32. Selective retina therapy (SRT) was applied to the area surrounding the pigment epithelial detachment (PED). Subretinal fluid (SRF) resolved markedly and PED was flattened prominently in 1 week. Both SRF and PED disappeared within 3 months. The BCVA improved to 20/20 in her right eye. (A) Large SRF (white arrowheads) with PED (yellow arrowhead) was observed on fundus fluorescein angiography (FFA) at baseline. (B) FFA demonstrated nine SRT laser spots (yellow arrows) surrounding PED at 1 week after SRT treatment. (C) Microperimetry (MP) performed 4 months after SRT treatment showed no significant decrease or scotoma change at SRT-treated regions. (D) Baseline optical coherence tomography (OCT) showed PED (red arrowhead) and large SRF. (E) PED and SRF were rapidly decreased at 1 week after SRT treatment. (F) The SRF and PED were completely resolved at 3 months after SRT.
FIGURE 6.
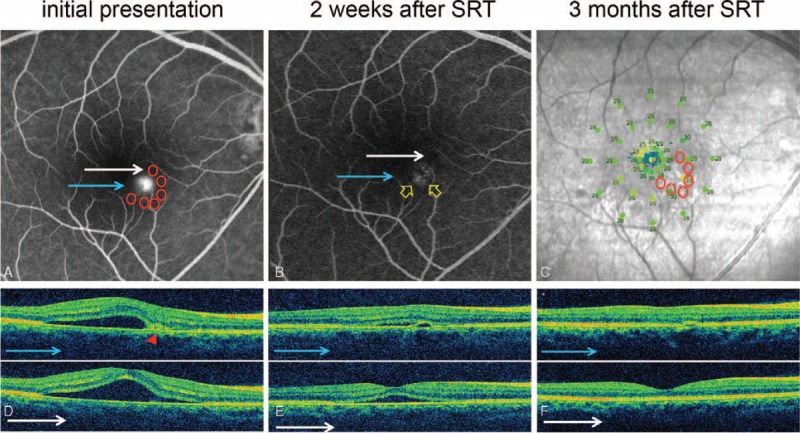
Case 9: A 41-year-old man presented with a 4-month history of central blur in the right eye. The best-correct visual acuity (BCVA) was 20/25. Previous 3 times of intravitreal injection of bevacizumab did not show any improvement. Selective retina therapy (SRT) was applied to the area surrounding the leaking point. Although both subretinal fluid (SRF) and pigment epithelial detachment (PED) disappeared completely, his BCVA remained unchanged. (A) Active leaking at PED was observed on fundus fluorescein angiography (FFA). Six arch-shaped SRT treatment spots (6 red circles) were applied around the leaking point. (B) No active leakage was found at PED (yellow arrow) on FFA. (C) Microperimetry (MP) demonstrated no scotoma on macular sensitivity. (D) Optical coherence tomography (OCT) showed PED (red arrowhead) and SRF at macular region at baseline. (E) OCT showed rapid resolution of SRF and PED 2 weeks after SRT treatment. (F) SRF completely disappeared and PED was nearly flattened at 3 months after SRT treatment.
Subfoveal Choroidal Thickness
SRT did not cause the change of subfoveal choroidal thickness. The mean subfoveal choroidal thickness was 351.9 ± 33.9 μm before SRT treatment, 352.1 ± 33.2 μm at 1 month (P = 0.986), 351.8. ± 33.2 μm at 2 months (P = 0.995), and 356.7 ± 36.4 μm at 3 months after SRT (P = 0.740).
SRT Retreatment
A single SRT treatment was enough to resolve chronic SRF in 8 eyes (66.7%). Eyes in this series were eligible for SRT retreatment if they had no response to initial therapy by month 2. Retreatment was performed in 4 eyes. One eye showed resolved SRF by month 3 after retreatment. Although the other 2 eyes showed marked improvements after retreatment, a minimal amount of SRF still remained at month 3, SRF was completely resolved by month 4. Remaining 1 eye did not respond to the retreatment. CSC completely resolved in this eye at month 6 after third SRT treatment. SRF in this eye persistently presented for a period of 17 months before study enrollment.
Safety of SRT
The mean number of applied SRT spots was 3.83. The range of pulse energy used for each treatment was between 65 and 90 μJ. All SRT laser spots were undetectable by color fundus photography and FFA 3 months after SRT treatment. Although SRT spots were invisible during the irradiation, some barely visible SRT spots were observed on fundus photography several minutes after SRT. However, these lesions faded and completely disappeared on fundus photography during follow-up. The autofluorescence of SRT spots was transiently increased after SRT treatment. The increased autofluorescence faded gradually during 3-month follow-up (Figure 7C and D). FFA was performed in order to confirm the success of laser treatment and ensure that sufficient energy was used in the selective damage of the RPE. The SRT spots were visible on FFA at 1 week, indicating some damage to the RPE, but all SRT laser spots had disappeared on FFA at 2 or 3 months, demonstrating recovery of the damaged area. Mean MS was 27.1 ± 0.95 dB after a resolution of SRF. The range of MS of SRT-treated lesions on MP was from 21 dB (the lowest) to 29 dB (the highest). The maximum difference of MS between SRT-treated lesion and untreated adjacent region was only 3 dB. Therefore, SRT did not cause any scotoma (Figure 8). In addition, no evidence of photoreceptor damage was observed on OCT in all SRT-treated regions.
FIGURE 7.
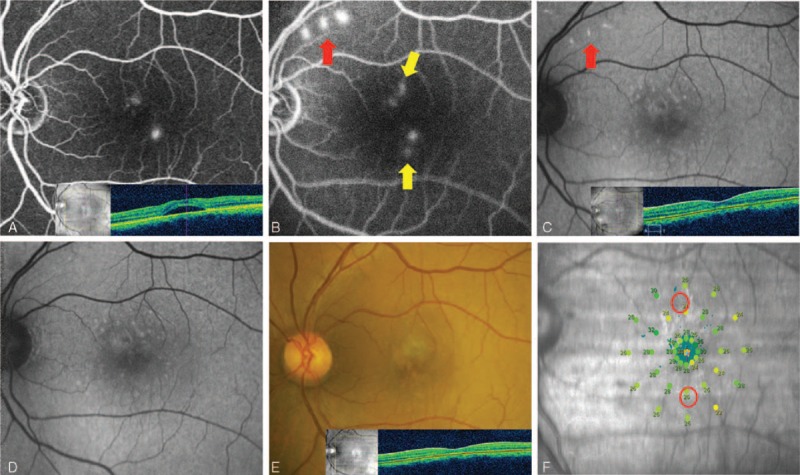
Case 3: A 45-year-old man presented with a 12-month history of central serous chorioretinopathy (CSC) in the left eye. His left best-correct visual acuity (BCVA) was 20/50. He had been treated with intravitreal injection of bevacizumab about 4 months previously. Selective retina therapy (SRT) was applied to the area of focal juxtafoveal angiographic leakages. His persistent subretinal fluid (SRF) completely resolved within 1 month. The BCVA remained unchanged. (A) Two leaking points on fundus fluorescein angiography (FFA) and SRF on optical coherence tomography (OCT) were observed at initial presentation. (B) Two SRT laser spots near 2 leaking points (yellow arrow) and 3 test spots (red arrow) were shown on FFA 30 minutes after SRT treatment. (C) Fundus autofluorescence (FAF) showed hyperautofluorescence at test spots at 1 month. (D) Hyperautofluorescence at test spots nearly disappeared at 3 months. (E) No scar was seen at SRT-treated sites. Complete resolution of SRF was shown on OCT 3 months after SRT. (F) Microperimetry (MP) showed no scotoma at 4 months after SRT.
FIGURE 8.
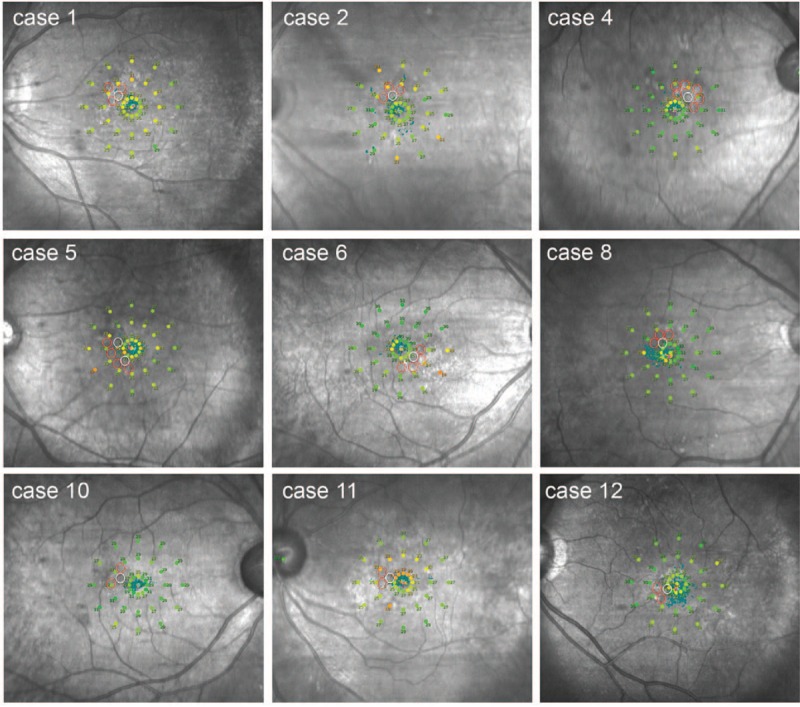
Microperimetry (MP) data were obtained after the complete resolution of subretinal fluid in selective retina therapy (SRT)-treated 12 patients. In all cases, there was no detectable scotoma in any of the SRT-treated lesions. Red circles indicate the SRT-irradiated points. White circles indicate leak points shown in fundus fluorescein angiography.
DISCUSSION
In most patients with acute CSC, SRF usually resolves spontaneously within months.1 However, a small portion of patients may develop chronic CSC, which can result in degenerative damage of the RPE and the overlying photoreceptors, and subsequently visual acuity loss.22 In several clinical studies, chronicity in CSC was defined as SRF for 3 months.23,24
Thus, chronic CSC lasting more than 3 months requires therapeutic intervention. SRT has previously been shown to result in absorption of SRF in CSC patients with good visual and structural outcomes.10,11 Although SRF was resolved by SRT, PED remained by the direct confluent treatment on PED. In the present study, SRT was shown to be a safe and effective treatment for persistent chronic CSC by indirect targeting method. In all cases, the complete resolution of SRF and/or PED was observed without any complications.
Unlike previous studies, we did not apply SRT directly to the point of leakage; rather, we irradiated at points immediately adjacent to, or circumferentially surrounding the leak. Previous studies reported that SRT could have an effect on adjacent to irradiated areas. In patients with drusen, disappearance of drusen was observed adjacent from the SRT irradiation.12 In animal experiments, reacting RPE was detected at the region surrounding the SRT-treated sites.18 Adjacent application of SRT spots in the present study showed a complete resolution of SRF and/or PED. These results suggest that SRT might induce RPE reaction near the laser-treated sites.
While SDM do not damage RPE cells, SRT can damage RPE selectively by using extremely shorter laser pulse duration than conventional laser in order to eliminate unwanted thermal damage and retain the therapeutic effects of laser therapy. Because of invisibility of SRT lesions, dosimetry of SRT system will allow clinicians to review real-time feedback by detecting transient microbubbles when the desired mechanical disruption of RPE cells occurs. In this study, 68 of 73 test spots were within therapeutic endpoints, and no overtreatment was observed in either test or treatment spots. By optimizing the algorithm of dosimetry through further clinical study, the precise laser dose can be determined for individual treatment endpoints without using FFA in the near future.
Prior SRT studies in Caucasian populations used pulse energies that ranged between 100 and 350 μJ, compared to the 65–90 μJ used in our study.10,11 All eyes in our series were of Asian ethnicity, which are recognized as having more pigment than Caucasian eyes. Although the racial differences of melanin density in RPE are not well understood, melanin pigment density must be considered during SRT therapy.
MP is notably useful to evaluate MS in patients with CSC. MS improves with the resolution of SRF, and areas of RPE irregularity or IS/OS disruption on OCT correlate with decreased MS.25 For these reasons, we believe that assessment of MS, after complete resolution of SRF, can demonstrate scotoma more clearly.
Previous SRT trials reported no central scotoma in SRT-treated CSC patients.10,11,16 However, scotoma was not evaluated by MP. In our series, all patients underwent MP for MS evaluation in order to distinguish scotoma occurrence of SRT-irradiated points rather than comparative improvement of MS. MS could be reduced even in resolved CSC.26,27 Although our data are not comparable with a previous study which measured MS of CSC cases with spontaneous resolution by MP, the mean MS (27.1 dB) of our patients was similar to that (25.8 dB) of the study.27 In our study, 6 of 12 eyes had a slightly lower range of 21 to 24 dB than the normal range at the SRT-treated lesion according to the protocol (V1.3) of MP. A clear differentiation of whether a slight decrease of MS around leak points was caused by CSC-mediated RPE abnormality or SRT-induced damage is limited. However, after SRT treatment, none of the patients showed a serious decrease of MS like scotoma during the follow-up. Moreover, repeated treatments among 4 patients did not cause any scotoma on MP. In these 4 eyes, we noted a slight reduction of the recorded reflectometry signal during irradiation of the treatment region as compared with test region. We speculate that undertreatment originally occurred, which may be associated with the limited treatment response. Based on the parameter of initial SRT treatment, laser energy during retreatment was marginally increased, resulting in the resolution of SRF in 3 among 4 eyes within 4 months.
Ahlers et al28 reported that multiple small PED were observed in more than 60% of CSC patients. In our study, of the 12 eyes, 7 and 2 eyes had small and large PED, respectively. Although the prognosis is usually quite good for eyes with PED, the presence of subfoveal PED has been shown to have a poorer prognosis. We were unable to demonstrate a change in very small, focal PED because the reattachment of the retina, as a result of SRF resolution, inhibits detection of small PED. We suspect that the detectable prompt flattening of larger PED over several weeks is reflective of the flattening or sealing of small PED. Since there was no change in subfoveal choroidal thickness, SRT might not affect choroidal hyperpermeability but induce RPE rejuvenation. Therefore, SRT could be another treatment option for treatment of PED in CSC.
Recent report29 revealed that current smokers have poor visual prognosis and show less favorable response to CSC treatment. One smoker was involved in our series, and SRF of the patient was completely resolved within 3 months after receiving SRT. We could not confirm the correlation between smoking and the treatment response.
We acknowledge that our study had several limitations, including its retrospective nature and the small number of cases. We were unable to evaluate recurrence rate of CSC in SRT-treated patients due to short-term follow-up. Our nonrandomized study cannot exclude the possibility of spontaneous resolution of SRF. Nevertheless, our SRT method and treatment pattern, targeting the surrounding area of leakage point or PED, improved visual acuity and led to the resolution of SRF without any risk of scotoma.
To our knowledge, this is the largest case series thus far to evaluate MS in CSC patients by MP after using RPE-targeting lasers, including SDM and SRT. Although further prospective studies are necessary to confirm the efficacy and safety of indirect targeting technique of SRT, these favorable visual and structural outcomes support the use of SRT treatment in the management of patients with chronic CSC.
Footnotes
Abbreviations: BCVA = best-correct visual acuity, CSC = central serous chorioretinopathy, FAF = fundus autofluorescence, FFA = fundus fluorescein angiography, logMAR = log of the minimum angle of resolution, MMP = maximum macular thickness, MP = microperimetry, MS = macular sensitivity, OCT = optical coherence tomography, OFV = optical feedback value, PDT = photodynamic therapy, PED = pigment epithelial detachment, RPE = retinal pigment epithelium, SDM = subthreshold micropulse laser, SRF = subretinal fluid, SRT = selective retina therapy.
This study was supported by the South Korean government-affiliated ministry of Trade, Industry and Energy (M000004912-00192937).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol 2008; 86:126–145. [DOI] [PubMed] [Google Scholar]
- 2.Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol 1986; 224:321–324. [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Goldbaum M, Wong DW, et al. Serous detachment of the retina. Retina 2003; 23:820–846. [DOI] [PubMed] [Google Scholar]
- 4.Liew G, Quin G, Gillies M, et al. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol 2013; 41:201–214. [DOI] [PubMed] [Google Scholar]
- 5.Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003; 23:752–763. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Soriano ME, García-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol 2008; 246:1235–1239. [DOI] [PubMed] [Google Scholar]
- 7.Huang WC, Chen WL, Tsai YY, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye 2009; 23:488–489. [DOI] [PubMed] [Google Scholar]
- 8.Koss MJ, Beger I, Koch FH. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye 2012; 26:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik KJ, Sampat KM, Mansouri A, et al. Low-intensity/high-density subthreshold micropulse diode laser for chronic central serous chorioretinopathy. Retina 2015; 35:532–536. [DOI] [PubMed] [Google Scholar]
- 10.Klatt C, Saeger M, Oppermann T, et al. Selective retina therapy for acute central serous chorioretinopathy. Br J Ophthalmol 2011; 95:83–88. [DOI] [PubMed] [Google Scholar]
- 11.Elsner H, Pörksen E, Klatt C, et al. Selective retina therapy in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2006; 244:1638–1645. [DOI] [PubMed] [Google Scholar]
- 12.Roider J, Brinkmann R, Wirbelauer C, et al. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol 2000; 84:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roider J, Liew SH, Klatt C, et al. Selective retina therapy (SRT) for clinically significant diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 2010; 248:1263–1272. [DOI] [PubMed] [Google Scholar]
- 14.Koinzer S, Elsner H, Klatt C, et al. Selective retina therapy (SRT) of chronic subfoveal fluid after surgery of rhegmatogenous retinal detachment: three case reports. Graefes Arch Clin Exp Ophthalmol 2008; 246:1373–1378. [DOI] [PubMed] [Google Scholar]
- 15.Prahs P, Walter A, Regler R, et al. Selective retina therapy (SRT) in patients with geographic atrophy due to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2010; 248:651–658. [DOI] [PubMed] [Google Scholar]
- 16.Klatt C, Elsner H, Pörksen E, et al. Selective retina therapy in central serous chorioretinopathy with detachment of the pigmentary epithelium. Ophthalmologe 2006; 103:850–855. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann R, Roider J, Birngruber R. Selective retina therapy (SRT): a review on methods, techniques, preclinical and first clinical results. Bull Soc Belge Ophtalmol 2006; 302:51–69. [PubMed] [Google Scholar]
- 18.Roider J, Michaud NA, Flotte TJ, et al. Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol 1992; 110:1786–1792. [DOI] [PubMed] [Google Scholar]
- 19.Roider J, Brinkmann R, Wirbelauer C, et al. Retinal sparing by selective retinal pigment epithelial photocoagulation. Arch Ophthalmol 1999; 117:1028–1034. [DOI] [PubMed] [Google Scholar]
- 20.Park YG, Seifert E, Roh YJ, et al. Tissue response of selective retina therapy by means of a feedback-controlled energy ramping mode. Clin Exp Ophthalmol 2014; 42:846–855. [DOI] [PubMed] [Google Scholar]
- 21.Kim HD, Han JW, Ohn YH, et al. Functional evaluation using multifocal electroretinogram after selective retina therapy with a microsecond-pulsed laser. Invest Ophthalmol Vis Sci 2014; 56:122–131. [DOI] [PubMed] [Google Scholar]
- 22.Koskela P, Laatikainen L, von Dickhoff K. Contrast sensitivity after resolution of central serous chorioretinopathy. Graefes Arch Clin Exp Opthalmol 1994; 232:473–476. [DOI] [PubMed] [Google Scholar]
- 23.Reibaldi M, Cardascia N, Longo A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a randomized clinical trial. Am J Ophthalmol 2010; 149:307–315. [DOI] [PubMed] [Google Scholar]
- 24.Shin JY, Woo SJ, Yu HG, et al. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina 2011; 31:119–126. [DOI] [PubMed] [Google Scholar]
- 25.Ojima Y, Tsujikawa A, Hangai M, et al. Retinal sensitivity measured with the micro perimeter 1 after resolution of central serous chorioretinopathy. Am J Ophthalmol 2008; 146:77–84. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, Oh J, Huh K. Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol 2012; 96:350–355. [DOI] [PubMed] [Google Scholar]
- 27.Chung HW, Yun CM, Kim JT, et al. Retinal sensitivity assessed by microperimetry and corresponding retinal structure and thickness in resolved central serous chorioretinopathy. Eye 2014; 28:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlers C, Geitzenauer W, Stock G, et al. Alterations of intraretinal layers in acute central serous chorioretinopathy. Acta Ophthalmol 2009; 87:511–516. [DOI] [PubMed] [Google Scholar]
- 29.Türkcü FM, Yüksel H, Sahin A, et al. Effects of smoking on visual acuity of central serous chorioretinopathy patients. Cutan Ocul Toxicol 2014; 33:115–119. [DOI] [PubMed] [Google Scholar]


