Abstract
Pregnancy-associated plasma protein-A (PAPP-A) level is an independent predictor of acute cardiovascular event occurrence. To test the hypothesis that increased PAPP-A levels would be associated with a higher burden of coronary thin-cap fibroatheroma (TCFA) thereby underlying the heightened risk for cardiovascular events in patients with coronary artery disease; 154 patients (462 vessels and 975 plaques) with stable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS) referred for percutaneous coronary intervention were assessed using 3-vessel virtual histology (VH)-intravascular ultrasound (IVUS). Thin-cap fibroatheroma virtual histology was defined as focal, necrotic core (NC)-rich (≥10% of cross-sectional area) plaques in contact with the lumen, and plaque burden ≥40%. Pregnancy-associated plasma protein-A levels were determined by sandwich enzyme-linked immunosorbent assay, and patients were divided into 3 groups based on PAPP-A level tertiles. Although the highest PAPP-A level tertile was not associated with 3-vessel plaque number, it was associated with 3-vessel VH-TCFA number and necrotic core volume. Patients with ≥3 VH-TCFAs had a higher PAPP-A level than patients with 1 to 3 VH-TCFAs or without any VH-TCFA (13.3 ± 11.8 versus 7.8 ± 4.7 versus 7.4 ± 4.7 mIU/L, P < 0.001, respectively). Moreover, PAPP-A level was an independent predictor of higher total number of VH-TCFAs (OR 1.18; 95% CI 1.07–1.29, P = 0.001). This VH-IVUS study demonstrated, for the first time to our knowledge, that higher PAPP-A levels are associated with higher 3-vessel TCFA burden in patients with coronary artery disease. Pregnancy-associated plasma protein-A, therefore, might be a useful serum biomarker to predict increased coronary TCFA burden and plaque instability.
INTRODUCTION
Pregnancy-associated plasma protein-A (PAPP-A), a zinc-binding matrix metalloproteinase that belongs to the metzincin superfamily of metalloproteinases, was initially discovered in the plasma of women in advanced stages of gestation1 but can also be produced in many nonplacental sites, including the cardiovascular system.2 Recently, increased activity of PAPP-A has been implicated in acute coronary syndrome (ACS), and circulating PAPP-A has been reported as an independent predictor of future adverse cardiovascular events and therefore a promising biomarker for risk stratification of ACS patients.2–4
Autopsy studies suggest that the majority of ACS occurs after rupture of a thin-cap fibroatheroma (TCFA) containing a thin fibrous cap overlying a large necrotic core.5–7 The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study found that in patients presenting with ACS the untreated nonculprit lesion most likely caused subsequent major adverse cardiac events (MACE); and almost always had at least 1 of 3 high-risk features: TCFA phenotype by virtual histology (VH)-intravascular ultrasound (IVUS), plaque burden (PB) ≥70%; and minimal luminal area (MLA) ≤4.0 mm.8 Furthermore, the vulnerable atherosclerosis (VIVA) study and the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis-Intravascular Ultrasound (ATHEROREMO-IVUS) study demonstrated that VH-TCFA in both culprit and nonculprit lesions was associated with overall MACE.9,10
Although PAPP-A is implicated in cardiovascular diseases, and increased PAPP-A immunostaining has been found within vulnerable plaques in autopsy patients and in animal models,2–4,11 the association between circulating PAPP-A level and coronary TCFA burden has not been demonstrated in vivo in patients. The current 3-vessel VH-IVUS study therefore tested the hypothesis that patients with elevated PAPP-A levels would have a higher coronary burden of VH-TCFAs that could explain their heightened risk for MACE.
METHODS
Patient Population
From January 2009 through December 2013, patients with either stable angina or non-ST-segment elevation ACS (NSTE-ACS) who were referred for percutaneous coronary intervention of a native, de novo coronary lesion were recruited. Exclusion criteria were ST-segment elevation myocardial infarction (STEMI); previous revascularization; anatomy unsuitable for 3-vessel VH-IVUS; any active inflammatory condition (ie, current infection, vasculitis, or rheumatic disease); and having received heparin before blood sampling for PAPP-A measurement. Data of angiograms, grayscale IVUS, and VH-IVUS were prospectively entered into the study database.
This study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board. Informed consent was obtained from all patients before the procedure.
Virtual Histology-intravascular Ultrasound Acquisition and Analysis
Virtual histology intravascular ultrasound was performed in all main coronary arteries-target vessels before and after percutaneous coronary intervention and all major epicardial nontarget vessels. All IVUS data were acquired with the Volcano S5 Imaging System (Volcano Therapeutics, San Diego, CA). No patient developed angina or had any complication (ie, distal embolization, no-reflow, or thrombus formation) during IVUS imaging, which was performed after intracoronary administration of nitroglycerin (100–200 mg). The IVUS catheter was advanced into the most distal safe position and withdrawn to the aorta-ostial junction using an automatic pullback system with a standard pull back speed 0.5 mm/s. Grayscale data was captured at 30 frames/s. Backscatter data were captured at the peak “R” wave using electrocardiographic triggering to adjust for catheter oscillation during myocardial contraction.
Each grayscale IVUS and VH-IVUS frame was co-registered with the corresponding angiographic roadmap with the use of side branches for alignment as previously described.11,12 Culprit lesions were identified on the basis of the association between angiographic lesion appearance and electrocardiographic changes or myocardial ischemia as detected during stress testing. All analyses were performed offline using Volcano Image Analysis Software without knowledge of PAPP-A levels and clinical information. External elastic membrane (EEM) and lumen borders were contoured for each slice. Quantitative IVUS measurements included EEM cross-sectional area (CSA), lumen CSA, plaque and media (P&M = EEM minus lumen) CSA, and plaque burden (P&M divided by EEM). Volumes were calculated using Simpson rule. Plaque volume burden was defined as plaque volume divided by analysis length.
A lesion on IVUS imaging was defined as at least 3 consecutive frames with a plaque burden of at least 40% and was classified by means of radiofrequency analysis consistent with published VH-IVUS classifications13: VH-TCFA; thick cap fibroatheroma (ThCFA); pathological intimal thickening (PIT); fibrotic plaque; and fibrocalcific plaque. Fibrotic plaque had mainly fibrous tissue with <10% confluent necrotic core, <10% confluent dense calcium, and <15% fibrofatty plaque. Fibrocalcific plaque had mainly fibrous tissue with >10% confluent dense calcium, but <10% confluent necrotic core. Pathological intimal thickening was a mixture of all plaque components, but dominantly fibrofatty plaque with <10% confluent necrotic core and <10% confluent dense calcium. Fibroatheroma (both VH-TCFA and ThCFA) was defined as >10% confluent necrotic core; because the resolution of VH-IVUS was 150–250 μm, it was not possible to detect fibrous cap thickness <65 μm (the typical pathologic definition of a thin fibrous cap) so that if there was >30° of necrotic core abutting to the lumen in 3 consecutive slices, the fibroatheroma was classified as VH-TCFA; otherwise, it was classified as ThCFA. The kappa coefficient of agreement for plaque classification for intraobserver variability was 0.90 (interval 3 months) and interobserver variability was 0.83 indicating excellent reproducibility. Intravascular ultrasound analysis was performed without knowledge of the findings of patient characteristics and blood measurement.
Blood Sampling and Pregnancy-associated Plasma Protein-A Measurement
Blood sampling for PAPP-A measurement was conducted at the time of hospital admission, before any administration of heparin and before angiography or coronary intervention to avoid preanalytical bias.14–16 All patients who had received heparin before sampling were excluded because heparin administration has been associated with a rapid and significant increase in PAPP-A level because of detachment of PAPP-A from vessel walls.17 Blood was drawn and centrifuged immediately, and serum was then placed into aliquots and stored at −80 °C. Circulating PAPP-A levels were determined by means of a modified sandwich enzyme-linked immunosorbent assay technique using the US DSL-10–27200 PAPP-A ELISA kit (Diagnostic Systems Laboratories, Inc, TX) with interassay and intraassay coefficients of variation of <5% and 10%, respectively.18 Technicians measuring PAPP-A level were blinded to clinical information. Levels of total, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, triglycerides, cardiac troponin I (cTnI), and high-sensitivity C reactive protein (hsCRP) were measured using standard methods.
Data Collection and Follow-up
Patients were followed up via clinical visits or telephone calls to the referring physician. Major adverse cardiac events included death, nonfatal myocardial infarction, and recurrent angina. The time to event was recorded and patients were studied for 1 year.
Statistical Analysis
Variables were compared between groups using t-test and analysis of variance or the Mann–Whitney nonparametric test (displayed as median and interquartile ranges [IQRs]) when the frequency distribution was skewed and not normally distributed.19 The Kaplan–Meier method was used for plotting time to event, and the log rank test was used to compare event-free survival curves.20 Simple logistic regression analysis was used to assess univariate associations between patient characteristics, biochemical measurements, grayscale IVUS parameters, VH-IVUS parameters, and tertiles of PAPP-A levels. Multiple logistic regression analysis was used to assess the independent adjusted relationship between PAPP-A levels and VH-TCFA; independent variables were those with P < 0.2 on univariate analysis. P < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SPSS15.0 software (SPSS Inc, Chicago, IL).
RESULTS
One hundred and fifty-four patients underwent 3-vessel VH-IVUS, and 975 plaques were classified. The interval between the latest onset of symptoms and first blood collection was 12.7 ± 6.4 hours. Median PAPP-A levels were 9.5 (interquartile: [3.7, 12.6]) mIU/L overall, and 2.9 mIU/L, 9.5 mIU/L, and 13.7 mIU/L in the low, intermediate, and high tertile groups, respectively. There was no significant difference in the interval between the latest onset of symptoms and blood collection among tertile groups: 13.4 ± 8.7 versus 12.6 ± 4.7 versus 12.2 ± 5.0 hours, respectively, P = 0.603.
Patient Characteristics
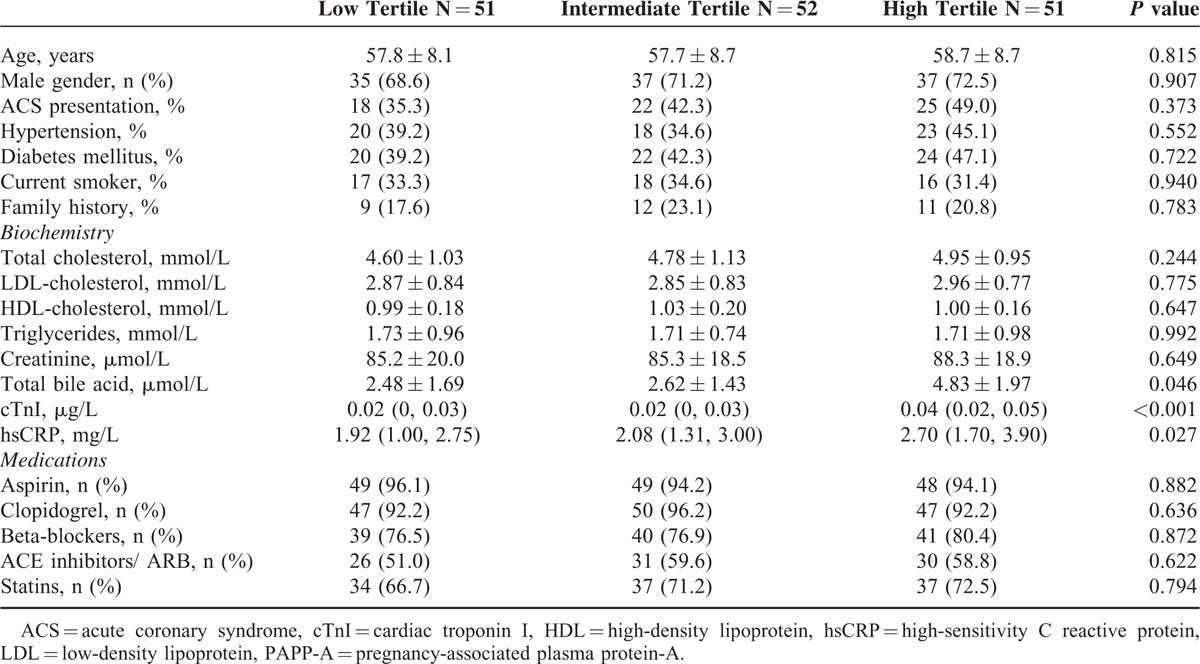
Baseline characteristics of patients are presented in Table 1. Distributions of established risk factors and medications at study entry were similar. Patient age was 58.1 ± 8.5 years, 70.8% were men, 42.9% had type 2 diabetes mellitus, and 42.2% presented with ACS. cardiac troponin I (P < 0.001) and hsCRP (P = 0.027) levels were higher in the high PAPP-A level tertile group.
TABLE 1.
Baseline Patient Characteristics According to Pregnancy-Associated Plasma Protein-A Level Tertiles
Grayscale and Virtual Histology-intravascular Ultrasound Analysis
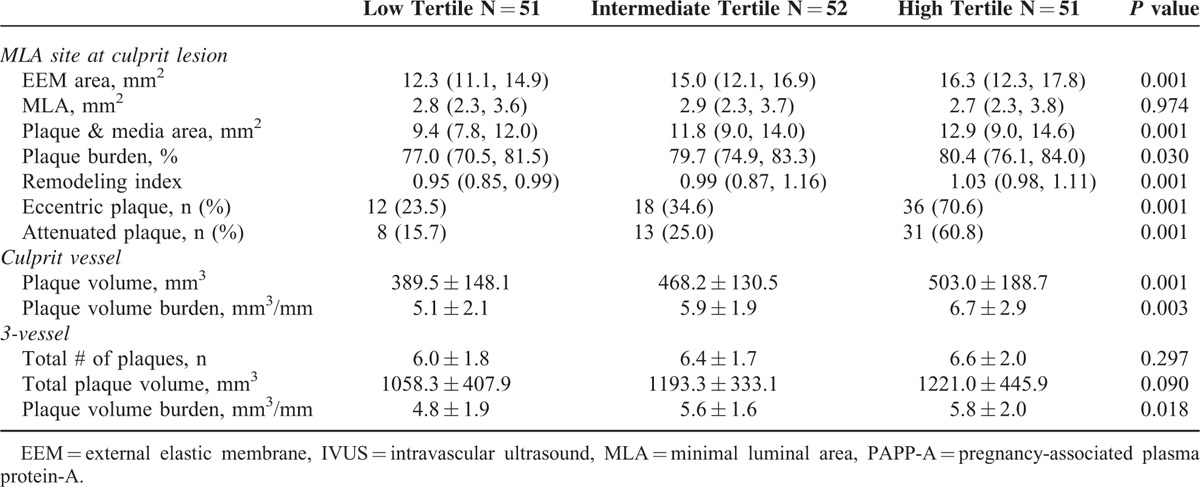
As shown in Table 2, there was a gradient in EEM CSA, P&M CSA, plaque burden, and remodeling index from low to high PAPP-A level tertiles at the MLA site; this was also true for plaque volume burden in the culprit vessel and 3 vessels, but not for the 3-vessel plaque number or total plaque volume.
TABLE 2.
Grayscale Intravascular Ultrasound Characteristics According to Pregnancy-associated Plasma Protein-A Level Tertiles
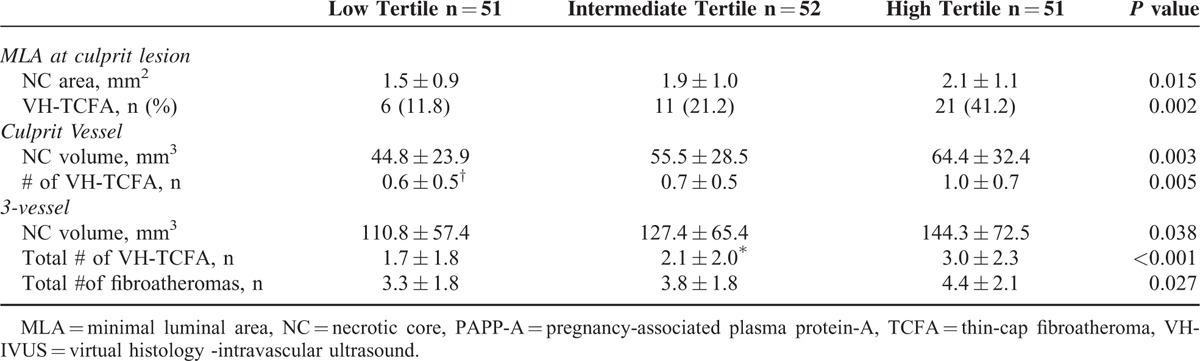
As shown in Table 3, there was a gradient in the VH-TCFA number and necrotic core amount at the MLA site, within the culprit vessel, and within all 3 vessels from the low to high PAPP-A level tertile.
TABLE 3.
Virtual Histology -intravascular Ultrasound Characteristics According to Pregnancy-associated Plasma Protein-A Level Tertiles
All patients were then divided into 3 groups according to the tertiles of necrotic core area at the MLA site, culprit vessel necrotic core volume, and 3-vessel necrotic core volume. Pregnancy-associated Plasma Protein-A levels were highest in the group with largest necrotic core tertiles versus the other two groups in all comparisons: MLA site, culprit vessel, and 3-vessel analysis (Figure 1).
FIGURE 1.
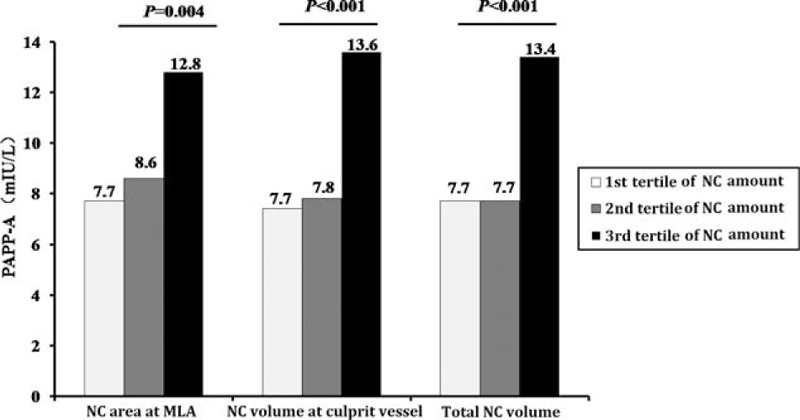
PAPP-A level for each tertile of necrotic core amount. Pregnancy-associated plasma protein-A level was significantly higher in the highest tertile than in other groups. MLA = minimum lumen area, NC = necrotic core, PAPP-A = pregnancy-associated plasma protein-A.
Thirty-eight patients (24.7%) had a VH-TCFA at the MLA site. Pregnancy-associated plasma protein-A levels were higher in patients with than without a VH-TCFA at the MLA site ([15.3 ± 13.2] mIU/L versus [7.7 ± 4.6] mIU/L, P < 0.001, respectively). In addition, the number of VH-TCFAs in the culprit vessel or among all 3 vessels strongly correlated with PAPP-A levels; the more VH-TCFAs, the higher the PAPP-A levels (Figure 2).
FIGURE 2.
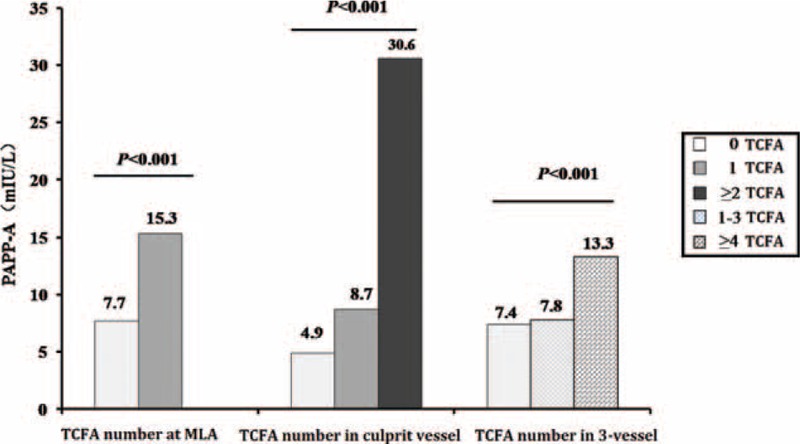
Pregnancy-associated plasma protein-A level for each number of TCFAs. The number of TCFAs at MLA, culprit vessel or 3-vessel strongly correlated with PAPP-A level; the more TCFAs, the higher the PAPP-A level. MLA = minimum lumen area, NC = necrotic core, PAPP-A = pregnancy-associated plasma protein-A, TCFA = thin-cap fibroatheroma.
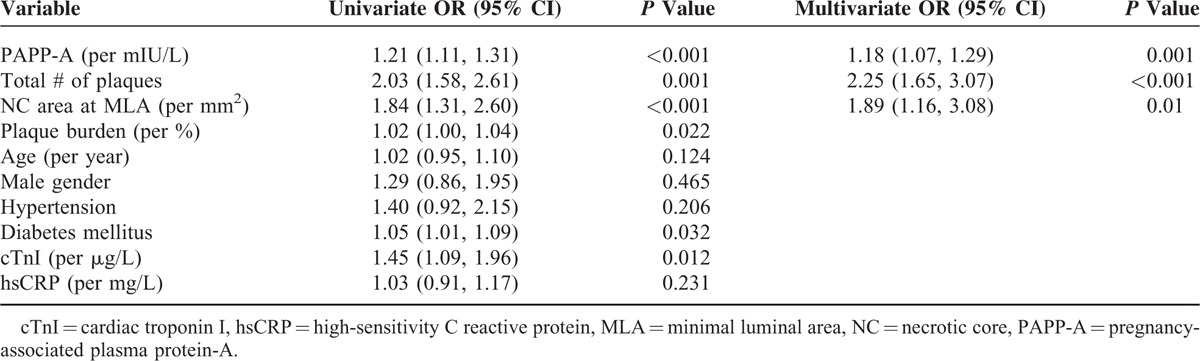
Table 4 summarizes the results of multivariate analysis for each of the clinical variables and IVUS, VH-IVUS measurements that could be independent predictors of higher total number of VH-TCFAs (n ≥ 3) in 3 vessels. After adjustment for all variables that were associated with higher total number of VH-TCFAs on univariate analysis (P < 0.2), PAPP-A level, total number of plaques in 3 vessels, and NC area at MLA had an independent significant association with higher total number of VH-TCFAs.
TABLE 4.
Multivariate Analysis
Clinical Outcome
Four patients (2.6%) were lost to follow-up (2 each for PAPP-A quartiles 1 and 2). Kaplan–Meier curves of event-free survival for MACE according to PAPP-A tertiles are shown in Figure 3. The log rank test showed a significant difference in the cumulative event-free survival among PAPP-A tertiles (P = 0.015).
FIGURE 3.
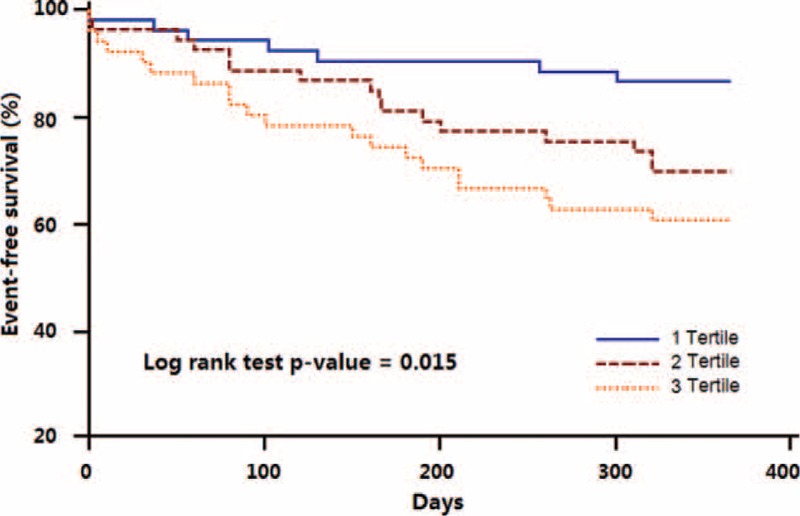
Kaplan–Meier curves of event-free survival for major adverse cardiac events according to pregnancy-associated plasma protein-A tertiles. The log rank test showed a significant difference in the cumulative event-free survival among pregnancy-associated plasma protein-A tertiles (P = 0.015).
DISCUSSION
The main findings of the current 3-vessel VH-IVUS study are that elevated serum PAPP-A level was significantly associated with: 3-vessel VH-TCFA number, culprit vessel VH-TCFA number, and MLA site VH-TCFA number; 3-vessel necrotic core volume, culprit vessel necrotic core volume, and MLA site necrotic core area; and 3-vessel plaque volume burden, but not with 3-vessel plaque number and total plaque volume in coronary arteries.
Pregnancy-associated plasma protein-A is a novel zinc metalloproteinase expressed in many circumstances beyond pregnancy, and has recently been implicated in cardiovascular diseases.2–4 Patients with ACS have elevated circulating PAPP-A levels,2 which allows risk stratification and risk prediction of future adverse cardiovascular events in ACS patients.2–4,21,22 The mechanisms underlying the association between PAPP-A and cardiovascular events are currently not fully understood. Studies on autopsy patients and mouse models have reported increased PAPP-A immunostaining in monocyte-macrophage cells and in the extracellular matrix in vulnerable atherosclerotic plaques.2,11 In animal studies, transgenic mice over expressing PAPP-A in arterial smooth muscle exhibited enhanced lesion development and increased vulnerable plaque phenotype,23 whereas deletion of the PAPP-A gene in mice resulted in resistance to the development of neointimal hyperplasia as an acute injury response.24 In in vitro studies, PAPP-A has been demonstrated to be a specific activator of insulin-like growth factor-I (IGF-I), which stimulates migration and proliferation of smooth muscle cells, production and accumulation of foam cells, and degradation of extracellular matrix.25–27 Our previous grayscale-IVUS study demonstrated that higher circulating PAPP-A levels were associated with echo-attenuated plaques,28 a grayscale IVUS marker of plaque instability.6,29 Grayscale IVUS, however, is limited to tissue characterization, especially for necrotic core-rich plaques. Virtual histology-intravascular ultrasound has been developed to improve on the plaque characterization ability of grayscale-IVUS, and VH-TCFAs have been associated with MACE in many recent studies.7–9 The current 3-vessel VH-IVUS study reinforces previous studies linking necrotic core parameters to high-risk features in ACS by showing that elevated PAPP-A level was associated with 3-vessel TCFA number and necrotic core volume, culprit vessel TCFA number and necrotic core volume, and MLA site TCFA and necrotic core area in patients with coronary artery disease.
Early detection of vulnerable plaque subtype is crucial for the prevention of acute cardiovascular events.30 The current study suggests the potential for PAPP-A as serum indicator for increased coronary TCFA burden and plaque instability in patients with coronary artery disease. Thus, identification of patients with elevated serum PAPP-A levels may allow targeting for aggressive systemic and possibly local therapies.
Of note, although VH-IVUS has a predictive accuracy of ∼93% for tissue characterization31 and a diagnostic accuracy of ∼76% for TCFA in ex vivo studies,32,33 its limited resolution (100–150 μm) precludes visualization of thin-fibrous cap. Optical coherence tomography (OCT) provides better resolution (10–20 μm) than IVUS, thereby allowing visualization of thin-fibrous cap and TCFA detection with better diagnostic accuracy (∼79%);32 however, because of its limited depth of penetration (<2 mm), it cannot detect the entire plaque structure and large/deep lipid cores. It has been reported that combined use of VH-IVUS and OCT might result in more precise detection of TCFA.32,34 Further studies with OCT or combined VH-IVUS/OCT are warranted to validate the results of the current study.
Several important limitations of our study should be taken into account to properly interpret its findings. First, although data in the current study were prospectively collected and blindly analyzed, the sample size (975 plaques in 462 vessels) was relatively small; however, overall, intracoronary imaging studies are more sensitive than population-based studies. Second, VH-IVUS has some technical limitations including the inferential identification of a TCFA because a thin-fibrous cap is below the resolution of IVUS; however, VH-TCFA was a predictor of events in PROSPECT, VIVA, and ATHEROREMO-IVUS studies.
CONCLUSIONS
Elevated PAPP-A levels were associated with increased coronary TCFA and necrotic core burden in patients with coronary artery disease. Pregnancy-associated plasma protein-A, therefore, might serve as a biomarker for predicting increased coronary TCFA burden and plaque instability.
Footnotes
Abbreviations: ACS = acute coronary syndrome, ATHEROREMO-IVUS = European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis-Intravascular Ultrasound, CSA = cross-sectional area, cTnI = cardiac troponin I, EEM = external elastic membrane, HDL = high-density lipoprotein, hsCRP = high-sensitivity C reactive protein, IQRs = interquartile ranges, IVUS = intravascular ultrasound, LDL = low-density lipoprotein, MACE = major adverse cardiac events, MLA = minimal luminal area, NSTE-ACS = non-ST-segment elevation ACS, OCT = optical coherence tomography, P&M = plaque and media, PAPP-A = pregnancy-associated plasma protein-A, PB = plaque burden, PCI = percutaneous coronary intervention, PIT = pathological intimal thickening, PROSPECT = Providing Regional Observations to Study Predictors of Events in the Coronary Tree, STEMI = ST-segment elevation myocardial infarction, TCFA = thin-cap fibroatheroma, ThCFA = thick cap fibroatheroma, VH = virtual histology, VIVA = vulnerable atherosclerosis.
This work was supported by grants from the Program of National Natural Science Foundation of China (81470492, 81000655, 81070176, 81330006, and 81270282), Program for New Century Excellent Talents in University from Ministry of Education of China (NCET-11–0898 and NCET-12–0352), Beijing Natural Science Foundation (7122057), Beijing health system health technology talents training plan (2013–3–010), and Medical Technology Program of Shanghai Committee of Science and Technology (15411963600), and The Importation and Development of High Caliber Talents Project of Beijing Municipal Institutions (CIT&TCD20150325).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lin TM, Galbert SP, Kiefer D, et al. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol 1974; 118:223–236. [DOI] [PubMed] [Google Scholar]
- 2.Bayes-Genis A, Conover CA, Overgaard MT, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med 2001; 345:1022–1029. [DOI] [PubMed] [Google Scholar]
- 3.Lund J, Qin QP, Ilva T, et al. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation 2003; 108:1924–1926. [DOI] [PubMed] [Google Scholar]
- 4.Heeschen C, Dimmeler S, Hamm CW, et al. CAPTURE Study Investigators. Pregnancy associated plasma protein-A levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol 2005; 45:229–237. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006; 47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 6.Pu J, Mintz GS, Biro S, et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2294 human coronary artery segments. J Am Coll Cardiol 2014; 63:2220–2233. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Ni M, Ma L, et al. Targeting blood thrombogenicity precipitates atherothrombotic events in a mouse model of plaque destabilization. Sci Rep 2015; 5:10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364:226–235. [DOI] [PubMed] [Google Scholar]
- 9.Calvert PA, Obaid DR, O'Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in vulnerable atherosclerosis) Study. JACC Cardiovasc Imaging 2011; 4:894–901. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J 2014; 35:639–647. [DOI] [PubMed] [Google Scholar]
- 11.Sangiorgi G, Mauriello A, Bonanno E, et al. Pregnancy-associated plasma protein-a is markedly expressed by monocytemacrophage cells in vulnerable and ruptured carotid atherosclerotic plaques: a link between inflammation and cerebrovascular events. J Am Coll Cardiol 2006; 47:2201–2211. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Maehara A, Mintz GS, et al. Virtual histology intravascular ultrasound analysis of non-culprit attenuated plaque detected by grayscale intravascular ultrasound in patients with acute coronary syndromes. Am J Cardiol 2010; 105:48–53. [DOI] [PubMed] [Google Scholar]
- 13.Pu J, Mintz GS, Brilakis ES, et al. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J 2012; 33:372–383. [DOI] [PubMed] [Google Scholar]
- 14.Hájek P, Macek M, Sr, Pešková M, et al. High positive predictive value of PAPP-A for acute coronary syndrome diagnosis in heparin-naïve patients. J Thromb Thrombolysis 2012; 34:99–105. [DOI] [PubMed] [Google Scholar]
- 15.Lund J, Qin QP, Ilva T, et al. Circulating pregnancy-associated plasma protein-a predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation 2003; 108:1924–1926. [DOI] [PubMed] [Google Scholar]
- 16.Heeschen C, Dimmeler S, Hamm CW, et al. Pregnancy-associated plasma protein-A levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol 2005; 45:229–237. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Zhang A, Han X, et al. Effect of routine heparins treatment in acute coronary syndrome on serum pregnancy-associated plasma protein a concentration. Ann Clin Lab Sci 2013; 43:274–277. [PubMed] [Google Scholar]
- 18.Rossen M, Iversen K, Teisner A, et al. Optimisation of sandwich ELISA based on monoclonal antibodies for the specific measurement of pregnancy-associated plasma protein (PAPP-A) in acute coronary syndrome. Clin Biochem 2007; 40:478–484. [DOI] [PubMed] [Google Scholar]
- 19.Ding S, Li Z, Ge H, et al. Impact of early ST-segment changes on cardiac magnetic resonance-verified intramyocardial haemorrhage and microvascular obstruction in st-elevation myocardial infarction patients. Medicine (Baltimore) 2015; 94:e1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Metrio M, Milazzo V, Rubino M, et al. Vitamin D plasma levels and in-hospital and 1-year outcomes in acute coronary syndromes: a prospective study. Medicine (Baltimore) 2015; 94:e857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonaca MP, Scirica BM, Sabatine MS, et al. Prospective evaluation of pregnancy associated plasma protein-a and outcomes in patients with acute coronary syndromes. J Am Coll Cardiol 2012; 60:332–338. [DOI] [PubMed] [Google Scholar]
- 22.Iversen KK, Teisner B, Winkel P, et al. CLARICOR Trial Group. Pregnancy associated plasma protein-A as a marker for myocardial infarction and death in patients with stable coronary artery disease: a prognostic study within the CLARICOR Trial. Atherosclerosis 2011; 214:203–208. [DOI] [PubMed] [Google Scholar]
- 23.Conover CA, Mason MA, Bale LK, et al. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol 2010; 299:H284–H291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 2007; 100:1696–1702. [DOI] [PubMed] [Google Scholar]
- 25.Tang SL, Chen WJ, Yin K, et al. PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRα through the IGF-I-mediated signaling pathway. Atherosclerosis 2012; 222:344–354. [DOI] [PubMed] [Google Scholar]
- 26.Renier G, Clement I, Desfaits AC, et al. Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-alpha production. Endocrinology 1996; 137:4611–4618. [DOI] [PubMed] [Google Scholar]
- 27.Jones JI, Prevette T, Gockerman A, et al. Ligand occupancy of the V3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci USA 1996; 93:2482–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Liu X, Dong J, et al. Pregnancy associated plasma protein-A as a marker of culprit lesion instability in unstable angina patients: An intravascular ultrasound study. Cardiology 2013; 126:244–251. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Mintz GS, Kubo T, et al. Relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction. J Am Coll Cardiol Intv 2011; 4:495–502. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Sun Y, Yi W, et al. A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J Pineal Res 2014; 57:357–366. [DOI] [PubMed] [Google Scholar]
- 31.Nair A, Margolis MP, Kuban BD, et al. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. Euro Intervention 2007; 3:113–120. [PubMed] [Google Scholar]
- 32.Brown AJ, Obaid DR, Costopoulos C, et al. Direct comparison of virtual-histology intravascular ultrasound and optical coherence tomography imaging for identification of thin-cap fibroatheroma. Circ Cardiovasc Imaging 2015; 8:e003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obaid DR, Calvert PA, Gopalan D, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging 2013; 6:655–664. [DOI] [PubMed] [Google Scholar]
- 34.Sawada T, Shite J, Garcia-Garcia HM, et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J 2008; 29:1136–1146. [DOI] [PubMed] [Google Scholar]


