Abstract
The aim of the study is to investigate the prevalence of high-risk human papillomavirus (hr-HPV) genotypes among Han women with high-grade cervical lesions in Beijing, China.
Cervical cell specimens from patients with histopathologically confirmed cervical lesions at 7 hospitals in Beijing were examined with a validated HPV kit for 13 hr-HPV genotypes during the study period. The patients were divided into a low-grade cervical lesions group (cervical intraepithelial neoplasia grade 1, CIN1) and a high-grade cervical lesions group (CIN2+, including cervical intraepithelial neoplasia grade 2, CIN2; cervical intraepithelial neoplasia grade 3, CIN3; squamous cervical cancer, SCC; and adenocarcinoma of the cervix, ACC) based on the histopathology results.
A total of 2817 eligible patients were enrolled, including 610 cases identified as CIN1 and 2207 as CIN2+. The hr-HPV positive rates in the CIN1 and CIN2+ groups were 78.2% (477/610) and 93.3% (2060/2207), respectively. The most frequently detected genotypes were HPV16, 58, 52 and18 in the CIN1 group and HPV16, 58, 33, and 52 in the CIN2+ group, in descending order of prevalence. In addition, the prevalence of HPV18 among the patients with ACC was 28.6% (14/49), significantly >7.2% (54/752) prevalence among the SCC patients (P < 0.001). Additionally, significantly more women in the CIN2+ group had multiple infections compared with those in the CIN1 group (38.1% and 24.9%, respectively; P < 0.001). However, as the cervical lesion grade increased, the prevalence of multiple hr-HPV infections gradually deceased to 44.2% in the CIN2 patients, 36.7% in the CIN3 patients, and 35.3% in the cervical cancer (CC) patients, which included SCC and ACC patients. In cases of multiple hr-HPV infections in the CIN2+ group, double infections accounted for ∼76.6%, and HPV16+58, HPV16+52, and HPV16+18 were the most common combinations, in descending order. The most frequent combination for triple infections was HPV16+58+31, with a rate of 4.2%. The highest positive rate occurred in the ≤24 year-old group for all types of cervical lesions.
The prevalence of HPV genotypes in the targeted population with high-grade cervical lesions differs from that of other countries. This information could be helpful for the prevention of CC in Beijing, China.
INTRODUCTION
Cervical cancer (CC) is a leading cancer among women worldwide. There were an estimated 527,600 new cervical cancers and 265,700 cervical cancer-related deaths worldwide in 2012, with an age-standardized incidence rate varying from 9.9 per 100,000 in developed countries to 15.7 per 100,000 in developing countries and mortality ranging from 3.3 per 100,000 in developed countries to 8.3 per 100,000 in developing countries.1 Moreover, nearly 90% of cervical cancer deaths occur in developing countries, such as China.1 China, with a population of ∼1.37 billion, is the world's most populous country; the cervical cancer incidence in China is high, with ∼132,300 new cases each year, yielding a rate of ∼27 per 100,000 women.2 Epidemiological studies and experimental data verify that persistent human papillomavirus (HPV) infection is considered to play a key role in the development of CC.3 The HPV infection prevalence for all types in CC varies widely, from as high as 95% in Oceania to 88% in Asia and 79% in Africa.4 To date, there are >200 HPV genotypes, which are divided into a “high-risk type,” known as oncogenic, and a “low-risk type” that generally only causes benign lesions. High-risk HPV (hr-HPV) can be detected in ∼90% of high-grade cervical lesions.5 Cervical intraepithelial neoplasia (CIN) reflects a continuous and progressive CC process, and high-grade squamous intraepithelial lesions (HSIL) with HPV infection, can develop and progress to CC over a period of 8 to 12 years.6
In recent years, significant progress has been made in CC prevention, including treatments and vaccines. For high-resource countries, the most efficacious way to prevent CC is to implement organized gynecological screening programs and appropriate treatments for detected precancerous lesions. However, in developing countries, this method is not possible because of limited resources and the complexity of appropriate screening. Therefore, vaccines against HPV are a promising means of reducing the CC incidence in a cost-effective manner. To date, 2 types of prophylactic HPV vaccines,7,8 the bivalent Cervarix® HPV vaccine (which primarily prevents HPV16 and 18) and the quadrivalent Gardasil® HPV vaccine (which targets HPV6, 11, 16, and 18), have been developed and are available in many countries worldwide but not in China.7,9,10 These vaccines address ∼70% of cervical cancers through protection from HPV16 and 18, which are the most common types of cervical cancer and account for approximately two-thirds of all cervical carcinomas worldwide.4,5,11,12 Recently, the efficacy and immunogenicity of a 9-valent HPV vaccine targeting HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 was reported. This type of vaccine prevented infection and disease related to HPV31, 33, 45, 52, and 58 in a susceptible population and generated an antibody response to HPV6, 11, 16, and 18 that was noninferior to that generated by the quadrivalent Gardasil® HPV vaccine.13
However, the prevalence of hr-HPV infection and the reported type-specific distribution varies greatly by geographic region and ethnicity.3–5,9–12 HPV16 is the most common infection type in patients with HSIL in all regions and among all ethnic groups; HPV58 and 52 are more common in patients with HSIL in developing and less developed countries; and HPV18, 33, and 45 are more common in patients with HSIL in more developed countries.6 In a retrospective cross-sectional worldwide study, the data indicated that the most prevalent HPV genotypes in CC were HPV16, 18, and 45, in decreasing order, in North America, Central South America, Africa, Asia, and Oceania.4 In a meta-analysis on the prevalence and attribution of HPV52 and 58 in cervical neoplasia worldwide, Chan et al found that the rates of HPV52 and 58 among cervical intraepithelial neoplasia grade 2 (CIN2), cervical intraepithelial neoplasia grade 3 (CIN3), and CC were significantly higher in Eastern Asia than in other regions worldwide.14 Furthermore, the data from mainland China indicated that HPV16, 18, 33, and 58 were the most common types in women with CC in Henan, central China,15 whereas HPV16, 58, 18, and 33 were the most prevalent types in CIN2+ (high-grade cervical lesions, including CIN2, CIN3, and CC) in women in Liaoning, northeast China16, and HPV16 and 58 were the most common types in CC and high-grade precancerous lesions in Chengdu, southwestern China.17 Another study investigating the distribution of HPV genotypes in the Eastern Inner Mongolian Autonomous Region, China, suggested that HPV16, 45, and 18 were the most prevalent genotypes in women with HSIL and CC, and that the prevalence of HPV45 were relatively higher than other regions of China and the prevalence of HPV18 in the CC of women from Mongolian was significantly higher than that in Han Chinese women.18
Beijing, with a population of 21.7 million, is the China's capital and one of its largest cities. Its population comprises 56 ethnic groups and Han Chinese, the largest ethnic group in China, accounts for 92% of the whole population. Although previous studies19–23 have reported the prevalence and distribution of HPV genotypes among women with cervical lesions in Beijing, China, generalization from these articles is difficult because they span different ethnicities and because patients were grouped by pathological diagnoses based on exfoliative cervical cells in some studies, or on tissues obtained through biopsy or surgery, the gold standard for the diagnosis of cervical lesions, in others. Hence, it is necessary to perform studies in Beijing, China, that are based on the gold standard.
This manuscript describes a hospital-based retrospective study on the prevalence of HPV genotypes in pathology-confirmed cervical lesions, especially high-grade cervical lesions, in Han women from 7 hospitals. This study aims to confirm the characteristics of the distribution of HPV genotypes among women in Beijing to help establish more cost-effective follow-up. It will have guiding significance for cervical cancer prevention.
MATERIALS AND METHODS
We identified patients from the medical records in the Statistics Departments and Medical Records Departments of 7 hospitals, distributed throughout 5 different regions of Beijing, China. We gathered data for patients who visited the hospitals for further diagnosis and treatment between January 2013 and July 2014. The inclusion criteria were patients (1) with cervical intraepithelial neoplasia grade 1 (CIN1) or greater, pathology-confirmed with cervical biopsy or hysterectomy or radical hysterectomy; (2) with HPV genotype testing results; (3) who were Han Chinese; (4) who had lived in Beijing for more than half a year. Cases were excluded when they met any of the following exclusion criteria: (1) re-examination after treatment of cervical lesions; (2) co-morbid endometrial lesion or ovary diseases or vaginal diseases; (3) pathological results of specimens from fractional curettage; (4) a history of cervical lesions; (5) a history of HPV infection; (6) immunocompromised condition (eg, infection with human immunodeficiency virus).
In these cases, tissue samples were collected from biopsies of colposcopy or advanced operations, including cervical conization and/or loop electrosurgical excisional procedure (LEEP) and/or hysterectomy or radical hysterectomy, performed by experienced gynecologists according to the disease stage. All specimens were evaluated by at least 2 experienced pathologists in their respective hospital's pathology department. CIN1, CIN2, CIN3, squamous cervical cancer (SCC), and adenocarcinoma of cervix (ACC) were diagnosed according to the standard criteria.24
In these cases, before colposcopy or any operation, consecutive liquid-based cervical cytology samples, obtained with a cytobrush from inside the endocervical canal and from the entire circumference of the ectocervix, were tested for 13 HPV genotypes, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. The testing was based on the polymerase chain reaction (PCR) and the Taqman technique using a commercially available High-risk Human Papillomavirus Genotyping Real Time PCR kit (Shanghai ZJ Bio-Tech Co., Ltd, China), which was validated and could identify these 13 types of hr-HPV.
Patient records/information were anonymized and de-identified before analysis. This protocol was approved by the ethical review board of the 7 participating hospitals.
The overall prevalence and type-specific hr-HPV prevalence were estimated for all cases and by 8 different age groups (≤24 years, 25–29 years, 30–34 years, 35–39 years, 40–44 years, 45–49 years, 50–55 years, and ≥55 years). Binary data are presented as number and percentage to characterize the number of women with cervical lesions, age at diagnosis, the prevalence of hr-HPV, and the prevalence of simple or multiple infections. Categorical data are reported for the histopathological results of CIN1/CIN2/CIN3/SCC/ACC and the hr-HPV genotype data. The data regarding simple and multiple hr-HPV infections in the CIN1 and CIN2+ groups were analyzed with Pearson's X2 test. The 2-sided P values were calculated, and P < 0.05 was considered statistically significant. All statistical analyses were conducted using the SPSS statistical analysis system software, version 17.0 (SPSS, Inc., Chicago, IL).
RESULTS
We identified 2817 eligible patients with a mean age of 43.5 years (range, 19–79) from the medical records. Of these patients, 610 were confirmed as CIN1, and 2207 were CIN2+, including 564 cases of CIN2, 815 cases of CIN3, 766 cases of SCC, and 62 cases of ACC. Of the total patient group, 90.1% (2537/2817) were infected with at least 1 type of hr-HPV. The hr-HPV positive rates in the CIN1 and CIN2+ groups were 78.2% (477/610) and 93.3% (2060/2207), respectively; the hr-HPV positive rate was significantly higher in CIN2+ than that in CIN1 (P < 0.001). The percentages of hr-HPV positive patients in the CIN2, CIN3, SCC, and ACC subgroups were 91.5% (516/564), 91.2% (743/815), 98.2% (752/766), and 79.0% (49/62), respectively.
The distribution of hr-HPV genotypes according to cervical lesions is shown in Figure 1. The 5 most common genotypes in patients with CIN1 were HPV16, 58, 52, 18, and 39. In the CIN2+ group, HPV16 was the most common genotype, accounting for 60.1% (1326/2207), followed by HPV58 (27.3%, 602/2207), HPV33 (10.0%, 221/2207), HPV52 (8.5%, 188/2207), and HPV18 (7.7%, 170/2207). The 5 most common hr-HPV genotypes were HPV16, 58, 52, 31, and 39 in CIN2; HPV16, 58, 33, 52, and 18 in CIN3; HPV16, 58, 33, 18, and 52 in SCC; and HPV16, 58, 18, 33, and 59 in ACC, in descending order. In addition, the prevalence of HPV18 in ACC was 28.6% (14/49), significantly >7.2% (54/752) prevalence in SCC (P < 0.001). A comparison of the prevalence of the 13 types of hr-HPV in women with high-grade cervical lesions in the present study and that reported in global statistics5 is presented in Table 1, which shows that worldwide, HPV16, 31, 33, 58, and 18 were the 5 most common genotypes in women with CIN2/CIN3; HPV16, 18, 33, 45, and 31 were the 5 most common genotypes in women with CC; and HPV16, 18, 31, 33, and 58 were the 5 most common genotypes in women with CIN2+, all in descending order.
FIGURE 1.

Distribution of high-risk human papillomavirus (hr-HPV) in CIN1 (cervical intraepithelial neoplasia grade 1), CIN2 (cervical intraepithelial neoplasia grade 2), CIN3 (cervical intraepithelial neoplasia grade 3), SCC (squamous cervical cancer), ACC (adenocarcinoma of the cervix), and CIN2+ (high-grade cervical lesions, including CIN2, CIN3, SCC, and ACC) patients. ACC = adenocarcinoma of the cervix, CIN1 = cervical intraepithelial neoplasia grade 1, CIN2 = cervical intraepithelial neoplasia grade 2, CIN2+ = (high-grade cervical lesions, including CIN2, CIN3, SCC, and ACC) patients, CIN3 = cervical intraepithelial neoplasia grade 3, hr-HPV = high-risk human papillomavirus, SCC = squamous cervical cancer.
TABLE 1.
Prevalence of 13 Types of High-Risk Human Papillomavirus in Women With High-Grade Cervical Lesions: A Comparison of the Current Study and Global Statistics

For all grades of cervical lesions, multiple hr-HPV infections were more common than single hr-HPV infection, and significantly more patients with CIN2+ had multiple hr-HPV infections compared with the CIN1 subgroup (38.1% and 24.9%, respectively; P < 0.001). However, in the CIN2+group, as the cervical lesion grade increased, the prevalence of multiple hr-HPV infections gradually deceased: 44.2% of the CIN2 patients had multiple infections, compared with 36.7% of the CIN3 patients and 35.3% of the CC patients (including SCC and ACC). In addition, the rate of multiple hr-HPV infections in the ACC subgroup was 79.6%, which was significantly >32.4% in the SCC subgroup (P < 0.001). In the CIN2+ group, the 5 most common genotypes with simple and multiple infections were HPV16, 58, 33, 52, 18, and HPV16, 58, 18, 33, 31, respectively. Moreover, in the CIN2+ group, all hr-HPV genotypes except HPV16 were more prevalent in women with multiple infections than that those with a simple infection. The prevalences of simple and multiple hr-HPV infections according to cervical lesion grade and genotype in the CIN2+ group were detailed in Figure 2A and B, respectively.
FIGURE 2.
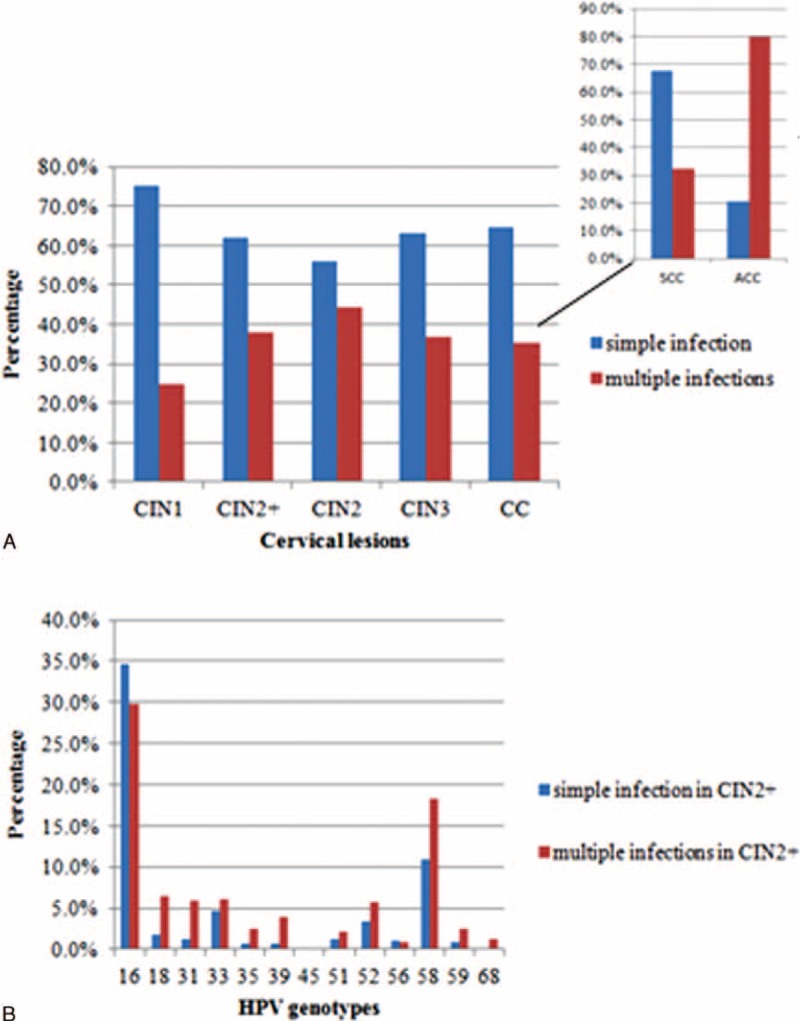
(A) Simple and multiple infections by high-risk human papillomavirus (hr-HPV) genotypes in cervical lesions; (B) simple and multiple infections by hr-HPV genotypes in CIN2+ (high-grade cervical lesions, including cervical intraepithelial neoplasia grade 2 [CIN2]), cervical intraepithelial neoplasia grade 3 (CIN3), cervical cancer (CC, including squamous cervical cancer [SCC] and adenocarcinoma of the cervix [ACC]) lesions. ACC = adenocarcinoma of the cervix, CC = cervical cancer, CIN2 = cervical intraepithelial neoplasia grade 2, CIN2+ = (high-grade cervical lesions, including CIN2, CIN3, SCC, and ACC) patients, CIN3 = cervical intraepithelial neoplasia grade 3, hr-HPV = high-risk human papillomavirus, SCC = squamous cervical cancer.
Some women with CIN2+ are infected with 2, 3, 4, or 5 types of hr-HPV simultaneously. Double infections accounted for the majority of multiple infections, ∼76.6% (601/785), of those, HPV16+58, HPV16+52 and HPV16+18 were the most common combinations, with rates of 29.0% (226/785), 6.0% (47/785), and 5.6% (44/785), respectively. The most frequent combinations in double HPV infections, in descending order, were HPV16+58, HPV16+59 and HPV16+31 in the CIN2 subgroup; HPV16+58, HPV16+18, and HPV16+52 in the CIN3 subgroup; HPV16+58, HPV16+52, and HPV16+18 in the SCC subgroup; and HPV16+58, HPV16+33, and HPV16+18 in the ACC subgroup. In the CIN2+ group, 22.4% (176/785) of patients had triple hr-HPV infections; the most frequent combination was HPV16+58+31, with a rate of 4.2% (33/785). The combinations and prevalences of the hr-HPV genotypes in multiple infections for each cervical lesion group and subgroup are specified in Table 2.
TABLE 2.
Combinations and Prevalence of High-Risk Human Papillomavirus Genotypes in Multiple Infections for Patients With High-Grade Cervical Lesions

The hr-HPV prevalence in the CIN1 and CIN2+ groups by the age group is shown in Figure 3A, and the prevalence and distributions of hr-HPV by the age group for patients with CIN2+, CIN2, CIN3, SCC, and ACC are presented in Figures 3B, C, D, E and F, respectively. For all cervical lesion types, the highest positive rate was observed in the ≤24 year group; this age group accounted for 90.5% of the CIN1patients; 93.8% of the CIN2 patients; 100% of the CIN3, SCC, and ACC patients; and 96.2% of the CIN2+ patients. In the CIN1 group, the hr-HPV positive rate decreased gradually until it reached its lowest point at 40 to 44 years; and then gradually increased as the age increased; in contrast, the hr-HPV infection rates in patients with CIN2+ were similar for all age groups, with a peak at ≤24 years. Moreover, in the CIN2+, CIN3, and ACC groups, HPV16 was most prevalent among younger women (≤39 years); in contrast, HPV58 and HPV18 were most prevalent among women in the ≥50-year and 40- to 44-year groups. Younger women in the CIN2 and SCC groups were more prone to infections with HPV16, 18, and 58, with the highest prevalence in patients aged ≤24 years.
FIGURE 3.
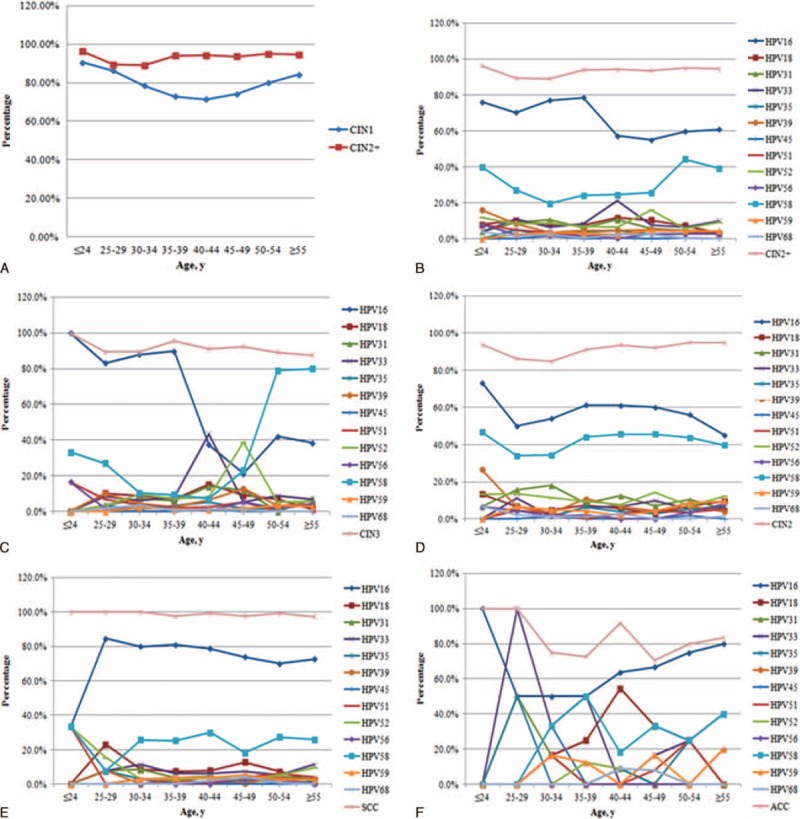
(A) Prevalence of high-risk human papillomavirus (hr-HPV) by age group in CIN1 (cervical intraepithelial neoplasia grade 1) and CIN2+ (high-grade cervical lesions, including cervical intraepithelial neoplasia grade 2 [CIN2], cervical intraepithelial neoplasia grade 3 [CIN3], squamous cervical cancer [SCC], and adenocarcinoma of the cervix [ACC]) patients; (B) age-specific distributions of hr-HPV genotypes in CIN2+ patients; (C) age-specific distributions of hr-HPV genotypes in CIN2 patients; (D) age-specific distributions of hr-HPV genotypes in CIN3 patients; (E) age-specific distributions of hr-HPV genotypes in SCC patients; (F) age-specific distributions of hr-HPV genotypes in ACC patients. ACC = adenocarcinoma of the cervix, CC = cervical cancer, CIN2 = cervical intraepithelial neoplasia grade 2, CIN2+ = (high-grade cervical lesions, including CIN2, CIN3, SCC, and ACC) patients, CIN3 = cervical intraepithelial neoplasia grade 3, hr-HPV = high-risk human papillomavirus, SCC = squamous cervical cancer.
DISCUSSIONS
Interestingly, we found that for Han population with high-grade cervical lesions in Beijing, China, HPV16 and 58 were the most 2 common genotypes, followed by HPV33, 52, and 18, in decreasing order. This coincides in part with Zhao's 19 and Li's 20 results, in which HPV16 and 58 were the most prevalent genotypes for the general female population in Beijing. Furthermore, another recent study demonstrated that HPV16 and 58 were the most common types in women with CIN2 or CIN3 in Beijing.21 Moreover, a worldwide meta-analysis reported that HPV 58 and 52 were relatively more prevalent in women with CIN2+ in Eastern Asia than those in Europe, North America, and Africa.14
The data on the prevalence and distribution of hr-HPV genotypes in cervical lesions worldwide are vast, and HPV16 is the most prevalent genotype.3–6,9–12,15–21 However, the results for other hr-HPV genotypes varied among some other cities in China and other parts of the world. HPV16, 45, and 18 were the most prevalent genotypes among patients with HSIL and CC from the Eastern Inner Mongolian Autonomous Region, China, and the prevalence of HPV18 in CCs in women from Mongolia was significantly higher than that in Han Chinese women.18 For Canada women, the 3 most frequent genotypes in order of decreasing frequency were HPV16, 52, and 31 in women with CIN2; HPV16, 31, and 18 in women with CIN3; and HPV16, 18, and 45 in women with CC.25 In a European multicenter study, Lucia et al reported that HPV16, 33, 31, and 18 were the 4 most common hr-HPV genotypes in CIN2+ patients.26 In a worldwide study, HPV 16, 18, 31, 33, 35, 45, 52, and 58, in descending order, were the most common genotypes in CC patients.12 Our findings suggest that HPV58 plays a significant role in the development of CIN and CC in Chinese women, especially Han Chinese women in Beijing, China.
In our survey, the hr-HPV-positive rates in CIN1 and CIN2+ patients were 78.2% (477/610) and 93.3% (2060/2207), respectively, which are both a little higher than those in a multiple-center US cervical cancer screening trial (65.6% (459/701) and 88.9% (442/497) in CIN1 and CIN2+, respectively),5 but lower than those for women in Denmark, who had hr-HPV prevalences of 89.5%(77/86) and 99.4% (182/183) in CIN1 and CIN2+ cases, respectively.10 This difference may be because the prevalence of HPV-positive status also vary varies among geographic locations and populations. Moreover, the prevalence highly depends on the detection methods used, as the studies by Susanne et al 10 and Keith et al confirm.27 Moreover, we carried out a retrospective study with a large sample size in which 2207 patients with CIN2+ were enrolled. According to the overall prevalence of CIN2+ (∼0.3%) in Wright TC's report,5 finding 2207 CIN2+ patients would require screening ∼735,667 women. However, all the data in our study were collected from medical records, and these patients underwent treatment for cervical lesions instead of cervical cancer screening, which reduced our screening workload on the one hand and may have resulted in an increased positive rate of HPV infection among the population with cervical lesions compared with a screening study. Moreover, ethnicity-dependent sensitivity may be another important factor in the frequency of HPV detection.
Our data suggested that multiple hr-HPV infections were associated with an increased risk of CIN2+ compared with CIN1(38.1% vs 24.9%, P < 0.001) and of ACC compared with SCC (79.6% vs 32.4%, P < 0.001); however, the rates of multiple hr-HPV infections in CIN2, CIN3, and CC parents showed a decreasing trend with severity of lesions. This finding is partially consistent with the results of previous studies, in which Francois et al25 found that the prevalence of multiple hr-HPV infections in CIN2+ parents was 49.1% (288/587), significantly <69.5% (323/465) in CIN1 parents; that the rates of multiple hr-HPV infections in CIN2, CIN3, and CC parents, similar to our results, declined gradually (72.2%, 61.3%, and 23.6%, respectively); and that the incidences of multiple infections were similar in patients with SCC and ACC (23.6% versus 21.0%). In a survey investigating multiple HPV infections in CC patients, Lee et al28 indicated that subjects infected with multiple hr-HPV types had a 31.8-fold higher risk of CC, whereas patients with single HPV infections had a 19.9-fold increased risk (P < 0.001). Moreover, for Chinese women, Hou et al23 and Li et al29 also observed higher prevalence of multiple hr-HPV infections than simple infections in CIN2+ patients, although the difference was not significant. In contrast, the data from another study reflected no relationship between multiple hr-HPV infections and the severity of cervical lesions, with prevalences of 67.2% and 76.7% in CIN1 and CIN2+ parents, respectively.30 Moreover, in a worldwide pooled analysis of 167 ACC patients, the multiple-infection rate was 8.9%, obviously lower than the simple infection prevalence of 91.1% .11 Thus, our data reflected a significant association between multiple hr-HPV infections and the progression of CIN1 to CIN2+ in Chinese women, especially the Han population in Beijing. Multiple hr-HPV infections maybe indeed be associated with the development of cervical lesions, or women with multiple HPV infections may be more susceptible to cervical carcinogenesis, or multiple HPV infections may produce conditions that confer immunological protection against persistent infection.28 In addition, the use of different assays to detect hr-HPV deoxyribonucleic acid (DNA) could also contribute to the diverse prevalence of multiple infections.31 Therefore, further studies of the relationship between multiple hr-HPV infections and the development of cervical lesions or different histological types are warranted.
Our data indicated that for CIN2+ patients infected with multiple hr-HPV genotypes, the combination of HPV16+58 plays a dominant role in all cervical lesion groups, contrary to findings in the Jewish Israeli population32 and in Austrians,26 but in agreement with findings in women in Beijing, China.23 Thus, the results of this study strongly support the key role of HPV16 and 58 in the development of CC and CIN in women in Beijing, China. In addition, in comparisons of the distribution of hr-HPV among pathological grades, co-infection with HPV16+18, similar to co-infection with HPV18, was more prevalent in women with ACC than in those with SCC, consistent with other studies. 4,5,11,25,27 In a large assessment of the prevalence of HPV genotypes, HPV16, 18, and 45 were detected in 443 out of 470 ACC patients from 38 countries worldwide4, and Xavier et al found that women with HPV18 had the highest risk of developing ACC compared with women with other hr-HPV types11. Furthermore, the ATHENA human papillomavirus study suggested that HPV18 was useful for identifying adenocarcinoma in situ.5 Thus, although the present study found that both HPV18 and HPV16+18 were the third most common infection or combination of double infections in the ACC group, we should pay close attention to patients with HPV18, given its role in causing ACC and to patients with HPV16 and 58.
Furthermore, we found that the highest hr-HPV positive rate occurred in the youngest patients and that among CIN1 patients, the rate decreased gradually as the age increased. This is similar to Wright TC's findings,5 which confirmed that the prevalence of hr-HPV peaked at age 21 to 24 years and decreased with increasing age in cervical lesion patients, especially those with CIN1 and CIN2. Furthermore, in another large population-based study in Denmark, Susanne et al reported that the HPV prevalence peaked in women aged 20 to 24 years and then decreased without a second peak in older women.10 However, several surveys in China >5 years ago reported that the HPV prevalence was highest in women aged 30 to 34 years and declined thereafter.19–20 In other studies, the researchers attributed the age-specific pattern to a relatively late age at the first intercourse led by the late marriage and late birth encouraged by a conservative society. It seems that hr-HPV is currently more prevalent in younger patients in China. China's rapid industrialization and urbanization over the last 4 decades is associated with an earlier age at first intercourse, a more active sexual life, smoking, and an increase in the number of sexual partners before marriage, which are the leading risk factors for hr-HPV infection and play key roles in the transmission of HPV and may be related to the current prevalence of hr-HPV infection.
In a population-based cohort study, Baandrup et al33 found that the prevalence of HPV16 in CIN2 and CIN3 was the highest among women aged 20 years and then decreased with increasing age to reach its lowest point among women aged 50 years. Similar results were found in this study: HPV16, with the highest prevalence at age ≤24 years in CIN2 patients, ≤24 years in CIN3 patients, 25 to 29 years in SCC patients, and ≤24 years in ACC patients, was more common among younger women; in comparison, HPV58, 52, 18, and 33 were more common in older women with CIN2+ (≥50 years, 45–49 years, 40–44 years, and 40–44 years, respectively). Nubia et al34 also found among CC patients that HPV16 was most prevalent in women aged ≤34 years, whereas both HPV58 and HPV52 were most common in women aged ≥50 years, and HPV18 and 33 had the highest frequency in women aged 35 to 49 years. In another study of HPV type-distribution among CC patients in China, Chen et al35 found that in patients with SCC, the prevalence of HPV16 at age ≤34 years was 85.4%, higher than that among patients aged 35 to 49 years and ≥50 years, whereas HPV18 was more common among patients aged 35 to 49 years and HPV52, 58, and 39 were all more common among patients aged ≥50 years. The age differences in the prevalence of HPV genotypes could reflect the different oncogenicity of different HPV types. For example, the higher prevalence of HPV16 in younger cervical lesion patients may indicate that females whose first intercourse occurs at a young age are susceptible to HPV16 36 or that HPV16 progresses more quickly to more serious, high-grade precancerous lesions or invasive cervical cancers than other hr-HPV types do.36,37 The possibility that lesions caused by HPV16 are larger than those caused by other hr-HPV types and therefore easier to detect could be another explanation.33 These differences might also reflect a change in the patterns of exposure to HPV infection among young Chinese women compared with older generations.35
There are some limitations in our study. First, we only enrolled Han women in Beijing, China. However, Han is the most common ethnicity in China; it accounts for >90% of the whole population and is the main ethnicity in Beijing. Moreover, we only included patients with cervical lesions, especially high-grade cervical lesions, because previous studies have reported that not all cervical lesions will progress to cervical cancer. In CIN1 cases, lesions generally regress, and the spontaneous regression rate is ∼60% to 85%, typically within 2 years. However, 40% of CIN2 lesions persist, whereas only ∼33% of CIN3 lesions regress, and 12% to 20% of them progress to cervical cancer.38,39 In contrast, a CIN1 diagnosis is not a significant risk factor for CIN3 beyond the risk attributed to molecular cause and genotype-specific HPV infection.40 In addition, other factors11,15 may be associated with the progression of CIN1 to CC, including the hr-HPV DNA load; age at first intercourse; high parity; smoking; endogenous and exogenous hormonal factors, such as parity, oral contraceptive use, and obesity; and infection with other sexually transmitted infectious agents, such as herpes simplex virus 2; however, these were not included or discussed in our study because HPV infection is the key risk factor in the development of cervical lesions and because no study has analyzed the hr-HPV distribution in the Han population of Beijing, China, based on histopathology results. In addition, we only detected 13 hr-HPV genotypes; others, such as HPV51 and 66, were not included because previous studies demonstrated that the 13 hr-HPV genotypes we included in our study account for >90% of CIN2+ and CC cases in China.15–17,21,22 Thus, detection that targets these HPV genotypes may improve cost-effectiveness.
Overall, our study provides updated knowledge related to ongoing research regarding prophylactic vaccines against HPV genotype-specific infections and cervical lesions in China. This study's findings will be beneficial for public health programs planning cervical cancer screening using hr-HPV DNA detection kits; specifically, our results suggest that the combined detection of HPV16 and 58 DNA would be more useful than the combined detection of HPV16 and 18 DNA in populations in Beijing, China. In addition, HPV vaccines could address 79.8% of CC and 74.1% of CIN2+ by providing protection against HPV16 and 58, the 2 most common types in patients with CC and CIN2+; these values are much higher than the 72.5% of CC cases and 64% of CIN2+ cases accounted for by HPV16 and 18 in the present study. Furthermore, in terms of cervical cancer prevention via HPV vaccines, the recently developed 9-valent HPV vaccine targeting HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 may be more efficacious for Chinese women, particularly those in Beijing than the bivalent Cervarix® HPV vaccine and the quadrivalent Gardasil® HPV vaccine.
Acknowledgments
The authors are very grateful to LiHong Bian, JinLan Jiao, Jing Liang, Qing Liu, ShengMei Xie, ChunLei Wang who helped with data collection in this collaborative study and are from Department of Obstetrics and Gynecology, the 307 Hospital of the People's Liberation Army; Department of Obstetrics and Gynecology, Air Force General Hospital of the People's Liberation Army; Department of Obstetrics and Gynecology, China-Japan Friendship Hospital; Department of Obstetrics and Gynecology, Beijing YouAn Hospital, Affiliated China Capital Medical University; Department of Obstetrics and Gynecology, Beijing AnZhen Hospital, Affiliated China Capital Medical University; Department of Infection Diseases and Clinical Microbiology, Beijing Chaoyang Hospital, Affiliated to China Capital Medical University, respectively. There were no funding sources for all of them.
Footnotes
Abbreviations: ACC = adenocarcinoma of cervix, CC = cervical cancer, CIN = cervical intraepithelial neoplasia, CIN1 = cervical intraepithelial neoplasia grade 1, CIN2 = cervical intraepithelial neoplasia grade 2, CIN2+ = high-grade cervical lesions, including CIN2, CIN3 and cervical cancer, CIN3 = cervical intraepithelial neoplasia grade 3, DNA = deoxyribonucleic acid, HPV = human papillomavirus, hr-HPV = high-risk human papillomavirus, HSIL = high-grade squamous intraepithelial lesions, LEEP = loop electrosurgical excisional procedure, PCR = polymerase chain reaction, SCC = squamous cervical cancer.
MX and QX contributed equally to this study. And both of them are considered as “first author.”
Funding: this study was supported in part by the Chinese High-tech R&D (863) Program.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lindsey AT, Freddie B, Rebecca LS, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Kidong K, Rongyu Z, Seok-Cheol C, et al. Current status of gynecological cancer in China. J Gynecol Oncol 2009; 2:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst 1995; 87:796–802. [DOI] [PubMed] [Google Scholar]
- 4.Silvia de S, Wim GVQ, Laia A, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CW, Mark HS, Catherine MB, et al. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol 2012; 206:46.e1–11. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26S:K1–K16. [DOI] [PubMed] [Google Scholar]
- 7.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by ocogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX. HPV vaccines and cervical cancer. Ann Oncol 2008; 19 Suppl 5:v48–v51. [DOI] [PubMed] [Google Scholar]
- 9.Diaz M, Kim JJ, Albero G, et al. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. Br J Cancer 2008; 99:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susanne KK, Gabrielle B, Christian M, et al. Population-based prevalence, type- and age- specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer 2008; 123:1864–1870. [DOI] [PubMed] [Google Scholar]
- 11.Castellsagué X, Díaz M, de Sanjosé S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst 2006; 98:303–315. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz SM, Daling JR, Shera KA, et al. Human papillomavirus and prognosis of invasive cervical cancer: a population-based study. J Clin Oncol 2001; 19:1906–1915. [DOI] [PubMed] [Google Scholar]
- 13.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–723. [DOI] [PubMed] [Google Scholar]
- 14.Chan PKS, Ho WCS, Chan MCW, et al. Meta-analysis on prevalence and attribution of human papillomavirus types 52 and 58 in cervical neoplasia worldwide. PLoS One 2014; 9:e107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong Sh, Jiaomei G, Yanqing L, et al. Epidemiology and genotype distribution of human papillomavirus (HPV) in women of Henan Province, China. Clinica Chimica Acta 2013; 415:297–301. [DOI] [PubMed] [Google Scholar]
- 16.Xia L, Shulan Zh, Qiang R, et al. Prevalence and type distribution of human papillomavirus in women with cervical lesions in Liaoning Province, China. Int J Gynecol Cancer 2010; 20:147–153. [DOI] [PubMed] [Google Scholar]
- 17.Jinke L, Dan Zh, Yi Zh, et al. Prevalence and genotype distribution of human papillomavirus in women with cervical cancer or high-grade precancerous lesions in Chengdu, western China. Int J Gynecol Obstet 2011; 112:131–134. [DOI] [PubMed] [Google Scholar]
- 18.En-qi W, Xianghui Y, Xiao Zh, et al. Distribution of human papillomavirus genotypes in archival cervical lesions in eastern inner Mongolian autonomous region, China. Int J Gynecol Cancer 2009; 19:919–923. [DOI] [PubMed] [Google Scholar]
- 19.Zhao R, Zhang WY, Wu MH, et al. Human papillomavirus infection in Beijing People's Republic of China: a population-based study. Br J Cancer 2009; 101:1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Changdong L, Minghui W, Jiandong W, et al. A population-based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing People's Republic of China. Cancer Epidemiol Biomarkers Prev 2010; 19:2655–2664. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Liu Z, Su J, et al. Human papillomavirus type-specific prevalence in women referred for colposcopic examination in Beijing. J Med Virol 2014; 86:1937–1943. [DOI] [PubMed] [Google Scholar]
- 22.Rong Zh, Christine V, Wen Ch, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grades 1 or worse among 4215 Chinese women in a population-based study. Cancer Epidemiol 2013; 37:939–945. [DOI] [PubMed] [Google Scholar]
- 23.Ren H, Caiyan X, Songwen Zh, et al. Distribution of human papillomavirus genotype and cervical neoplasia among women with abnormal cytology in Beijing, China. Int J Gynaecol Obstet 2012; 119:257–261. [DOI] [PubMed] [Google Scholar]
- 24.Kurman RJ, Ellenson LH, Ronnett BM. Blaustein's Pathology of the Female Genital Tract. 5th edn.New York, NY: Springer; 2002. [Google Scholar]
- 25.Francois C, Samuel R, Agnihotram VR, et al. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J Med Virol 2011; 83:1034–1041. [DOI] [PubMed] [Google Scholar]
- 26.Lucia R, Olaf R, Reinhard H, et al. Human papillomavirus in high-grade cervical lesions Austrian data of a European multicentre study. Wien Klin Wochenschr 2013; 125:591–599. [DOI] [PubMed] [Google Scholar]
- 27.Lo KWK, Yong YF, Chan MKM, et al. Prevalence of human papillomavirus in cervical cancer: a multicenter study in China. Int J Cancer 2002; 100:327–331. [DOI] [PubMed] [Google Scholar]
- 28.Sang Ah L, Daehee K, Sang SS, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer Lett 2003; 198:187–192. [DOI] [PubMed] [Google Scholar]
- 29.Ying L, Yipeng W, Chanwei J, et al. Detection of human papillomavirus genotypes with liquid bead microarray in cervical lesions of northern Chinese patients. Cancer Genet Cytogenet 2008; 182:12–17. [DOI] [PubMed] [Google Scholar]
- 30.Pista A, Oliveira A, Verdasca N, et al. Single and multiple human papillomavirus infections in cervical abnormalities in Portuguese women. Clin Microbiol Infect 2011; 17:941–946. [DOI] [PubMed] [Google Scholar]
- 31.Jack C, Linda H, George T, et al. Individual detection of 14 high risk human papilloma virus genotypes by the PapType test for the prediction of high grade cervical lesions. J Clin Virol 2014; 60:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laskov I, Grisaru D, Efrat G, et al. Are the human papillomavirus genotypes different in cervical cancer and intraepithelial neoplasia in Jewish Israeli women, a low-risk population? Int J Gynecol Cancer 2013; 23:730–734. [DOI] [PubMed] [Google Scholar]
- 33.Baandrup L, Munk C, Andersen KK, et al. HPV16 is associated with younger age in women with cervical intraepithelial neoplasia grade 2 and 3. Gynecol Oncol 2012; 124:281–285. [DOI] [PubMed] [Google Scholar]
- 34.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527. [DOI] [PubMed] [Google Scholar]
- 35.Wen C, Xun Zh, Anco M, et al. Human papillomavirus type-distribution in cervical cancer in China: the importance of HPV 16 and 18. Cancer Causes Control 2009; 20:1705–1713. [DOI] [PubMed] [Google Scholar]
- 36.Rolando H, Allan H, Concepcion B, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474. [DOI] [PubMed] [Google Scholar]
- 37.Carolina P, Ana CR, Allan H, et al. HPV types by age in cervical cancer precursors: predominance of HPV 16 in young women. Cancer Epidemiol Biomarkers Prev 2009; 18:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azuma Y, Hagiwara D, Lu W, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin Oncol 2014; 44:910–917.doi:10.1093/jjco/hyu112. [DOI] [PubMed] [Google Scholar]
- 39.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993; 12:186–192. [PubMed] [Google Scholar]
- 40.Philip EC, Julia CG, Cosette MW, et al. The clinical meaning of a cervical intraepithelial neoplasia grade 1 biopsy. Obstet Gynecol 2011; 118:1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]


