Abstract
The aim of this prospective study was to propose a new rating system using a risk model including conventional ultrasound (US) and acoustic radiation force impulse (ARFI) elastography for predicting central lymph node metastasis (LNM) in patients with papillary thyroid microcarcinoma (PTMC).
A total of 252 patients with PTMCs were enrolled, who were preoperatively evaluated by US and ARFI elastography including virtual touch tissue imaging (VTI) and virtual touch tissue quantification (VTQ). Risk factors of independent variables for central LNM were analyzed by univariate and multivariate analyses. A multivariate analysis was performed to create a predicting model and rating system.
Of the 252 patients, 72 (28.6%) had central LNMs. Multivariate analysis revealed that rare internal flow (odds ratio [OR]: 4.454), multiple suspicious foci on US (OR: 5.136), capsule involvement (OR: 20.632), and VTI area ratio (VAR) > 1 (OR: 5.621) were independent risk factors for central LNM. The final predicting model was obtained and the risk score (RS) was defined as 1.5 × (if rare internal flow) + 1.6 × (if multiple suspicious foci on US) + 1.7 × (if VAR > 1) + 3.0 × (if capsule involvement). The rating system was divided into 5 stages. Stage I, <1.5; Stage II, 1.5 to 3.0; Stage III, 3.1 to 4.7; Stage IV, 4.8 to 6.3; and Stage V, 6.4 to 7.8. The risk rates of central LNM were 3.4% (2/59) in Stage I, 13.3% (13/98) in Stage II, 54.2% (39/72) in Stage III, 72.2% (13/18) in Stage IV, and 100% (5/5) in Stage V (P < 0.001).
The results indicated that rare internal flow, multiple suspicious foci, capsule involvement on US, and VAR > 1 on ARFI elastography are the risk factors for predicting central LNM. The risk model developed in the study clearly predicts the risk of central LNM in patients with PTMC and thus has a potential to avoid unnecessary central compartment node dissection.
INTRODUCTION
Papillary thyroid microcarcinoma (PTMC) is defined as papillary carcinoma measuring ≤1.0 cm diameter.1 With the widespread use of thyroid ultrasound (US) and US-guided fine-needle aspiration biopsy (US-FNAB), the incidence of PTMC has increased remarkably.2 The prognosis of PTMC patients is generally excellent.3 However, the frequency of central lymph node metastasis (LNM) in PTMC is as high as 37% to 64% and is comparable with that of conventional papillary thyroid carcinoma (PTC).4,5 The guidelines from the American Thyroid Association (ATA) and European Thyroid Association (ETA) recommended therapeutic central compartment node dissection (CCND) in high-risk thyroid cancer patients, and prophylactic CCND in low-risk patients was regarded as a useful strategy for guiding subsequent treatment and follow-up.6,7 The routine prophylactic CCND suggested by ATA guidelines is largely due to the low diagnostic accuracy of imaging studies such as US and higher incidences of central LNM. Although preoperative neck US has been shown to be helpful for identifying the stage of PTMC, the sensitivities (29–62%) of preoperative US for central LNM were lower than those (sensitivities, 41–94%) for lateral LNM.8–10 However, the improvement in locoregional control or disease-specific survival is uncertain in PTMC patients with prophylactic CCND.11 In addition, the risk of surgical complications such as permanent hypoparathyroidism and recurrent laryngeal nerve injury may be increased with prophylactic CCND.12,13 Thus, whether to perform prophylactic CCND or not is still controversial in PTMC patients. It has also been shown that the recurrence rate of PTMC is associated with central LNM number, size, and the presence of extranodal extension.14,15 Therefore, a reliable predicting staging system of central LNM is needed for identifying patients at risk for central LNM and avoiding unnecessary CCND in PTMC patients with low risk of central LNM.
The suspicious US features for LNM include loss of fatty hilum, cystic change, round shape, calcification, hyper-echogenicity, and peripheral vascularization.16–18 On the other hand, approximately 90% of cervical LNMs with false-negative US characteristics cannot be detected preoperatively,6 especially for central LNM.19 To improve the detection of central LNM before operation, some authors explored the possibility of predicting central LNM using preoperative US features of PTMC and it was found that some US features of PTMC such as larger tumor size (>5 mm), bilateral foci, capsular invasion, and lateral LNM were reported to be related to central LNM.20,21 In addition, based on the relationship between clinicopathologic characteristics and tumor stiffness, it was reported that US elastography was helpful to predict cervical LNM or extrathyroidal extension.22–24
Acoustic radiation force impulse (ARFI) is a new US-based elastography technique, of which acoustic radiation force is emitted from the US transducer and the resultant tissue displacement and transverse shear wave transmission within the region of interest (ROI) is tracked. The former is denoted with qualitative virtual touch tissue imaging (VTI) whereas the latter is denoted with quantitative virtual touch tissue quantification (VTQ).25–30 In comparison with conventional US strain elastography techniques, ARFI is more operator-independent and reproducible.28–32 To our knowledge, no studies have been carried out to investigate the association between ARFI elastography findings and prognostic factors in patients with PTMC. And it is supposed that ARFI elastography features of PTMC might be helpful to predict central LNM as an adjunct to conventional US. The purpose of our study was to identify the risk factors for central LNM and then to propose a new rating system using a risk model including conventional US and ARFI elastography for predicting central LNM in patients with PTMC.
MATERIALS AND METHODS
Patients
The institutional review board approved this prospective study. Informed consent was obtained from all patients for their data scientifically analyzed, US-FNAB and surgery before each procedure.
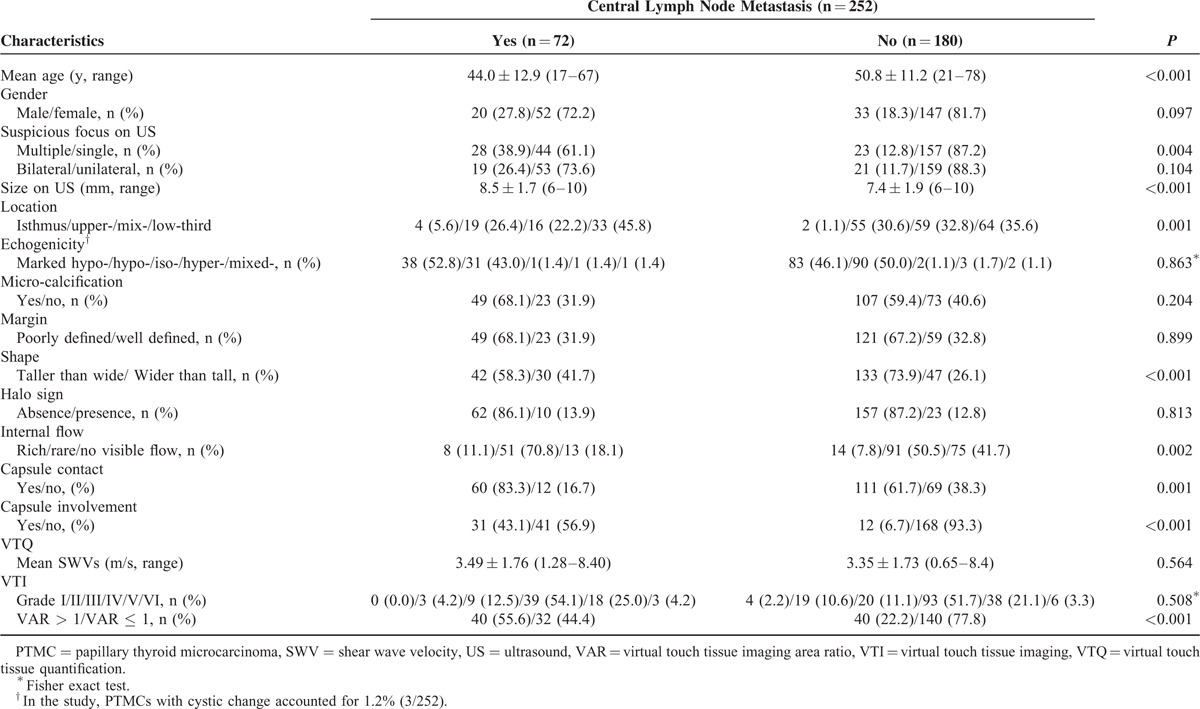
Preoperative staging US and ARFI elastography for thyroid nodule has been routinely performed at our institution before the patients undergo US-FNAB or surgery since 2011. From June 2012 to August 2014, 582 patients with suspicious nodules on US were observed. The inclusion criteria for the patients were as follows: the tumor size was 6 to 10 mm on US; without macro-carcinoma larger than 10 mm on US; and the tumor was confirmed by histopathology. Among the 582 patients, 309 patients had nodules with 6 to 10 mm in maximal diameter. Excluded patients were as follows: 12 patients had incomplete preoperative US and ARFI elastography data; 3 patients had undergone a previous head or neck operation; 3 patients had malignancies from other organs; 21 patients were excluded because the PTMCs had internal macro-calcifications (≥2 mm in diameter)26; and 18 patients who were proven to be benign nodules by US-FNAB or surgery and CCND was not performed. Two hundred three patients were diagnosed as malignancy or suspicious of malignancy on preoperative US-FNAB and 49 patients were diagnosed as suspicious malignancy on US without preoperative FNAB because the patients selected direct surgery. Finally, 252 patients (53 males and 199 females; mean age 48.8 ± 12.1 years, range 17–78 years) with PTMCs were included in this study. The basic characteristics of the patients were listed in Table 1.
TABLE 1.
Risk Factors of Clinic and US Characteristics for Patients With Papillary Thyroid Microcarcinoma
Conventional US and ARFI Elastography
Conventional US and ARFI elastography were performed by 1 of 2 board-certified investigators with 9 years’ experience in head and neck imaging. All imaging were obtained using the same S2000 US scanner (Siemens Medical Solutions, Mountain View, CA) with a 6 to 15 MHz linear transducer for conventional US and a 4 to 9 MHz linear transducer for ARFI elastography. Suspicious nodule on US was selected for imaging. The suspicious US features, as described in previous reports,6,26 were as following: taller than wide shape, hypo- or marked hypo-echogenicity, microcalcifications, poorly defined margin, and capsule involvement. The most suspicious nodule or the largest 1 was selected when there were multiple nodules. Both longitudinal and transverse scans were routinely performed and the largest dimension of tumor was measured. Findings on conventional US and ARFI elastography were prospectively recorded. US parameters of the index tumor as following were recorded: multiple or single suspicious foci, bilateral or unilateral suspicious foci, location (upper-, mid-, low-third in the lobe, and isthmus), echogenicity (hypo-, iso-, hyper-echogenicity, compared to surrounding thyroid tissue; marked hypo-echogenicity, echogenicity lower than the nearby strap muscle; mixed-echogenicity, containing 2 or more echogenic changes within the tumor), micro-calcification (<2 mm in diameter),26 margin (poorly defined margin, including microlobulated or speculated shape; well-defined margin, regular shape and distinct borderline between tumor and adjacent tissue), taller than wide shape (greater anteroposterior diameter than transverse or longitudinal diameter), halo sign (hypo-echoic rim at the periphery of the tumor), internal flow (rich internal flow, more than 3 linear or tree-like signals on color Doppler US; rare internal flow, dotted signals on color Doppler US; no internal flow, without signals on color Doppler US), capsule contact (tumor in contact with the adjacent thyroid capsule more than 25%), and capsule involvement (a loss of echogenicity in the adjacent thyroid capsule).
ARFI elastography was subsequently performed for the index tumor, and elastography imaging was obtained on longitudinal plane. Firstly, VTI mode was carried out. Under VTI mode, the split-screen mode including VTI and gray scale images are shown simultaneously. By comparing the nodule and thyroid tissue nearby, the gray scale value in VTI image is classified into black or white value. The scale ranges from white, showing areas of greatest strain (ie, softest component), to black, showing no strain (ie, hardest component). Based on the Xu's VTI grading method,26,28–30 VTI elastography images were divided into 6 grades according to the proportion of blackness and whiteness shown in the lesion, in which higher grade of VTI elastography corresponds to harder tissue. Then, the nodules area ratio was calculated using both VTI and B-mode images. The detailed method for measuring the ratio was as follows according to Xu et al reported28: A1 is defined as the area of the nodule drawn around the nodule margin on conventional US image. And A2 is defined as the area of the nodule drawn around the nodule margin on VTI. The VTI area ratio (VAR) was obtained by the ratio of A2 to A1.
Then, VTQ measurement was performed. In general, ROI was placed at the central portion of the tumor. The ARFI ROI is fixed at 5 × 6 mm so that for small tumor, it should be ensured that the push line (ie, the left line of the sampling ROI box) is within the tumor. The same site of tumor was measured 7 times with SWVs, the values of which were expressed in m/s as unit. For VTQ, the highest value and lowest values in the 7 measurements were excluded and the left 5 values were used for the average calculation. The higher the value of SWV is, the harder the tissue is. The VTQ measurement results of “x.xx m/s” occasionally happened in some extremely hard MPTCs. With excluding other factors such as improper pressing strength, patient breath and movement, or inappropriate ROI placement, the measurement result of “x.xx m/s” was assigned a value of 8.4 m/s per the suggestion of the equipment manufacturer and relevant recent reports.26–30
Conventional US and ARFI elastography features were prospectively reviewed by 1 investigator with more than 10 years’ experience in thyroid imaging. When multiple PTMCs were confirmed in the surgical specimen, the nodule corresponding to the index tumor on US was selected for analysis.
Thyroid Surgery and Reference Standard
The extent of thyroid surgery is determined in accordance with the guidelines of the ATA.6 Patients with multiple, bilateral tumors, and extrathyroidal invasion underwent total or near total thyroidectomy. Central compartmental neck dissection for lymph nodes, such as paratracheal, pretracheal, and prelaryngeal lymph nodes (LNs) was performed routinely when thyroid malignancy was diagnosed by preoperative cytology or intraoperative frozen-section analysis.
The final reference was based on the histopathological result. All histopathological findings were analyzed by the board-certified pathologist with 20 years’ experience in thyroid histopathology. Histopathological findings were initially analyzed according to intraoperative frozen-section, and then hematoxylin–eosin staining and immunohistochemistry were performed to determine the final result. Both histological subtypes and tumor node metastasis (TNM) staging were evaluated according to the American Joint Committee on Cancer/International Union against Cancer pathologic TNM classification criteria.33 Multifocality was defined as 2 or more malignant foci in 1 lobe, and bilaterality was defined as 1 or more malignant foci in both lobes. Extrathyroidal extension was defined when there was extrathyroid infiltration or cervical LNM.
Statistical Analysis
Statistical analysis was performed with SPSS software (version 13.0; SPSS Inc. Chicago, IL). Continuous quantitative data were expressed as mean ± standard deviation (SD) if in normal distribution and otherwise as range. Patients were divided into 2 groups according to the presence or absence of central LNM. Categorical variables of clinical characteristics were analyzed using Chi-squared test or Fisher exact test. Continuous variables in 2 groups were analyzed with Student t test. Univariate analysis was used to analyze the correlation between the predicting factors and central LNM. Multivariate logistic regression analysis was performed to determine the risk factors for central LNM. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated and the equation for predicting central LNM was created based on the statistically significant factors defined by multivariate analysis. Receiver-operating characteristic (ROC) curve of a predictive equation was measured to approve the reliability of multivariate analysis result. Then a model of rating system was established to predict the risk of central LNM. All tests were 2-sided and statistical significance was defined as P < 0.05.
RESULTS
Histopathological Findings
Based on the histopathological examination, all the 252 patients were confirmed to have PTMCs. Among them, 63 (25.0%) patients had multifocal foci and 44 (17.5%) had bilateral foci. Extrathyroidal extension was found in 2 (0.8%) of the patients. Cervical LNM was present in 73 (29.0%) patients, among whom 72 (28.6%) had central LNM and 1 (0.4%) had both central and lateral LNMs. A total of 86 (34.1%) patients had chronic lymphocytic thyroiditis on pathology. The mean number of central LNM was 2.3 ± 1.9 (median, 2; range, 1–9). Distant metastasis was not found in any of the patients. Among 252 patients, 20 patients had suspicious features for LNMs on US before surgery, such as loss of fatty hilum, cystic change, round shape, calcification, hyper-echogenicity, and peripheral vascularization; whereas only 11 (11/20, 55%) were confirmed by histology.
Univariate Analysis
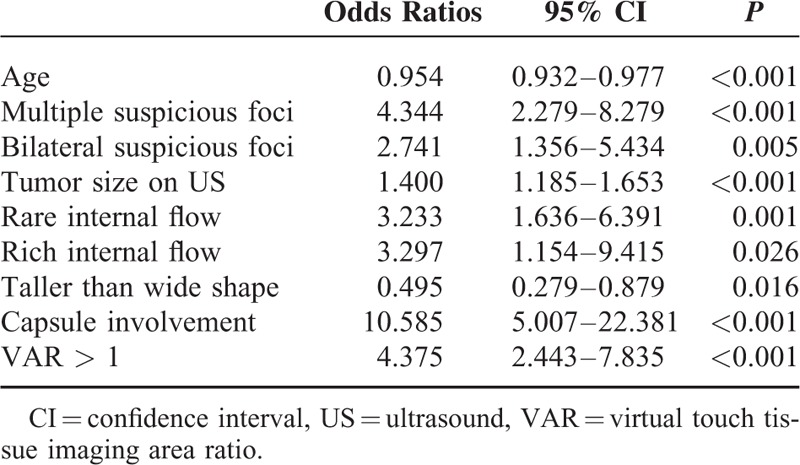
Univariate analysis demonstrated that the predictors such as multiple suspicious foci on US (OR: 4.344, P < 0.001), bilateral suspicious foci on US (OR: 2.741, P = 0.005), tumor size on US (OR: 1.400, P < 0.001), internal flow (rare internal flow, OR: 3.233, P = 0.001; rich internal flow, OR: 3.297, P = 0.026), capsule involvement (OR: 10.585, P < 0.001), and VAR > 1 (OR: 4.375, P < 0.001) were significantly associated with central compartment LNM. Age (OR: 0.954, P < 0.001) and taller than wide shape (OR: 0.495, P = 0.016) were significantly associated with low risk for central compartment LNM. None of the predictive factors such as gender, echogenicity, microcalcification, margin, halo sign, capsule contact, SWV value on VTQ, and VTI grade were found to be statistically significant in predicting central LNM in PTMC patients (all P > 0.05; Table 2).
TABLE 2.
Univariate Analyses of Clinical and US Factors for Predicting Central Lymph Node Metastasis
Multivariate Logistic Regression Analysis
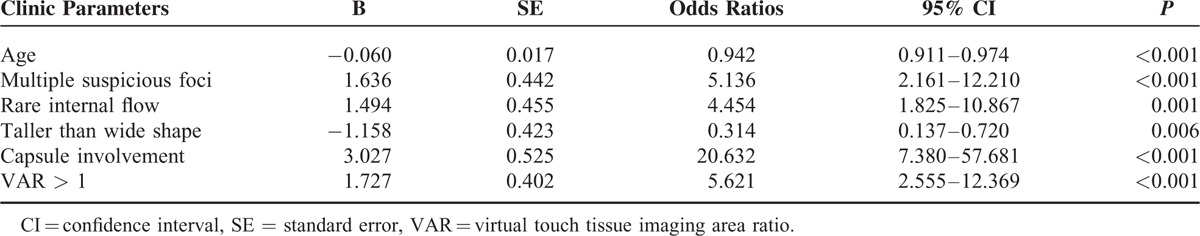
Multivariate logistic regression analysis entering all independent variables in this study was performed and determined that the predictors such as multiple suspicious foci on US (OR: 5.136, P < 0.001), rare internal flow (OR: 4.454, P = 0.001), capsule involvement (OR: 20.632, P < 0.001), and VAR > 1 (OR: 5.621, P < 0.001) were independent risk factors for predicting central compartment LNM (Figure 1). Age (OR: 0.942, P < 0.001) and taller than wide shape (OR: 0.314, P = 0.006) were significantly associated with low risk for central compartment LNM (Table 3).
FIGURE 1.
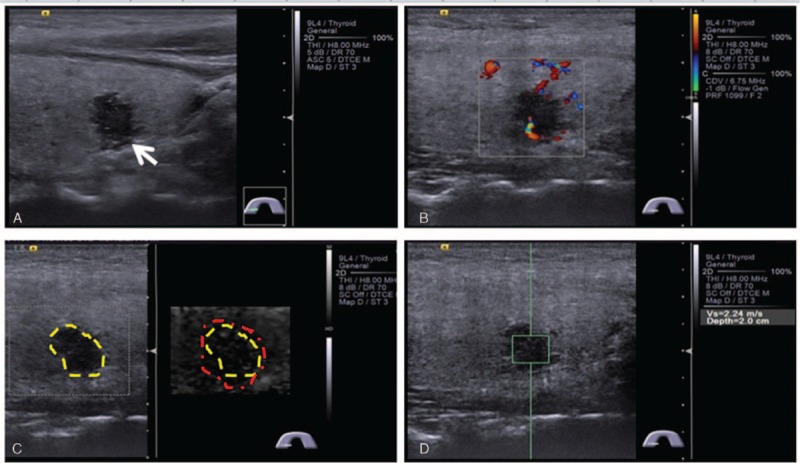
Conventional ultrasound (US) and acoustic radiation force impulse (ARFI) elastography findings of central lymph node metastasis (LNM) in a 36-year-old female patient with right papillary thyroid microcarcinoma (PTMC). (A) A longitudinal US image shows that a 10-mm PTMC invades thyroid capsule with a loss of echogenicity (arrow). (B) Rare internal flow is found on color Doppler flow image of the PTMC. (C) A virtual touch tissue imaging (VTI) image shows that the VTI area ratio (VAR) (linered/lineyellow) is larger than 1. (D) SWV of 2.24 m/s is displayed on virtual touch tissue quantification (VTQ) image. SWV = shear wave velocity.
TABLE 3.
Multivariate Analysis in Predicting Central Lymph Node Metastasis
A multivariate logistic regression equation was then created with the significant predictive variables and the ROC curve was plotted. The equation was established as following: P = 1/1 + ExpΣ [−0.09 + 0.06 × age (years) −1.636 × (if multiple suspicious foci on US) −1.494 × (if rare internal flow) + 1.158 × (if taller than wide shape) −3.027 × (if capsule involvement) −1.727 × (if VAR > 1)]. The diagnostic value of predictive equation for the population was accurate and discriminative with an area under the ROC curve of 0.888 (CIs, 0.844–0.932) (Figure 2). The sensitivity and specificity were 88.9% and 74.4%, respectively. The cut-off value for predicting central LNM in the equation was 0.22.
FIGURE 2.
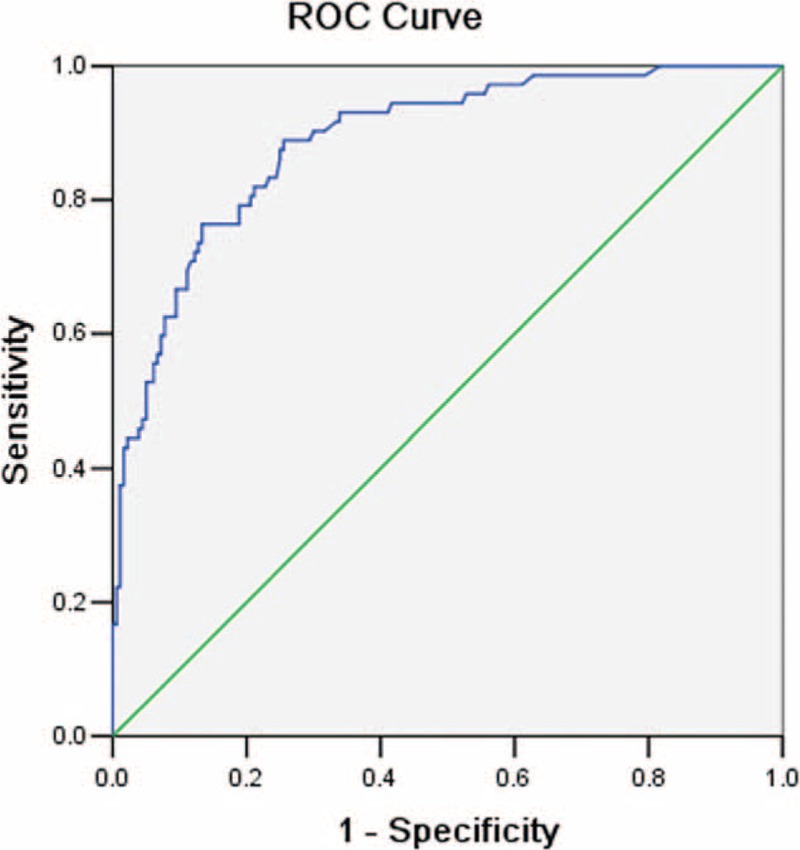
Receiver operating characteristic (ROC) curve. The equation for prediction of central lymph node metastasis (LNM) was accurate and discriminating, with an area under the ROC curve of 0.888. The point of cutoff value for predicting central LNM in the equation was defined as 0.22. The sensitivity and specificity was 88.9% and 74.4%, respectively.
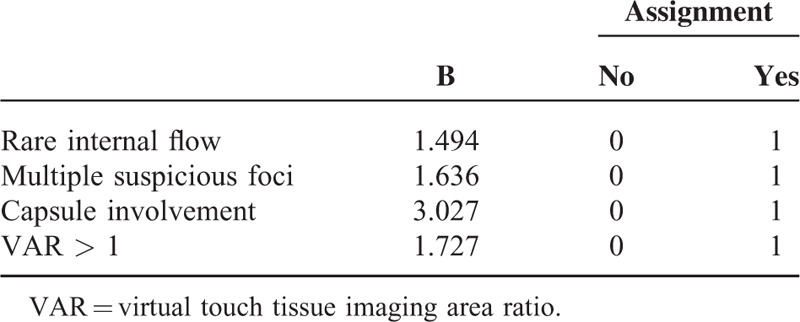
Scoring System Using a Predicting Model
A final predicting model was created based on the 4 risk factors selected by multivariate logistic regression analysis (Table 4). Risk score (RS) was defined as follows: RS = 1.5 × (if rare internal flow) + 1.6 × (if multiple suspicious foci on US) + 1.7 × (if VAR > 1) + 3.0 × (if capsule involvement). The rating system was divided into 5 stages: Stage I was marked low-risk group corresponding to those that the score was <1.5 and none of 4 risk factors was enrolled, included 59 patients (23.4%); Stage II was low-risk group corresponding to those that the score was 1.5 to 3.0 and any 1 of 4 risk factors was enrolled, included 98 patients (38.9%); Stage III was intermediate-risk group corresponding to those that the score was 3.1 to 4.7 and any 2 of 4 risk factors were enrolled, included 72 patients (28.6%); Stage IV was high-risk group corresponding to those that the score was 4.8 to 6.3 and any 3 of 4 risk factors were enrolled, included 18 patients (7.1%); and Stage V was marked high-risk group corresponding to those that the score was 6.4 to 7.8 and all of 4 risk factors were enrolled, included 5 patients (2.0%). The risk rates of central LNM were 3.4% (2/59) in Stage I, 13.3% (13/98) in Stage II, 54.2% (39/72) in Stage III, 72.2% (13/18) in Stage IV, and 100% (5/5) in Stage V (P < 0.001).
TABLE 4.
Predicting Model for Central Lymph Node Metastasis
DISCUSSION
The predictive factors for central LNM in patients with PTMC have not been well defined; although some studies reported that some clinical characteristics and US features were associated with central LNM.20,21 The present study included 17 clinical characteristics, US features, and ARFI elastography features as potential predictors for central LNM. The results indicated that the presence of central LNM was depending on the features such as rare internal flow, multiple suspicious foci, and capsule involvement on US, as well as VAR > 1 on ARFI. Among those independent predictors, capsule involvement (OR: 20.632, P < 0.001) on US was the most potent risk factor for predicting central LNM, which was in agreement with previous studies.20,21 Rare internal flow (OR: 4.454, P = 0.001) and multiple suspicious foci on US (OR: 5.136, P < 0.001) as the other 2 US features were also associated with central LNM. Until present, none of the studies had identified them as the predictors for central LNM. Histopathological characteristics such as the vascular infiltration, extension to the adjacent parenchyma or the thyroid capsule, multiple foci, are all associated with metastatic spread, which may explain the above-mentioned results.34,35
As a noninvasive technique, ARFI elastography including VTI and VTQ is a complementary tool for conventional US to evaluate the hardness of the tissue. In the present study, hard tumor indicated by VTI grade and SWV value on VTQ was not associated with central LNM. The findings were in agreement with some previous studies, which showed that a hard malignancy on elastography scores was not associated with central or lateral LNM.22–24 On the other hand, VAR > 1 (OR: 5.621, P < 0.001), as a new parameter on ARFI, was identified as a risk factor for central LNM. In comparison with VTI or VTQ that only stiffness of the PTMC is reflected, VAR reveals the stiffness of both the target tumor and the surrounding thyroid parenchyma. On pathology, some PTMC may infiltrate the adjacent thyroid tissue, which might be invisible on conventional US while visible on ARFI. The infiltration into the surrounding thyroid tissue might increase the stiffness of the surrounding tissue, which leads to enlarge stiff area on VTI. Therefore, the area on VTI is larger than that on conventional US and VAR > 1 is achieved. Recently, it has been confirmed that there is a positive association between the infiltrative margin found on histopathology and LNM.35–37 It may be the potential mechanism for VAR > 1 as a significant predictor for central LNM.
In the present study, age (OR: 0.942, P < 0.001) seemed to be the predictors of low-risk central LNM, as found in previous studies that central LNM is found more commonly in young patients.20,21 Taller than wide shape as an independent predictor of low-risk central LNM has not been reported before. The histologic feature of lateral tubular growth is an independent predictor of LNM in PTC, which may explain the result.35 In univariate analyses, predictive factors of bilateral suspicious foci on US, tumor size on US, and rich internal flow were associated with central LNM; however, they were not independent predictors after adjustment for other factors in multivariate logistic analysis. In addition, a multivariate logistic regression equation was established in the present study. The reliability of multivariate analysis result was confirmed by measuring ROC curve with an area under curve of 0.888, which was higher than that of 0.772 reported by Yang et al.20
Although many studies have demonstrated that patients with central LNM risk factors necessitate CCND,20,21 it is still difficult to decide which patients should undergo prophylactic CCND because of variegated risk factors for central LNM. Therefore, we proposed a rating system using a risk model including 4 predictive factors: rare internal flow, multiple suspicious foci on US, capsule involvement, and VAR > 1 based on multivariate logistic regression analysis. To our knowledge, there has been no such risk model so far.
By entering afore-mentioned 4 independent risk factors, a risk model with 5 stages was established. The addition of ARFI elastography feature may increase the discriminatory power. The model analyses showed that for the PTMC patients at Stages I, II, III, IV, and V, the risks of central LNM were 3.4%, 13.3%, 54.2%, 72.2%, and 100%, respectively. The results indicated that none or 1 of 4 predictive factors was expected to yield a low-risk stage of central LNM in patients with PTMC, and more than 2 of 4 predictive factors led to a high-risk stage. Thus, based on the model, prophylactic CCND was not suggested in patients at Stage I and II, whereas was suggested in patients at Stage IV and V. For Stage III patients, central LNM was probably indeterminate, and combining other risk factors such as male gender, larger tumor size (>5 mm), bilateral foci reported by previous studies might be helpful to determine the strategy of surgery.20,21 The risk model we proposed here is potential to clinical practice.
There were several limitations in this study. First, a selection bias may be present that only patients with 6 to 10 mm nodules enrolled in this prospective study because the ARFI ROI is fixed at 5 × 6 mm. On the other hand, many studies suggested that in nodules <10 mm US-FNAB or thyroidectomy might be not necessary. This viewpoint is controversial in that although PTMCs generally have an excellent outcome, a considerable percentage may have a more aggressive disease and worse prognosis, particularly in a subset of patients with BRAF (V600E) mutation and PTMCs >5 mm.38,39 Second, 6.3% (16/252) nodules showed results of “X.XX m/s” on VTQ, thus the true stiffness of those nodules were uncertain. Third, it is well known that microscopic or macroscopic central LNMs have different prognostic values. However, in this study, microscopic or macroscopic central LNMs were not subclassified because prophylactic CCND was routinely performed in all enrolled patients. Until now, whether to perform prophylactic CCND or not is still controversial in PTMC patients and many studies do not advice CCND in PTMCs. In addition, the LNMs were not evaluated by ARFI because the detection rate for central LNMs by US before surgery is relatively low. Finally, tumor recurrence and survival after central compartment dissection was not studied because long-term follow-up was not available in some enrolled patients.
CONCLUSION
In summary, rare internal flow, multiple suspicious foci, capsule involvement on US, and VAR > 1 on ARFI elastography are the risk factors for predicting central LNM. The risk model developed in the study could clearly predict the risk of central LNM in patients with PTMC and thus might be helpful to avoid unnecessary CCND.
Footnotes
Abbreviations: ARFI = acoustic radiation force impulse, CCND = central compartment node dissection, CI = confidence interval, LN = lymph node, LNM = lymph node metastasis, OR = odds ratio, PTMC = papillary thyroid microcarcinoma, ROC = receiver-operating characteristic, ROI = region of interest, RS = risk score, SWV = shear wave velocity, TNM = tumor node metastasis, US = ultrasound, US-FNAB = ultrasound-guided fine-needle aspiration biopsy, VTI = virtual touch tissue imaging, VTQ = virtual touch tissue quantification.
This work was supported in part by Grant SHDC12014229 from Shanghai Hospital Development Center, Grants 14441900900 and 15411969000 from Science and Technology Commission of Shanghai Municipality, and Grants 81401417 and 81501475 from the National Natural Science Foundation of China.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.DeLellis RA, Lloyd RV, Heitz PU, et al. WHO Classification of Tumors. Pathology and Genetics. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006; 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003; 13:381–387. [DOI] [PubMed] [Google Scholar]
- 4.Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg Oncol 2003; 237:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.So YK, Son YI, Hong SD, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery 2010; 148:526–531. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 7.Pacini F, Schlumberger M, Dralle H, et al. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006; 154:787–803. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope 2011; 121:487–491. [DOI] [PubMed] [Google Scholar]
- 9.Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol 2012; 76:131–136. [DOI] [PubMed] [Google Scholar]
- 10.Mulla MG, Knoefel WT, Gilbert J, et al. Lateral cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the lateral compartment. Clin Endocrinol 2012; 77:126–131. [DOI] [PubMed] [Google Scholar]
- 11.Hartl DM, Travagli JP. The updated American Thyroid Association Guidelines for management of thyroid nodules and differentiated thyroid cancer: a surgical perspective. Thyroid 2009; 19:1149–1151. [DOI] [PubMed] [Google Scholar]
- 12.White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg 2007; 31:895–904. [DOI] [PubMed] [Google Scholar]
- 13.Garcia A, Palmer BJ, Parks NA, et al. Routine prophylactic central neck dissection for low-risk papillary thyroid cancer is not cost-effective. Clin Endocrinol 2014; 81:754–761. [DOI] [PubMed] [Google Scholar]
- 14.Cho SY, Lee TH, Ku YH, et al. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery 2015; 157:111–118. [DOI] [PubMed] [Google Scholar]
- 15.Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012; 22:1144–1152. [DOI] [PubMed] [Google Scholar]
- 16.Antonelli A, Miccoli P, Ferdeghini M, et al. Role of neck ultrasonography in the follow-up of patients operated on for thyroid cancer. Thyroid 1995; 5:25–28. [DOI] [PubMed] [Google Scholar]
- 17.Park JS, Son KR, Na DG, et al. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol 2009; 192:66–72. [DOI] [PubMed] [Google Scholar]
- 18.Leboulleux S, Girard E, Rose M, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab 2007; 92:3590–3594. [DOI] [PubMed] [Google Scholar]
- 19.Choi YJ, Yun JS, Kook SH, et al. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg 2010; 34:1494–1499. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Chen C, Chen Z, et al. Prediction of central compartment lymph node metastasis in papillary thyroid microcarcinoma. Clin Endocrinol 2014; 81:282–288. [DOI] [PubMed] [Google Scholar]
- 21.Kim KE, Kim EK, Yoon JH, et al. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg 2013; 37:385–391. [DOI] [PubMed] [Google Scholar]
- 22.Moon HJ, Kim EK, Yoon JH, et al. Clinical implication of elastography as a prognostic factor of papillary thyroid microcarcinoma. Ann Surg Oncol 2012; 19:2279–2287. [DOI] [PubMed] [Google Scholar]
- 23.Park YJ, Kim JA, Son EJ, et al. Quantitative shear wave elastography as a prognostic implication of papillary thyroid carcinoma (PTC): elasticity index can predict extrathyroidal extension (ETE). Ann Surg Oncol 2013; 20:2765–2771. [DOI] [PubMed] [Google Scholar]
- 24.Jin ZQ, Lin MY, Hu WH, et al. Gray-scale ultrasonography combined with elastography imaging for the evaluation of papillary thyroid microcarcinoma: as a prognostic clinicopathology factor. Ultrasound Med Biol 2014; 40:1769–1777. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich-Rust M, Romenski O, Meyer G, et al. Acoustic radiation force impulse-imaging for the evaluation of the thyroid gland: a limited patient feasibility study. Ultrasonics 2012; 52:69–74. [DOI] [PubMed] [Google Scholar]
- 26.Xu JM, Xu HX, Xu XH, et al. Conventional US, US elasticity imaging, and acoustic radiation force impulse imaging for prediction of malignancy in thyroid nodules. Radiology 2014; 272:577–586. [DOI] [PubMed] [Google Scholar]
- 27.Gu J, Du L, Bai M, et al. Preliminary study on the diagnostic value of acoustic radiation force impulse technology for differentiating between benign and malignant thyroid nodules. J Ultrasound Med 2012; 31:763–771. [DOI] [PubMed] [Google Scholar]
- 28.Xu JM, Xu XH, Xu HX, et al. Prediction of cervical lymph node metastasis in patients with papillary thyroid cancer using combined conventional ultrasound, elastic imaging, and acoustic radiation force impulse (ARFI) elastography. Eur Radiol 2015; November 11 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Xu JM, Xu HX, Xu XH, et al. Solid hypo-echoic thyroid nodules on ultrasound: the diagnostic value of acoustic radiation force impulse elastography. Ultrasound Med Biol 2014; 40:2020–2030. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YF, He Y, Xu HX, et al. Virtual touch tissue imaging on acoustic radiation force impulse elastography: a new technique for differential diagnosis between benign and malignant thyroid nodules. J Ultrasound Med 2014; 33:585–595. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia KS, Tong CS, Cho CC, et al. Shear wave elastography of thyroid nodules in routine clinical practice: preliminary observations and utility for detecting malignancy. Eur Radiol 2012; 22:2397–2406. [DOI] [PubMed] [Google Scholar]
- 32.Lim DJ, Luo S, Kim MH, et al. Interobserver agreement and intraobserver reproducibility in thyroid ultrasound elastography. Am J Roentgenol 2012; 198:896–901. [DOI] [PubMed] [Google Scholar]
- 33.Edge SB, Byrd DR, Comptom CC, et al. AJCC Cancer Staging Manual. 7th edNew York, NY: Springer; 2010. [Google Scholar]
- 34.Zhao Q, Ming J, Liu C, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol 2013; 20:746–752. [DOI] [PubMed] [Google Scholar]
- 35.Jacquot-Laperrière S, Timoshenko AP, Dumollard JM, et al. Papillary thyroid microcarcinoma: incidence and prognostic factors. Eur Arch Otorhinolaryngol 2007; 264:935–939. [DOI] [PubMed] [Google Scholar]
- 36.Jung YY, Lee CH, Park SY, et al. Characteristic tumor growth patterns as novel histomorphologic predictors for lymph node metastasis in papillary thyroid carcinoma. Hum Pathol 2013; 44:2620–2627. [DOI] [PubMed] [Google Scholar]
- 37.Kim KJ, Hong SW, Lee YS, et al. Tumor margin histology predicts tumor aggressiveness in papillary thyroid carcinoma: a study of 514 consecutive patients. J Korean Med Sci 2011; 26:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastos AU, Oler G, Nozima BH, et al. BRAF V600E and decreased NIS and TPO expression are associated with aggressiveness of a subgroup of papillary thyroid microcarcinoma. Eur J Endocrinol 2015; 173:525–540. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Wei S, Han Y, et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF (V600E) mutational status of 977 cases. Ann Surg Oncol 2013; 20:2266–2273. [DOI] [PubMed] [Google Scholar]


