Abstract
With an increasing use of traditional Chinese medicine (TCM) in type 2 diabetes mellitus (T2DM), evidence of long-term benefit with adjunctive TCM treatment is limited. This study investigated whether the concurrent TCM treatment reduces the risk of vascular complications in T2DM patients by using a large population from National Health Insurance Research Database (NHIRD).
We identified 33,457 adult patients with newly diagnosed T2DM using anti-diabetic agents from a random sample of one million beneficiaries in the NHIRD between January 1, 2000 and December 31, 2011. We recruited 1049 TCM users (received TCM over 30 days with a diagnosis of T2DM) and randomly selected 4092 controls as the non-TCM cohort at a ratio of 1:4 frequency-matched by age, sex, hypertension, hyperlipidemia, and index year. We investigated the prescription pattern of TCM and conducted a Cox proportional hazards regression to calculate the hazard ratios (HRs) of stroke, chronic kidney diseases (CKD), and diabetic foot between the 2 cohorts.
In the TCM cohort, the prescription pattern of TCM was different between insulin and noninsulin patients. The most common herbs were Dan-Shen (Radix Salviae Miltiorrhizae) in noninsulin group and Da-Huang (Radix et Rhizoma Rhei) in insulin group. The most common formulae were Liu-Wei-Di-Huang-Wan in noninsulin group and Yu-Quan-Wan in insulin group. Although no significant reduction in the hazard ratio of CKD and diabetic foot, the incidence rate of stroke was 7.19 per 1000 person-years in the TCM cohort and 10.66 per 1000 person-years in the control cohort, respectively. After adjustment of age, sex, hypertension, hyperlipidemia, and antidiabetes agent use (including sulfonylureas, α-glucosidase, metformin, meglitinide, thiazolidinediones, and insulin), TCM cohorts were found to have a 33% decreased risk of stroke (95% CI = 0.46–0.97; P < 0.05).
This population-based retrospective study showed that the complementary TCM therapy might associate with the decreased risk of stroke in T2DM, suggesting TCM as an adjunctive therapy for T2DM to prevent subsequent stroke.
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) has increased steadily worldwide, estimated to be 2.8% in 2000 and predicted to be 4.4% in 2030.1 Patients with diabetes are at risk of developing a spectrum of vascular complications, such as stroke, nephropathy, retinopathy, and foot ulcers. The current treatment for vascular complications is glycemic control. However, most patients who have T2DM develop vascular complications despite intensive glycemic control with a variety of antidiabetes medications and disease monitoring.2 Thus T2DM continues to be an important public health concern because of substantial morbidity and mortality as well as long-term complications. With no specific treatments for vascular complications other than glucose control, alternative therapies have become increasingly popular as adjunctive treatments.
Traditional Chinese medicine (TCM) is a form of complementary and alternative medicine that has been widely applied for centuries in Asian countries. In Taiwan, TCM is a widely used form of medical treatment as well as Western medicine.3–6 Both forms of treatment are covered by National Health Insurance (NHI), which is a government-run, single-payer program that covers more than 99% of Taiwanese citizens and over 93% of Taiwanese healthcare institutes.7 TCM therapy has gained popularity as a complementary and alternative therapy for diabetes and its complications in Taiwan. Although previous studies implied potential hypoglycemic effects of TCM,8,9 evidence of benefit with long-term adjunctive TCM treatment is limited. Chronic subclinical inflammation is associated with insulin resistance and many TCM herbs have been reported to exert a hypoglycemic effect via their anti-inflammatory mechanism. In addition to blood glucose and glycated hemoglobin (HbA1c), the incidence of vascular complications could be a possible outcome measurement for the long-term TCM treatment effect.
To fill in the gap between TCM therapy and its long-term benefit on T2D, we conducted a large-scale clinical analysis of TCM on vascular complications in T2DM patients. By using a large population-based database from the NHI research database, we investigated whether the integration of TCM and Western medicine reduced the risk of vascular complications among T2DM patients.
METHODS
Data Source
This study was designed as a population-based cohort study analyzing from a sample of 1 million subjects (Longitudinal Health Insurance Database 2000, LHID2000) which was randomly selected from the 23 million beneficiaries of the National Health Insurance Research Database (NHIRD) in Taiwan to determine the risk of complications while combining TCM treatment with Western medicine. This National Health Insurance program was established on March 1, 1995 by Taiwan's Bureau of National Health Insurance (NHI) and it covers over 22.60 million people in Taiwan (total population = 22.96 million).7 The LHID2000 comprises medical records such as inpatient and outpatient claims, administered treatments, and personal information such as sex, date of birth, and the date that patients enrolled in the NHI program. The identification of insurants in the LHID2000 was encrypted before being sent to the researchers. We defined disease on the basis of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM).
Study Subjects
The subjects were selected from the random 1 million individuals as follows (Figure 1). We considered patients to be T2DM sufferers if antidiabetic medication had been prescribed. First, we included patients who were newly diagnosed T2DM (ICD-9-CM 250.00, 250.000, and 250.02) and treated with antidiabetic agents between January 1, 2000 and December 31, 2011 (n = 33,457). Patients using TCM over 30 days with a diagnosis of T2DM were defined as TCM users, whereas those treated for less than 30 days were considered as non-TCM users. Their use of antidiabetes agent, including sulfonylureas, α-glucosidase, metformin, meglitinide, thiazolidinediones, and insulin, was also analyzed. The index date in TCM users was defined the 30th day under TCM treatment. We excluded patients with a history of stroke (ICD-9-CM 430–438), chronic kidney diseases (CKD) (ICD-9-CM 585, 250.40 or 250.42), diabetic foot (ICD-9-CM 682.7, 682.9, 444.22, 728.86, 707.0–707.7 or operation for amputation, debridement, or ostectomy), trauma, or fracture within 180 days before the date of index date, leaving 1049 diabetic patients. To assemble a comparison cohort, we randomly selected controls who were matched with the study cohort at a ratio of 1:4 on frequency of age, sex, hypertension, hyperlipidemia, and index year as the comparison cohort, using the same exclusion criteria during the same period. Finally, we selected 1049 patients as the TCM-combined cohort (mean age = 53.4 years, SD = 11.1 years). The 4092 controls (mean age = 53.7 years, SD = 10.7 years) comprised patients who received TCM <30 days following their T2DM diagnosis and their index dates were randomly assigned as TCM users. The Institutional Review Board of the China Medical University and Hospital approved this study (CMU-REC-101–102).
FIGURE 1.
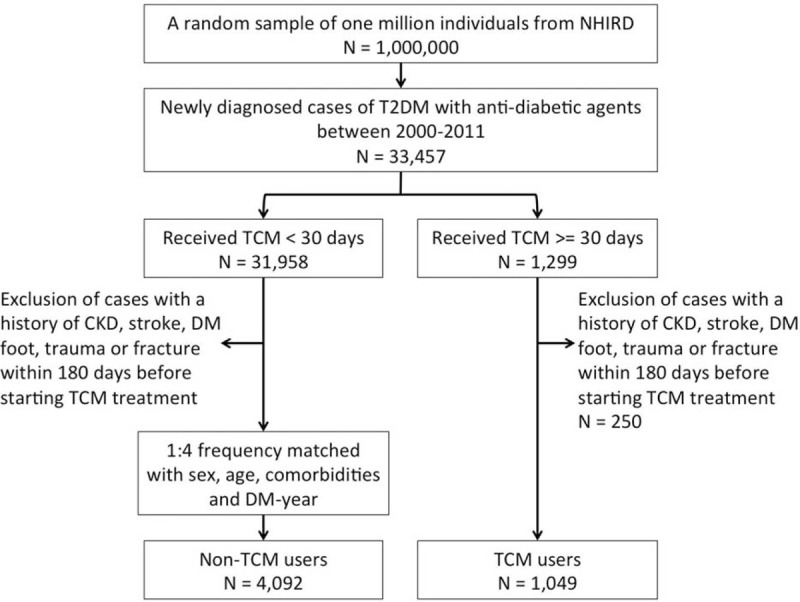
Flow recruitment chart of newly diagnosed type 2 diabetes mellitus patients from a random sample of 1 million individuals from the National Health Insurance Research Database in Taiwan. NHIRD = National Health Insurance Research Database; T2DM = type 2 diabetes mellitus; TCM = traditional Chinese medicine.
Endpoint
The data were examined from the index date until the endpoint, which was based on whichever of the following occurred first: the date a patient was diagnosed of stroke (ICD-9-CM 430–438), CKD (ICD-9-CM 585, 250.40, or 250.42), or diabetic foot (ICD-9-CM 682.7, 682.9, 444.22, 728.86, 707.0–707.7 or operation for amputation, debridement, or ostectomy); the date a patient withdrew from the NHI program; and the end of 2011.
Statistical Analyses
To test the differences in distribution between the 2 cohorts, we used the χ2 test for the categorical variable and the t test for continuous variable. The χ2 tests were carried out to examine the differences in the distribution of demographic factors and comorbidities between TCM users (using TCM and Western medicine) and non-TCM users (using Western medicine only). To understand the utility of different TCM therapies in T2DM patients, we displayed the frequency distribution. The most frequently prescribed single herbs and herbal formulae were described. For subjects who did not receive insulin at baseline in both groups, we also examine the incidence of insulin use in the follow-up duration. We counted person-years on the basis of the index date to the endpoint for each patient. The incidence of stroke, CKD, and diabetic foot in 2 cohorts was calculated. To compare the TCM and non-TCM cohorts, we estimated the incidence rate ratio and 95% confidence intervals (CIs) of complications by using Poisson regression. The multivariate model was controlled using age, sex, hypertension, hyperlipidemia, and anti-diabetes agent use (including sulfonylureas, α-glucosidase, metformin, meglitinide, thiazolidinediones, and insulin) by Cox's proportional hazard regression.
RESULTS
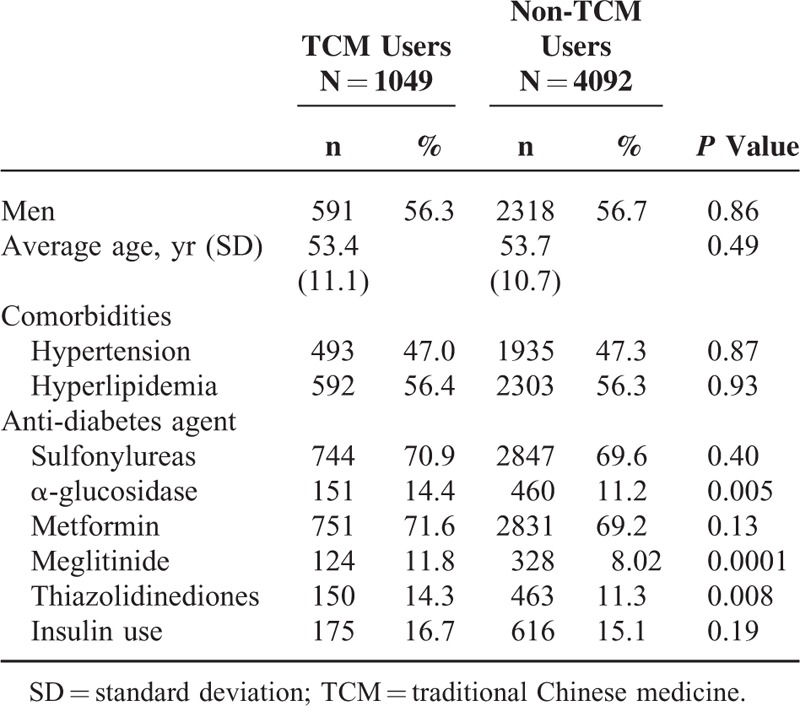
The TCM and non-TCM cohorts comprised data for 1049 and 4092 patients, respectively. Details of the demographic distribution and comorbidities of 2 groups are presented in Table 1. There are more men than women in both groups. Because we matched the distribution of age stratum (every 5 years), sex, and comorbidities (including hypertension and hyperlipidemia), there was no significant difference in those variables. Regarding Western medicine used at baseline, TCM users were more likely to receive α-glucosidase, meglitinide, and thiazolidinediones. To understand the utility of different TCM therapies in T2DM patients, we displayed the frequency distribution. Among total 121,096 TCM clinical visits in T2DM patients, the most frequent TCM therapy was Chinese herbal products (CHPs) (78.2%), followed by acupuncture (12.6%) and manipulative therapy (6.61%). There are few TCM users who use 2 or more different TCM therapies.
TABLE 1.
Demographic Characteristics Between Type 2 Diabetes Users With and Without Traditional Chinese Medicine
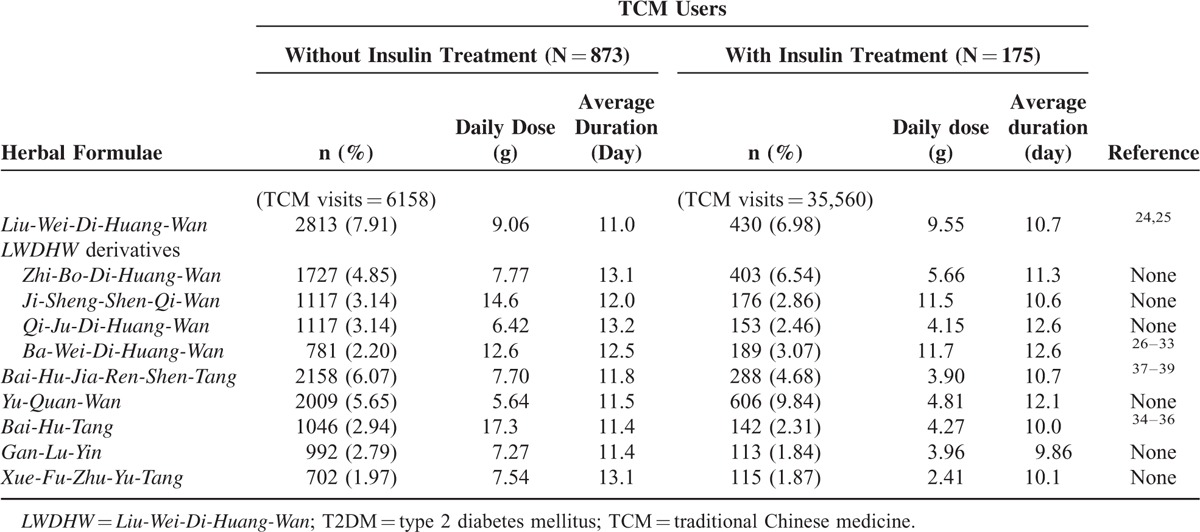
To investigate the prescription pattern for TCM users, we divided the TCM cohort into 2 groups, 1 with insulin use and 1 without. In the group without insulin use, the most common single herbs were Dan-Shen, Tian-Hua-Fen, and Huang-Qi (Table 2), and the most common herbal formulae were Liu-Wei-Di-Huang-Wan, Bai-Hu-Jia-Ren-Sheng-Tang, and Yu-Quan-Wan (Table 3). In the group receiving insulin treatment, the most common single herbs were Da-Huang, Tian-Hua-Fen, Dan-Shen, and the most common herbal formulae were Yu-Quan-Wan, Liu-Wei-Di-Huang-Wan, and Zhi-Bo-Di-Huang-Wan.
TABLE 2.
Top 10 Common Single Herbs Prescribed for Type 2 Diabetes in Users With Traditional Chinese Medicine
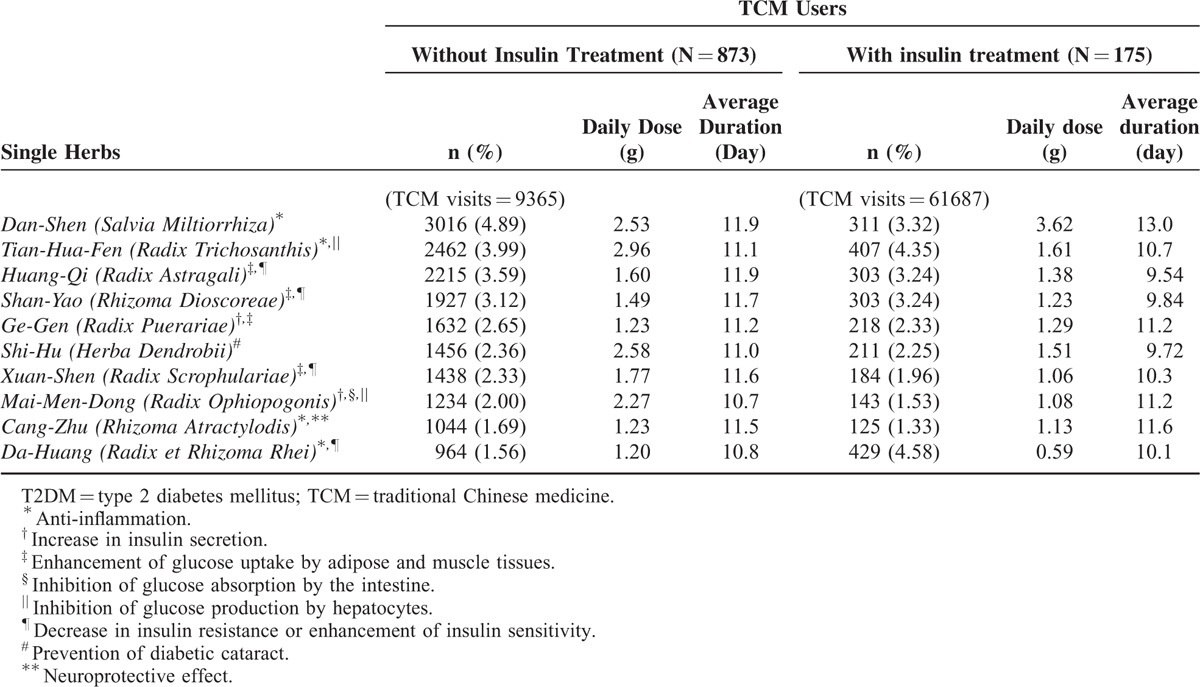
TABLE 3.
Top 10 Common Herbal Formulae Prescribed for Type 2 Diabetes in Users With Traditional Chinese Medicine
We also examine whether adjunctive TCM could decrease the incidence of subsequent insulin use. Among 3476 non-TCM users and 874 TCM users who did not receive insulin at baseline, there are 647 and 177 patients who use insulin in the following of the study, respectively. In patients who did not receive insulin at baseline, the incidence of subsequent insulin use in TCM and non-TCM cohorts was 50.03 and 46.24 per 1000 person-years, respectively. The risk for subsequent insulin use was not significantly different between 2 cohorts (95% CI = 0.91–1.27).
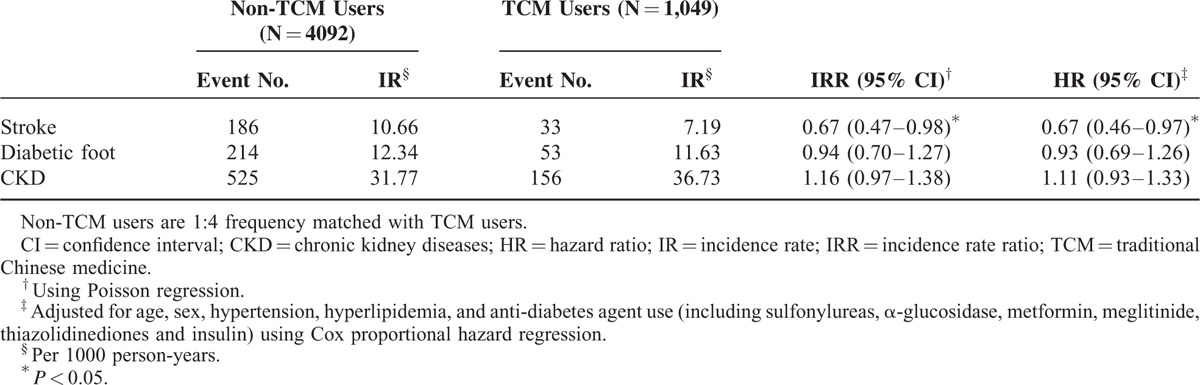
Comparing TCM and non-TCM groups, we examine the incidence of 3 vascular complications (stroke, CKD, and diabetic foot) to evaluate the long-term effect of adjunctive TCM treatment (Table 4). During the follow-up, the incidence of CKD and diabetic foot between 2 groups did not achieve statistical significance. However, 33 TCM patients and 186 non-TCM patients had stroke, and the incidence rates of stroke among the TCM and control cohorts were 7.19 and 10.66 per 1000 person-years, respectively. Compared with the non-TCM cohort, patients who received adjunctive TCM to treat their T2DM exhibited a 33% lower risk for stroke (95% CI = 0.46–0.97) after adjusting for age, sex, hypertension, hyperlipidemia, and anti-diabetes agent use (including sulfonylureas, α-glucosidase, metformin, meglitinide, thiazolidinediones, and insulin).
TABLE 4.
Incidence and Hazard Ratio of Stroke, Diabetic Foot and CKD Among TCM and Non-TCM Users
DISCUSSION
In this population-based, retrospective study, we found that combined use of TCM and Western medicine to treat T2DM exhibited a 33% reduced risk of stroke than using Western medicine alone. To our knowledge, this is the first population-based pharmacoepidemiological study that focused on the clinical benefit of adjunctive TCM in patients with T2DM to prevent subsequent stroke. Among various TCM therapies, our study showed that the most frequent TCM therapy in T2DM patients was CHPs (78.2%), followed by acupuncture (12.6%) and manipulative therapy (6.61%).
Chronic subclinical inflammation is associated with insulin resistance, a situation of increased risk for developing diabetes and cardiovascular events.10,11 Clinical evidence showed that the risk and outcome of stroke is highly associated with inflammatory burden.12 In our study, the top 4 herbs most commonly prescribed in the treatment of T2DM patients have been reported to exert a hypoglycemic effect via their anti-inflammatory mechanism, including Dan-Shen (Salvia Miltiorrhiza),13Da-Huang (Radix et Rhizoma Rhei),14Tian-Hua-Fen (Radix Trichosanthis),15 and Huang-Qi (Radix Astragali).16 Other than Chinese herbal medicine, acupuncture could also take part in the anti-inflammatory mechanism by enhancing vagus nerve activity.17 Since inflammation participates in the pathogenesis of T2DM and subsequent stroke,18 TCM with anti-inflammation mechanisms could exert not only hypoglycemic effects but also potential effects to prevent subsequent stroke through anti-inflammatory mechanisms. Previous study also elucidated that the type 2 ryanodine receptor, a calcium release channel, exists in cardiomyocytes and pancreatic β cells and plays a crucial role in oxidative stress, which mechanically explains the relationship between cardiovascular disease and diabetes.19 Further studies focus on the mechanism how TCM work on the calcium channel is a potential research direction.
In the clinical practice, insulin injection means an increased severity of T2DM. For TCM practitioners, insulin use also means a progressed stage that needs more intensive treatment. We divided TCM users into 2 groups, 1 with insulin and 1 without, to observe the different prescription pattern between the 2 groups. In our study, the most common single herb for T2DM was Dan-Shen (Radix Salviae Miltiorrhizae) in patients without insulin use, compared with Da-Huang (Radix et Rhizoma Rhei) in patients who received insulin treatment. Dan-Shen, the roots and rhizomes of Radix Salviae Miltiorrhizae, is an important Chinese medicine widely used to promote blood circulation, remove blood stasis, and treat coronary heart diseases. It is often used in herbal formulae to treat diabetic complications. The combination of Radix Salviae Miltiorrhizae and Cortex Moutan prevents diabetes-induced vascular damage and produces significant cerebrovascular protective effects through the reduction of oxidative stress and through intracellular calcium regulatory mechanisms.20 Danshensu, the main active component of Dan-Shen, protects endothelial cells from the damage induced by H2O2 and inhibits apoptosis.21 On the other hand, Da-Huang, the roots and rhizomes of Rheum palmatum L., Rheum tanguticum Maxim. ex Balf., or Rheum officinale Baill., is used as laxative to clear heat, remove toxicity, activate blood, and dispel stasis according to TCM theory. Emodin, the major bioactive component of Da-Huang, significantly decreases the levels of blood glucose, triglyceride, and total cholesterol in STZ-induced diabetic mice through the activation of PPAR-γ and the modulation of metabolism-related genes.22 The glucose tolerance and insulin sensitivity in the emodin-treated group were significantly improved compared with the controls. Furthermore, emodin is considered a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase type 1 which is an attractive therapeutic target of T2DM.23 As a result, using insulin treatment as a cut point of different stages in T2DM, TCM practitioners use Dan-Shen to protect vascular endothelium in the early stage without insulin treatment and use Da-Huang to enhance hypoglycemic effect in the late stage with insulin use. We found difference in single herb prescription pattern between insulin and noninsulin patients, indicating corresponding TCM strategies for various conditions in T2DM.
In addition, there is also a different herbal formula prescription pattern between insulin and noninsulin population. The most common herbal formula for T2DM was Liu-Wei-Di-Huang-Wan in patients without insulin use, compared with Yu-Quan-Wan in patients who received insulin treatment. According to the concepts of TCM, T2DM is a systemic disease with yin-deficiency, which causes heat accumulation and blood stasis in the body and eventually leads to subsequent complications. In TCM theory, Liu-Wei-Di-Huang-Wan is used to nourish yin and tonify the liver and kidney. Yu-Quan Wan is used to treat polydipsia by clearing heat, generating body fluids, quenching thirst, and moistening dryness, which are obvious symptoms in hyperglycemic state. Among the top 10 common herbal formulae, 4 CHPs, Zhi-Bo-Di-Huang-Wan, Ji-Sheng-Shen-Qi-Wan, Qi-Ju-Di-Huang-Wan, and Ba-Wei-Di-Huang-Wan, are derivative formulae of Liu-Wei-Di-Huang-Wan. Similar to another study in Taiwan,3Liu-Wei-Di-Huang-Wan and its derivatives were found to be the most common herbal formulae prescribed by TCM doctors for the treatment of diabetes in Taiwan. Recent scientific evidence has suggested that Liu-Wei-Di-Huang-Wan reduced blood glucose, relieved neuropathy and nephropathy in diabetes,24 and improved the lipid profile to reduce cardiovascular risk.25 Animal studies revealed that Ba-Wei-Di-Huang-Wan increased insulin synthesis and release,26,27 suppressed the release of glucose from the liver,26 normalized or suppressed the small intestinal disaccharidase activity,28 reduced the damage caused by oxidative stress,29,30 and prevented diabetic nephropathy.31–33
Another formula, Bai-Hu-Tang, and its derivative, Bai-Hu-Jia-Ren-Shen-Tang, were also frequently used in T2DM. Bai-Hu-Tang, traditionally used to reduce fever heat and promote generation of body fluids, played an important role in immunity protection and anti-inflammation.34,35 Moreover, Bai-Hu-Tang stimulated glucose uptake in adipocytes via PPAR-γ activation rather than the insulin signaling pathway.36Bai-Hu-Jia-Ren-Shen-Tang (same as Byakko-ka-ninjin-to in Kampo medicine) has hypoglycemic effect and acts as a facilitatory agent on salivary secretion to reduce xerostomia in T2DM patients.37–39 Evidence of Gan-Lu-Yin focused on antitumor effect, including inhibiting angiogenesis40 and suppressing vascular smooth muscle cell migration.41Xue-Fu-Zhu-Yu-Tang, traditionally used to promote the circulation of blood to remove blood stasis and activate the flow of Qi to relieve pain, demonstrated potency to antiplatelet aggregation,42 decrease in the serum total triglyceride concentration, and suppression of proinflammatory cytokines in high cholesterol-fed rats.43 Lack of scientific research on Yu-Quan-Wan, Gan-Lu-Yin, and Xue-Fu-Zhu-Yu-Tang in treating T2DM, further investigation on these 3 herbal formulae in T2DM is necessary.
Other than CHPs, we found that acupuncture is also a widely used treatment in T2DM. Previous randomized controlled trials showed that electroacupuncture (EA) and rosiglitazone combined therapy improved insulin resistance through a reduction in plasma free fatty acid.44 EA enhanced insulin sensitivity and improved glucose tolerance through activating cholinergic nerves and nitric oxide synthase, which lower plasma free fatty acid levels.45–47 Moreover, acupuncture stimulation showed potential neuroprotective effects through suppression of cerebral blood flow response in the increased plasma osmolality and extracellular changes in glutamate in diabetic rats under ischemic conditions.48 In the above studies, the most common acupoints used in T2DM were located at the abdomen and legs, including Zhongwan (CV-12), Gwanyuan (CV-4), Zusanli (ST-36), Yanglingquan (GB-34), and Xuanzhong (GB-39).
One of this study's strengths is that we obtained the data from the NHI database, which is a government-run, single-payer NHI program that covers more than 97% of Taiwanese citizens and over 99% of Taiwanese healthcare institutes.7 This ensures that the present study is representative of the general population with minimal selection bias, thereby offering a comprehensive account of the subsequent stroke of both TCM and non-TCM users. The large sample size gave adequate power for stratification into subgroups for statistical analysis. There are several limitations. First, we did not choose 2 other common complications, neuropathy and retinopathy, as the endpoint. It was difficult to define DM-related neuropathy and retinopathy since they are progressive and the diagnosis codes could be varied among different physicians in different clinical conditions. Second, although we did not have the exact value of blood glucose or glycated hemoglobin (HbA1c) of every patient because of the limitation of the NHI database, we could evaluate the long-term treatment effect by the incidence of subsequent vascular events. Third, people using TCM may tend to focus on maintaining good health and have healthier lifestyle modification. Because of the nature of NHIRD, some variables that contribute to the propensity to stroke, such as exposure to cigarette smoke, obesity, and alcohol consumption, were not collected in this database. A future study including information on lifestyle will be helpful. We are currently designing a long-term follow-up study of diabetes patients in our hospital to evaluate the protective effects of adjunctive TCM therapy in diabetes patients. Meanwhile, this population-based large-scale retrospective analysis of the NHIRD still provides some valuable information on the association of TCM therapy and vascular complications among patients with T2DM.
In conclusion, this population-based, retrospective cohort study shows that the concurrent TCM use may decrease the risk of stroke in T2DM patients. Our study results suggest that the use of TCM as an adjunctive therapy in patients with T2DM may prevent subsequent stroke. Further long-term prospective studies are required to validate these findings.
Acknowledgment
This study was supported by China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan. This study was also supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002). This study was based in part on data from the National Health Insurance Research Database, provided by the National Health Insurance Administration, Ministry of Health and Welfare, and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare, or National Health Research Institutes.
Footnotes
Abbreviations: CHPs = Chinese herbal products, CI = confidence interval, CKD = chronic kidney diseases, EA = electroacupuncture, FFA = free fatty acid, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IR = incidence rate, IRR = incidence rate ratio, LHID = Longitudinal Health Insurance Database, LWDHW = Liu-Wei-Di-Huang-Wan, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, SD = standard deviation, T2DM = type 2 diabetes mellitus, TCM = traditional Chinese medicine.
A-LL and B-CC contributed equally as co-first authors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 2.Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med 2010; 123 (3 Suppl):S3–S11. [DOI] [PubMed] [Google Scholar]
- 3.Huang CY, Tsai YT, Lai JN, et al. Prescription pattern of Chinese herbal products for diabetes mellitus in Taiwan: a population-based study. Evid Based Complement Alternat Med 2013; 2013:201329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CY, Lai WY, Sun MF, et al. Prescription patterns of traditional Chinese medicine for peptic ulcer disease in Taiwan: a nationwide population-based study. J Ethnopharmacol 2015; 176:311–320. [DOI] [PubMed] [Google Scholar]
- 5.Huang MC, Pai FT, Lin CC, et al. Characteristics of traditional Chinese medicine use in patients with rheumatoid arthritis in Taiwan: a nationwide population-based study. J Ethnopharmacol 2015; 176:9–16. [DOI] [PubMed] [Google Scholar]
- 6.Yen HR, Liang KL, Huang TP, et al. Characteristics of traditional Chinese medicine use for children with allergic rhinitis: a nationwide population-based study. Int J Pediatr Otorhinolaryngol 2015; 79:591–597. [DOI] [PubMed] [Google Scholar]
- 7.Introduction to the National Health Insurance Research Database (NHIRD), Taiwan. http://nhird.nhri.org.tw/date_01.html Accessed [Dec 19, 2015] [Google Scholar]
- 8.Feng S, Song L, Liu Y, et al. Hypoglycemic activities of commonly-used traditional Chinese herbs. Am J Chin Med 2013; 41:849–864. [DOI] [PubMed] [Google Scholar]
- 9.Hui H, Tang G, Go VL. Hypoglycemic herbs and their action mechanisms. Chin Med 2009; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98–107. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM. Pre-diabetes, insulin resistance, inflammation and CVD risk. Diabetes Res Clin Pract 2003; 61:S9–S18. [DOI] [PubMed] [Google Scholar]
- 12.Murray KN, Buggey HF, Denes A, et al. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci 2013; 53:14–25. [DOI] [PubMed] [Google Scholar]
- 13.Fan GW, Gao XM, Wang H, et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol 2009; 113:275–280. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Zhou J, Jia Z, et al. Hypoglycemic effect of Rehmannia glutinosa oligosaccharide in hyperglycemic and alloxan-induced diabetic rats and its mechanism. J Ethnopharmacol 2004; 90:39–43. [DOI] [PubMed] [Google Scholar]
- 15.Hartog A, Hougee S, Faber J, et al. The multicomponent phytopharmaceutical SKI306X inhibits in vitro cartilage degradation and the production of inflammatory mediators. Phytomedicine 2008; 15:313–320. [DOI] [PubMed] [Google Scholar]
- 16.Ryu M, Kim EH, Chun M, et al. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J Ethnopharmacol 2008; 115:184–193. [DOI] [PubMed] [Google Scholar]
- 17.Oke SL, Tracey KJ. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann N Y Acad Sci 2009; 1172:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab 2011; 12:289–301. [DOI] [PubMed] [Google Scholar]
- 19.Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015; 125:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Li YL, Li ZL, et al. Chronic supplementation of paeonol combined with danshensu for the improvement of vascular reactivity in the cerebral basilar artery of diabetic rats. Int J Mol Sci 2012; 13:14565–14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang GD, Zhang H, Lin R, et al. Down-regulation of CD40 gene expression and inhibition of apoptosis with Danshensu in endothelial cells. Basic Clin Pharmacol Toxicol 2009; 104:87–92. [DOI] [PubMed] [Google Scholar]
- 22.Xue J, Ding W, Liu Y. Anti-diabetic effects of emodin involved in the activation of PPARgamma on high-fat diet-fed and low dose of streptozotocin-induced diabetic mice. Fitoterapia 2010; 81:173–177. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Huang SL, Dou W, et al. Emodin, a natural product, selectively inhibits 11beta-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Br J Pharmacol 2010; 161:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon TY, Ong KL, Cheung BM. Review of the effects of the traditional Chinese medicine Rehmannia Six Formula on diabetes mellitus and its complications. J Diabetes 2011; 3:184–200. [DOI] [PubMed] [Google Scholar]
- 25.van Wietmarschen HA, van der Greef J, Schroen Y, et al. Evaluation of symptom, clinical chemistry and metabolomics profiles during Rehmannia six formula (R6) treatment: an integrated and personalized data analysis approach. J Ethnopharmacol 2013; 150:851–859. [DOI] [PubMed] [Google Scholar]
- 26.Hirotani Y, Ikeda K, Myotoku M. Effects of the herbal medicine Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) on insulin secretion and glucose tolerance in type 2 diabetic Goto-Kakizaki rats. Drug Discov Ther 2010; 4:129–134. [PubMed] [Google Scholar]
- 27.Hirotani Y, Ikeda T, Yamamoto K, et al. Effects of Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) on hyperglycemia in streptozotocin-induced diabetic rats. Biol Pharm Bull 2007; 30:1015–1020. [DOI] [PubMed] [Google Scholar]
- 28.Hirotani Y, Ikeda T, Ikeda K, et al. Effects of Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) on intestinal function in streptozotocin-induced diabetic rats. Yakugaku Zasshi 2007; 127:1509–1513. [DOI] [PubMed] [Google Scholar]
- 29.Kim HY, Yokozawa T, Cho EJ, et al. Protective effects of the Chinese prescription Hachimi-jio-gan against diabetic oxidative stress. J Pharm Pharmacol 2004; 56:1299–1305. [DOI] [PubMed] [Google Scholar]
- 30.Yamabe N, Yokozawa T. Protective effect of Hachimi-jio-gan against the development of pancreatic fibrosis and oxidative damage in Otsuka Long-Evans Tokushima Fatty rats. J Ethnopharmacol 2007; 113:91–99. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa T, Yokozawa T, Yamabe N, et al. Long-term treatment with Hachimi-jio-gan attenuates kidney damage in spontaneously diabetic WBN/Kob rats. J Pharm Pharmacol 2005; 57:1205–1212. [DOI] [PubMed] [Google Scholar]
- 32.Yamabe N, Yokozawa T. Activity of the Chinese prescription Hachimi-jio-gan against renal damage in the Otsuka Long-Evans Tokushima fatty rat: a model of human type 2 diabetes mellitus. J Pharm Pharmacol 2006; 58:535–545. [DOI] [PubMed] [Google Scholar]
- 33.Yokozawa T, Yamabe N, Cho EJ, et al. A study on the effects to diabetic nephropathy of Hachimi-jio-gan in rats. Nephron Exp Nephrol 2004; 97:e38–e48. [DOI] [PubMed] [Google Scholar]
- 34.Lin CJ, Su YC, Lee CH, et al. Bai-hu-tang, ancient chinese medicine formula, may provide a new complementary treatment option for sepsis. Evid Based Complement Alternat Med 2013; 2013:193084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Wang D, Wang X, et al. Aqueous extract of Bai-Hu-Tang, a classical Chinese herb formula, prevents excessive immune response and liver injury induced by LPS in rabbits. J Ethnopharmacol 2013; 149:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CC, Hsiang CY, Chiang AN, et al. Peroxisome proliferator-activated receptor gamma transactivation-mediated potentiation of glucose uptake by Bai-Hu-Tang. J Ethnopharmacol 2008; 118:46–50. [DOI] [PubMed] [Google Scholar]
- 37.Kimura I, Nakashima N, Sugihara Y, et al. The antihyperglycaemic blend effect of traditional Chinese medicine Byakko-ka-ninjin-to on alloxan and diabetic KK-CA(y) mice. Phytother Res PTR 1999; 13:484–488. [DOI] [PubMed] [Google Scholar]
- 38.Niijima A, Kubo M, Ichiki H, et al. Effect of byakko-ka-ninjin-to on the efferent activity of the autonomic nerve fibers innervating the sublingual gland of the rat. J Auton Nerv Syst 1997; 63:46–50. [DOI] [PubMed] [Google Scholar]
- 39.Yanagi Y, Yasuda M, Hashida K, et al. Mechanism of salivary secretion enhancement by Byakkokaninjinto. Biol Pharm Bull 2008; 31:431–435. [DOI] [PubMed] [Google Scholar]
- 40.Pan CH, Hsieh IC, Liu FC, et al. Effects of a Chinese herbal health formula, “Gan-Lu-Yin”, on angiogenesis. J Agric Food Chem 2010; 58:7685–7692. [DOI] [PubMed] [Google Scholar]
- 41.Chien YC, Sheu MJ, Wu CH, et al. A Chinese herbal formula “Gan-Lu-Yin” suppresses vascular smooth muscle cell migration by inhibiting matrix metalloproteinase-2/9 through the PI3K/AKT and ERK signaling pathways. BMC Complement Altern Med 2012; 12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Jiang W, Xiao Y, et al. Multiobjective optimization on antiplatelet effects of three components combination by quantitative composition-activity relationship modeling and weighted-sum method. Chem Biol Drug Des 2014; 84:513–521. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Cheng Y, Zhang H. Phytochemical analysis of anti-atherogenic constituents of Xue-Fu-Zhu-Yu-Tang using HPLC-DAD-ESI-MS. Chem Pharm Bulletin (Tokyo) 2004; 52:1295–1301. [DOI] [PubMed] [Google Scholar]
- 44.Lin RT, Pai HC, Lee YC, et al. Electroacupuncture and rosiglitazone combined therapy as a means of treating insulin resistance and type 2 diabetes mellitus: a randomized controlled trial. Evid Based Complement Alternat Med 2013; 2013:969824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishizaki N, Okushi N, Yano T, et al. Improvement in glucose tolerance as a result of enhanced insulin sensitivity during electroacupuncture in spontaneously diabetic Goto-Kakizaki rats. Metabolism 2009; 58:1372–1378. [DOI] [PubMed] [Google Scholar]
- 46.Lee YC, Li TM, Tzeng CY, et al. Electroacupuncture-induced cholinergic nerve activation enhances the hypoglycemic effect of exogenous insulin in a rat model of streptozotocin-induced diabetes. Exp Diabetes Res 2011; 2011:947138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin RT, Chen CY, Tzeng CY, et al. Electroacupuncture improves glucose tolerance through cholinergic nerve and nitric oxide synthase effects in rats. Neurosci Lett 2011; 494:114–118. [DOI] [PubMed] [Google Scholar]
- 48.Choi S, Lee GJ, Chae SJ, et al. Potential neuroprotective effects of acupuncture stimulation on diabetes mellitus in a global ischemic rat model. Physiol Meas 2010; 31:633–647. [DOI] [PubMed] [Google Scholar]


