Abstract
Carbon monoxide (CO) poisoning may cause toxicity of the central nervous system and heart. However, the association between CO poisoning and long-term dementia risk remains unestablished. We investigated the incidence of dementia in patients with CO poisoning in Taiwan and evaluated whether they had a higher risk of dementia than did the general population.
A nationwide population-based cohort study was conducted among patients with CO poisoning identified using Taiwan's National Health Insurance Research Database (NHIRD) during 2004 to 2013. CO poisoning was defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification codes. The study cohort comprised patients with CO poisoning between 2005 and 2010 (N = 14,590). Each patient was age-, sex-, and index date-matched with 4 randomly selected controls from the comparison cohort (N = 58,360). All patients were followed from the study date until dementia development, death, or the end of 2013. Cox proportional hazards regressions were performed for comparing the hazard ratios for dementia between the 2 cohorts.
Incident cases of dementia were identified from the NHIRD.
After adjustment for potential confounders, the study cohort was independently associated with a higher dementia risk (adjusted hazard ratio, 2.75; 95% confidence interval, 2.26–3.35).
This population-based cohort study indicated that patients with CO poisoning have a higher risk of dementia than do people without CO poisoning.
INTRODUCTION
Dementia, a progressive and incurable disorder, is defined by impaired memory with at least 1 cognitive deficit including aphasia, apraxia, agnosia, or impaired executive function. In patients with advanced dementia, the year before death is characterized by a series of persistently severe disabilities, including profound memory impairment such as inability to recognize family members, minimal verbal abilities, inability to independently ambulate, inability to perform activities of daily living, and urinary or fecal incontinence.1,2 Although the etiology is not well known, risk factors reported for dementia include advanced age, a family history of dementia, and genetic factors. It was estimated ∼5 million Alzheimer disease patients in the United States in 2009. By 2050, the number is expected to increase to ∼13 million Americans with dementia.3 Because of its progressive neurodegenerative characteristics, dementia increasingly affects a considerable proportion of the aging population.4 Public health approaches to reduce burden and costs related to dementia are receiving increasing attention.
Carbon monoxide (CO), a toxic gas, is colorless, odorless, tasteless, and initially nonirritating, thus complicating its detection. The most common sources of accidental CO poisoning are faulty or inadequately ventilated gas heating appliances, fires, mining accidents, and automobile exhaust fumes. Homes in Taiwan do not usually have central heating and additional appliances may be required during the winter season. Natural gas, home delivered, is preferred for cooking and water heating. Moreover, CO generated from the smoke of burning charcoal can be intentionally utilized for committing suicide.5 Symptoms of mild acute CO poisoning include lightheadedness, confusion, headaches, vertigo, and flu-like effects; exposure for long periods can cause severe toxicity of the central nervous system and heart and even death. In most patients, most of the aforementioned symptoms completely resolve with the inhalation of oxygen or exposure to hyperbaric oxygen (HBO). However, there are ∼30% of CO poisoning patients exhibit chronic neurological symptoms because of late encephalopathy even 1 year after poisoning.6 Late encephalopathy in patients with CO poisoning causes neuropsychiatric symptoms, such as mental deterioration, parkinsonism, incontinence, mutism, and gait disturbance.7
However, a literature search revealed that only a few studies have addressed the association between CO exposure and dementia in adults. Therefore, we conducted a retrospective cohort study for determining the association between CO poisoning and dementia risk. This study provides data on the incidence of dementia after CO poisoning from a longitudinal health insurance database.
MATERIALS AND METHODS
Longitudinal Health Insurance Database
Taiwan's National Health Insurance program, established in 1995, provides mandatory universal health insurance. In 2007, the program covered nearly 99% (>25 million people) of the entire Taiwanese population. The database contains detailed patient information, including that on sex; date of birth; residential or work area; dates of clinical visits; the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes; details on prescriptions; expenditure amounts; and outcomes at hospital discharge (recovered, died, or transferred). The study was approved from full review by the Joint Institutional Review Board of Taipei Medical University.
Study Population
The study cohort comprised all patients diagnosed with CO poisoning (ICD-9-CM 986, E868.8)8 between January 1, 2005 and December 31, 2010. The exclusion criterion was dementia diagnosis (ICD-9-CM 290.0–290.4, 294.1, 331.0–331.2) before the CO exposure index date. Patients with missing variables, such as birth date and sex, were also excluded from the study. We also identified patients diagnosed with dementia in at least 3 consecutive examinations to ensure the accuracy of diagnosis. The resulting study cohort comprised 14,590 patients with CO poisoning. Each patient was then individually followed from the index ambulatory visit until dementia development, death, or December 31, 2013.
Matched Control Sample
The comparison cohort comprised the remaining patients in Taiwan's National Health Insurance Research Database (NHIRD) between January 1, 2005, and December 31, 2010. Patients diagnosed with CO poisoning during 2004 to 2013 were excluded from the comparison cohort. Patients diagnosed with dementia before index date matching were not included in the comparison cohort. A total of 58,360 patients were randomly stratified, and 4 patients were age ( ± 1 year-), sex-, and index date-matched with each patient from the study cohort. The index date was defined as the first day of CO poisoning, and this date of the comparison cohort corresponded to that of the study cohort.
Study Endpoint
Each patient was followed from his or her entry date until dementia development, death, or December 31, 2013. Dementia diagnosis in Taiwan involves a complete assessment of cognitive functions, followed by a complete physical examination, including neurological examination. Inpatient and outpatient diagnoses were reviewed to enable controlling for comorbid medical diseases, namely diabetes mellitus (DM), hypertension (HTN), coronary artery disease (CAD), stroke, hyperlipidemia, and cancer.
The National Cause of Death Registry contains data on all deaths; we assessed data until available December 2013. We used national identification numbers for linking data from the included patients with those obtained from the aforementioned registry.
Statistical Analysis
We compared the demographic data and comorbidities between the study and comparison cohorts. Differences in demographic characteristics and comorbidities were examined using Pearson's chi-squared test or the t test. The Cox proportional hazards regression model was used for comparing hazard ratios (HRs) for dementia between the study and comparison cohorts after adjustment for potential confounders, namely DM, CAD, HTN, stroke, hyperlipidemia, cancer, and the Charlson comorbidity index (CCI). Moreover, dichotomous variables in the model were assessed for proportionality by using exploratory diagnostic log–log survival plots to meet the proportional hazards assumption. We performed sensitivity analysis excluding patients diagnosed with dementia <1 to 2 years after CO poisoning. The study cohort was also reanalyzed after stratification by age (18–44, 45–64, > 65 years), sex, comorbidity status, and CCI (≤1 or >1). Moreover, we used Kaplan–Meier analysis for assessing the proportion of patients without dementia in each cohort. The dementia cumulative incidence curves based on the Kaplan–Meier estimate of the survival function for the study and comparison cohorts were plotted. All analyses were performed using the SAS statistical package (SAS System for Windows, Version 9.3.1, SAS Institute Inc., Cary, NC) or SPSS 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). P < 0.05 was considered significant.
RESULTS
Demographic characteristics and comorbidities among the study and comparison cohorts are shown in Table 1. The study cohort had fewer urban residents but higher rates of comorbidities before the index date than did the comparison cohort. The study cohort was more likely to have DM, CAD, stroke, cancer, HTN, and hyperlipidemia and a higher CCI (P < 0.001 for all) than was the comparison cohort (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Study Population
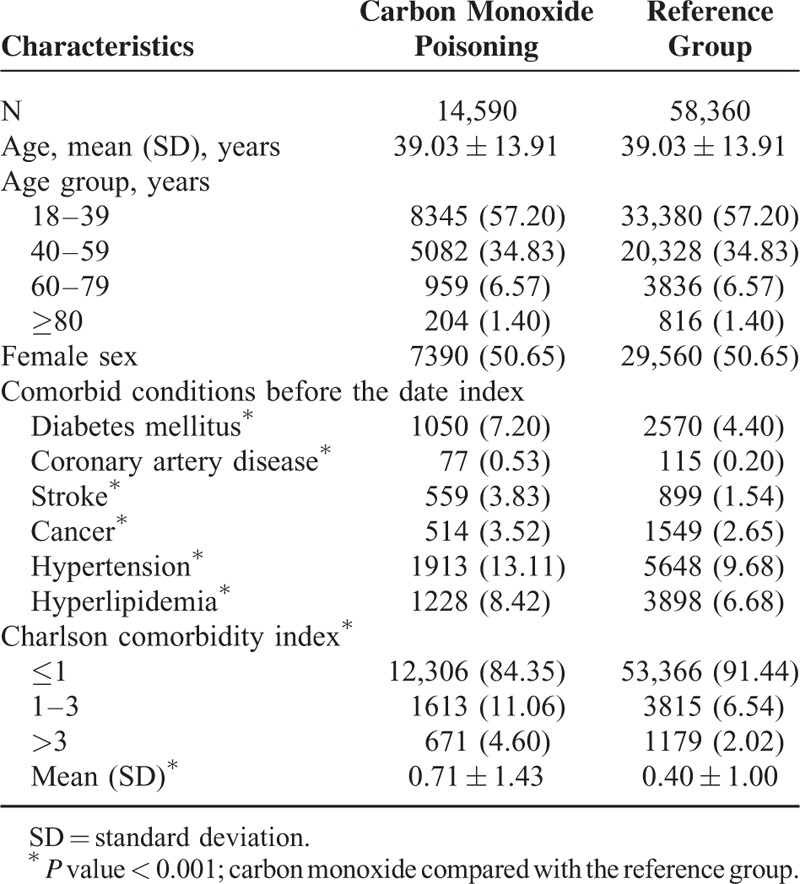
Table 2 presents the incidence and crude and adjusted HRs for dementia of both cohorts. Of all patients in the study cohort, 189 [23.33 per 10,000 person-years] experienced dementia during the 81,017.09 person-year follow-up period. The crude hazard ratio (HR) for dementia was 3.60 (95% confidence interval [CI], 2.97–4.37), and the adjusted HR was 2.75 (95% CI, 2.26–3.35) after adjustment for DM, CAD, HTN, stroke, hyperlipidemia, cancer, and CCI.
TABLE 2.
Incidence and Adjusted Hazard Ratios for Dementia During the 9-Year Follow-up Period
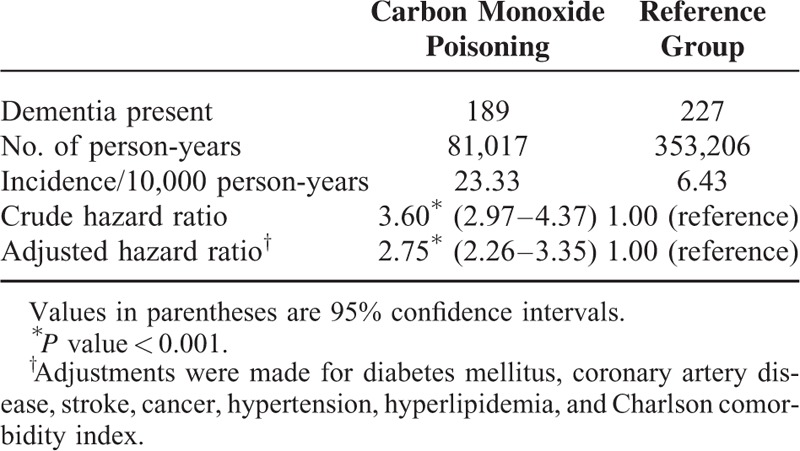
Sensitivity analysis results obtained using the Cox proportional hazards regression model for examining the risk of dementia after CO poisoning are shown in Table 3. We performed sensitivity analysis after excluding patients diagnosed with dementia <1 to 2 years after CO poisoning. The association between CO poisoning and dementia remained consistent. Kaplan–Meier analysis revealed survival curves based on the Cox proportional hazards regression model for the cumulative incidence of dementia in the study and comparison cohorts during the mean 5-year follow-up period. The cumulative incidence of dementia during the follow-up for the study cohort was significantly higher than that for the comparison cohort (P < 0.001; Figure 1).
TABLE 3.
Sensitivity Analysis of Cox Regression Model for Dementia
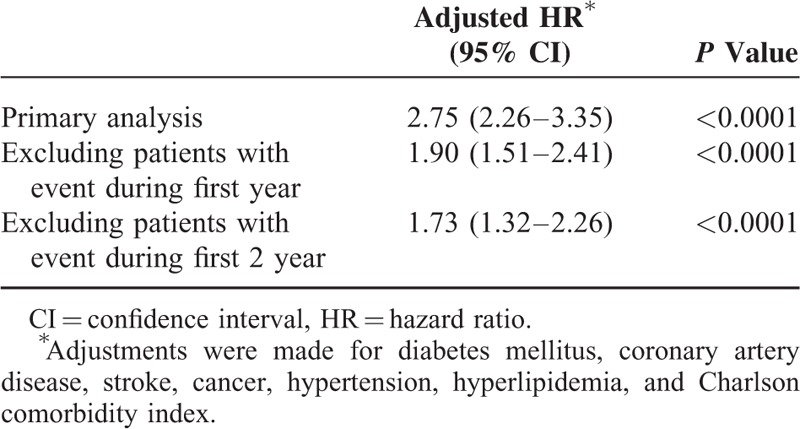
FIGURE 1.
Plot of dementia hazard curves based on the Cox model analysis for patients with carbon monoxide intoxication and comparison cohort.
After age stratification (Figure 2), the adjusted HR for dementia was 10.72 (95% CI, 4.76–24.13) for patients aged 18 to 44 years in the study cohort, which was higher than that reported for patients in the comparison cohort. Similarly, the adjusted HRs for dementia were 6.87 (95% CI, 4.23–11.15) and 2.63 (95% CI, 2.07–3.34) in patients aged 45 to 64 years and > 65 years, respectively, in the study cohort, which were higher than those for patients in the same age groups in the comparison cohort. We analyzed the data stratified by sex, comorbidity status, and CCI (≤1 or >1). The adjusted HR for dementia in the CO poisoning cohort stratified by sex, comorbidity status, and CCI (≤1 or >1) during the 9-year follow-up period were all higher than that in the comparison cohort, respectively. We further analyzed the data stratified by the existence of stroke or not. However, because of a small sample size and lack of power, the adjusted HR was not significantly higher (HR: 1.34, 95% CI, 0.91–1.99) for patients with stroke in the study cohort than for patients in the comparison group. The distribution of patients diagnosed with CO poisoning by the time of the emergency department visit is shown in the Figure 3. The frequency of CO poisoning were increased in winter months every year.
FIGURE 2.

Subgroup analysis of risk of dementia among patients with carbon monoxide intoxication and matched cohort.
FIGURE 3.
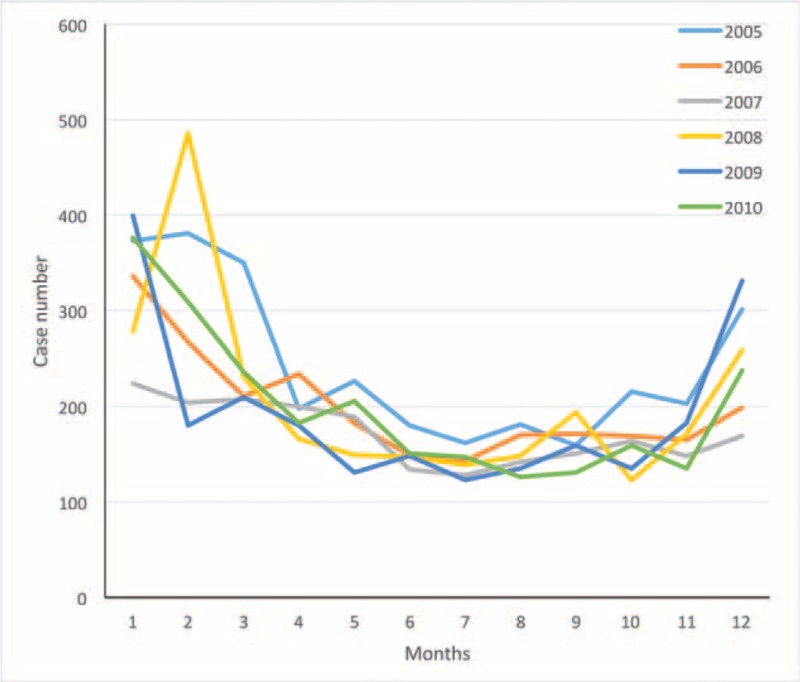
The curve represent patients with carbon monoxide poisoning in the time frame.
DISCUSSION
Several studies have indicated that CO poisoning is associated with a risk of toxicity of the central nervous system and heart. The present population-based cohort study conducted using Taiwan's NHIRD revealed a high dementia risk among patients with CO poisoning. The study also revealed a significant risk of dementia in a long-term follow-up after adjustment for age, sex, and comorbidity. The multivariate analysis of dementia aimed to achieve a balance between variables among the study and comparison cohorts through adjustment for DM, stroke, cancer, HTN, hyperlipidemia, and CCI thus eliminating bias. A major strength of this study was the use of a comprehensive national database to follow up the subsequent dementia development in patients with CO poisoning over a 9-year period. In this population-based study, we demonstrated that CO poisoning is associated with dementia in patients aged 18 to 64 years.
The mechanism and causality underlying the association between CO poisoning and dementia has to be elucidated. CO exposure reduces both the oxygen-carrying and -delivering capacities of the blood. It also induces marked oxidative stress and inflammatory responses via multiple hypoxia-independent pathways. The direct hypoxic effects, subsequent oxidative stress, and inflammatory responses cause varying degrees of end-organ damage.9 Magnetic resonance imaging of the brain may reveal imaging abnormalities, including increased numbers of T2-weighted hyperintensities, basal-ganglia lesions, and atrophy of the hippocampi after CO poisoning, which are associated with an increased risk of early cognitive decline.10–12 Although CO poisoning can cause neurological and neuropsychological problems, the incidence of sequelae after poisoning is not clearly known. Information on the long-term sequelae beyond the first year after CO exposure is limited.13–15 Weaver et al reported that 19% of patients had cognitive problems16 and 37% had abnormal neurological findings ∼6 years after CO poisoning.17 Our findings indicate that the incidence rate of dementia was 26.15 per 10,000 person-year in patients with CO poisoning. We performed a time-trend analysis for evaluating the risk of dementia by stratifying the event time. The effects of CO poisoning were significant during the follow-up period, implying a long-term dementia risk after the poisoning.
Elderly patients in the study cohort exhibited a high HR; younger patients also exhibited a considerable risk of dementia after CO exposure, implying that these patients require exclusive clinical care. Intentional or suicide attempt in the young is the potential cause but it needs further study to confirm the possibility. The standard treatment for CO poisoning includes the administration of oxygen and general supportive care.18 Two studies have demonstrated the benefits of HBO on memory.13,19 However, a meta-analysis revealed that randomized trials have not established the beneficial effects of HBO on neurological outcomes in patients with CO poisoning.20 HBO may be beneficial for more severe poisoning.
Frequent emergency department visits due to CO poisoning in winter months were observed in the literature.21 The distribution of patients diagnosed with CO poisoning in the NHIRD, which covers 99% of residents in Taiwan, also showed increased frequency of CO poisoning in winter months during 2005 to 2010. It may be related to unintentional poisoning due to the use of natural gas. CO poisoning causes neurological dysfunction, effects that can be irreversible. Developing current public health interventions such as CO alarms could reduce ongoing exposures to CO from common sources, such as those in the residential setting.
We used the NHIRD, which covers 99% of residents in Taiwan. The major strength of our study is the relatively large sample sizes in the study and comparison cohorts. However, this study has several limitations. First, dementia diagnosis based on ICD-9-CM codes may be less accurate than that obtained through a complete interview and neurological examination. To validate the dementia diagnoses in this study, we selected only patients who were diagnosed with dementia at least 3 consecutive times by clinical physicians. However, the study did not consider patients with dementia who did not seek medical care or whose diagnosis was miscoded. Furthermore, for comorbidities, we identified chronic diseases on the basis of patients making at least 3 visits to the hospital because of the same disease. Without conducting biological examination for disease validation, inaccurate diagnosis is likely, increasing the possibility of bias. Second, data on some variables, such as smoking, body mass index, and laboratory data were not available in the claims database. Although we matched the cohorts to balance their demographics and conducted stratified analysis to confirm the robustness of our results, unmeasured confounders possibly affecting both groups may exist. Third, clinical data on the neurological functions of patients, severity of CO poisoning and mortality rate could not be obtained from the database. Future prospective studies focusing on these concerns are warranted.
Footnotes
Abbreviations: CAD = coronary artery disease, CCI = Charlson comorbidity index, CI = confidence interval, CO = carbon monoxide, DM = diabetes mellitus, HBO = hyperbaric oxygen, HR = hazard ratio, HRs = hazard ratios, HTN = hypertension, ICD-9-CM = International Classification of Diseases Ninth Revision Clinical Modification, NHIRD = National Health Insurance Research Database.
Author contributions: CSW and Y-CL—study concept and design; LYH, T-TC, and H-PM—acquisition of data; CSW, T-TC, Y-HH, and S-HT —analysis and interpretation; CSW, Y-FL, and M-YW—critical revision of the manuscript for important intellectual content; M-YW—study supervision
Dr. Wu and all co-authors report no disclosures.
REFERENCES
- 1.Gill TM, Gahbauer EA, Han L, et al. Trajectories of disability in the last year of life. N Engl J Med 2010; 362:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouryamout L, Dams J, Wasem J, et al. Economic evaluation of treatment options in patients with Alzheimer's disease: a systematic review of cost-effectiveness analyses. Drugs 2012; 72:789–802. [DOI] [PubMed] [Google Scholar]
- 5.Shie HG, Li CY. Population-based case-control study of risk factors for unintentional mortality from carbon monoxide poisoning in Taiwan. Inhal Toxicol 2007; 19:905–912. [DOI] [PubMed] [Google Scholar]
- 6.Weaver LK, Hopkins RO, Elliott G. Carbon monoxide poisoning. N Engl J Med 1999; 340:1290.author reply 1292. [DOI] [PubMed] [Google Scholar]
- 7.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983; 40:433–435. [DOI] [PubMed] [Google Scholar]
- 8.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 9.Weaver LK. Carbon monoxide poisoning. N Engl J Med 2009; 360:1217–1225. [DOI] [PubMed] [Google Scholar]
- 10.Gale SD, Hopkins RO, Weaver LK, et al. MRI, quantitative MRI, SPECT, and neuropsychological findings following carbon monoxide poisoning. Brain Inj 1999; 13:229–243. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson RB, Hopkins RO, Cleavinger HB, et al. White matter hyperintensities and neuropsychological outcome following carbon monoxide poisoning. Neurology 2002; 58:1525–1532. [DOI] [PubMed] [Google Scholar]
- 12.Pulsipher DT, Hopkins RO, Weaver LK. Basal ganglia volumes following CO poisoning: a prospective longitudinal study. Undersea Hyperb Med 2006; 33:245–256. [PubMed] [Google Scholar]
- 13.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med 2002; 347:1057–1067. [DOI] [PubMed] [Google Scholar]
- 14.Jasper BW, Hopkins RO, Duker HV, et al. Affective outcome following carbon monoxide poisoning: a prospective longitudinal study. Cogn Behav Neurol 2005; 18:127–134. [DOI] [PubMed] [Google Scholar]
- 15.Weaver LK, Valentine KJ, Hopkins RO. Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am J Respir Crit Care Med 2007; 176:491–497. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins ROWL. Cognitive outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb Med 2008; 35:258. [Google Scholar]
- 17.Weaver LKHR, Churchill S, Deru K. Neurological outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb Med 2008; 35:258–259. [Google Scholar]
- 18.Wolf SJ, Lavonas EJ, Sloan EP, et al. Clinical policy: critical issues in the management of adult patients presenting to the emergency department with acute carbon monoxide poisoning. J Emerg Nurs 2008; 34:e19–e32. [DOI] [PubMed] [Google Scholar]
- 19.Thom SR, Taber RL, Mendiguren II, et al. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med 1995; 25:474–480. [DOI] [PubMed] [Google Scholar]
- 20.Buckley NA, Juurlink DN, Isbister G, et al. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev 2011; Cd002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract 2011; 13:1–14.quiz 14. [PubMed] [Google Scholar]


