Abstract
To determine the prevalence of antineutrophil cytoplasmic antibodies (ANCA) in patients with infective endocarditis (IE) in internal medicine; and to compare clinical and biochemical features and outcome between patients exhibiting IE with and without ANCA.
Fifty consecutive patients with IE underwent ANCA testing. The medical records of these patients were reviewed.
Of the 50 patients with IE, 12 exhibited ANCA (24%). ANCA-positive patients with IE exhibited: longer duration between the onset of first symptoms and IE diagnosis (P = 0.02); and more frequently: weight loss (P = 0.017) and renal impairment (P = 0.08), lower levels of C-reactive protein (P = 0.0009) and serum albumin (P = 0.0032), involvement of both aortic and mitral valves (P = 0.009), and longer hospital stay (P = 0.016). Under multivariate analysis, significant factors for ANCA-associated IE were: longer hospital stay (P = 0.004), lower level of serum albumin (P = 0.02), and multiple valve involvement (P = 0.04). Mortality rate was 25% in ANCA patients; death was because of IE complications in all these patients.
Our study identifies a high prevalence of ANCA in unselected patients with IE in internal medicine (24%). Our findings further underscore that ANCA may be associated with a subacute form of IE leading to multiple valve involvement and more frequent renal impairment. Because death was due to IE complications in all patients, our data suggest that aggressive therapy may be required to improve such patients’ outcome.
INTRODUCTION
Antineutrophil cytoplasmic antibodies (ANCA) directed against proteinase 3 (PR3) and myeloperoxydase (MPO) are strongly associated with primary systemic vasculitis, including granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis.1,2 Although, ANCA have also been described in other conditions, especially connective-tissue diseases, inflammatory bowel diseases, malignancies, and drug-induced vasculitis.3
Interestingly, ANCA have further been reported during the course of various infections, such as: viral (eg, hepatitis B and C, Epstein Barr virus, parvovirus B19, human immunodeficiency virus), bacterial (eg, Staphylococcus, Streptococcus, Bartonella, Gram-negative bacteria), fungal (eg, Aspergillus, Histoplasma), and parasitic (eg, Plamodium, Entamoeba histolytica) infections.4 Previous authors have speculated that infection-associated ANCA may be triggered by many immune dysfunction in response to microbial peptides leading to: upregulation of autoantigen genes, molecular mimicry between pathogen microorganisms and self-antigens, formation of neutrophil extracellular traps, interaction of pathogen microorganism components with toll-like receptors.5–8 More recently, the association between infective endocarditis (IE) and formation of ANCA has also been reported.9–11 To date, only a few series, however, have analyzed the prevalence and the outcome of ANCA-positive patients with IE, which prompted us to conduct the current retrospective study. Our aims were to: determine the prevalence of ANCA in patients with IE; and compare clinical and biochemical features and outcome between patients exhibiting IE with and without ANCA.
PATIENTS AND METHODS
Patients
From January 2010 to December 2014, 162 consecutive patients with IE were seen in the Department of Internal Medicine at the university of Rouen medical center. The definite diagnosis of IE was based on the modified Duke criteria.12 Ethical approval was obtained from the local ethical committee (Comité d’Ethique en recherche non interventionnelle for the Comité de protection des personnes de Haute-Normandie), and informed consent was obtained from all patients.
First, the medical records of patients with IE were reviewed for patients general characteristics at diagnosis: age and sex; comorbidities, such as arterial hypertension (cutoff 140/90 mm Hg), diabetes mellitus and cancer; previous medical history of: intravenous drug abuse, predisposing valvular disease (which was defined as having a native valve affected by regurgitation or stenosis), IE, endocavitary device, including pacemaker, cardioverter-defibrillator, left ventricular assist device, prosthetic material, that is, prosthetic heart valve, intravenous graft material, prosthetic joint, bone plate/screw, orthopedic rod; and immunosuppressive therapy for >30 days at time of IE diagnosis, including steroids, cytotoxic drugs, antitumor necrosis factor α, and rituximab.
All the patients had undergone the same routine clinical evaluation to investigate IE, as follows:
Constitutional symptoms: fever ≥38 °C, chills, asthenia, weight loss
Systemic manifestations, especially: congestive heart failure defined according to the New York Heart Association classification system,13 heart murmurs; vascular features, including arterial emboli, septic pulmonary infarctus, mycotic aneurysms, purpura, and Janeway lesions; rheumatologic signs: arthralgia, myalgia; and immunologic features, that are Osler nodes and renal impairment. In these patients, renal involvement was dichotomized into: acute renal failure and progressive renal failure. Kidney biopsies were designated as having the following patterns: mesangial proliferative glomerulonephritis (GN), focal necrotizing and crescentic GN, diffuse necrotizing and crescentic GN, focal proliferative or diffuse proliferative GN, membranoproliferative GN, thrombotic microangiopathy, and acute tubular injury; immunofluorescence pattern was determined, using fluorescein-tagged polyclonal rabbit antihuman antibodies to: C3, IgA, IgG, IgM, fibrinogen, and κ/λ-light chains. The outcome of renal involvement was determined as follows: complete resolution, characterized by normalization of serum creatinine values; persistent renal impairment characterized by persistent increase of serum creatinine 0.2 mg/L above baseline values; and end-stage renal involvement, requiring renal dialysis.14
Second, all patients had undergone transthoracic and/or transoesophageal echocardiography to detect: valvular impairment: aortic, mitral, tricuspid; localization and size of vegetations. Site of IE acquisition was defined following International collaboration on endocarditis15; and complications of IE such as paravalvular abscess and valvular perforation.
Third, the medical records of patients with IE were reviewed for laboratory characteristics at diagnosis of IE: C-reactive protein (mg/L), hemoglobin level (g/dL), leukocytosis (G/L), creatininemia (μmol/L), serum albumin (g/L); urinanalysis: hematuria, proteinuria; microbiological data: number of positive blood cultures, identification of pathogen microorganism.
Infective endocarditis features also included the timing of initial symptoms and patients’ hospitalization. Thus, IE was classified as: community-acquired IE, when IE was diagnosed at the time of admission or ≤48 hours of admission; health care-associated IE, when IE was diagnosed within 48 hours of admission in outpatients as follows: intravenous therapy, wound care or specialized nursing care at home ≤30 days before IE onset; attendance at hospital and/or intravenous chemotherapy ≤30 days before IE onset; hospitalization for ≥2 days in the 3 months days before IE onset; and residence in a long-term care facility; and nosocomial IE, when IE developed in patients hospitalized for >48 hours.13,15 Prosthetic valve IE was determined as an infection, involving: valve prothesis, reconstructed native heart valves whether a mechanical prosthesis and/or a bioprosthetic xenograft, stented or unstented, and/or a repaired native valve with implantation of an annular ring.15,16
Furthermore, all patients with IE received specific therapy. Data regarding patients’ therapy were reviewed for treatment used at any time during the course of IE, that is:
Antimicrobial therapy
Immunosuppressive therapy, including steroids and cytotoxic drugs
And surgical procedures of IE complications
In addition, all patients underwent follow-up to determine the outcome of IE as follows: resolution; onset of complications; and in-hospital mortality (because of IE or to another cause than IE). Finally, the duration of hospital stay was also checked for all patients.
Antineutrophil Cytoplasmic Antibodies
Antineutrophil cytoplasmic antibodies testing was performed using indirect immunofluorescence assay in ethanol-, formalin-, and methanol-fixed neutrophils. The ANCA staining pattern [cytoplasmic (c-ANCA) and perinuclear (p-ANCA)] was also determined by indirect immunofluorescence assays (Euroimmun, Lubeck, Germany). Furthermore, PR3 and MPO-ANCAs were detected by commercially available enzyme-linked immunosorbent assay (ELISA) (“anti-PR3-hn-hr IgG” and “anti-MPO-hn-hr IgG” Euroimmun, Lubeck, Germany).17–20 Values above 20 IU/mL were considered positive.
Comparison of Antineutrophil Cytoplasmic Antibodies-positive and Antineutrophil Cytoplasmic Antibodies-negative Patients With Infective Endocarditis
We compared various features between the group of IE patients with and without ANCA, that is, median age, sex, comorbidities, previous medical history, constitutional, and systemic manifestations; biochemical findings; characteristics of IE; outcome of IE; and median duration of hospital stay.
Statistical Analysis
Statistical analyses were conducted to assess the features of ANCA-associated IE. Patients were divided into 2 groups consisting of IE patients with and without ANCA.
For group comparison involving binary data, we used the Fisher exact test. Comparisons involving continuous data were performed using: Student test when distribution of variables was normal and Mann–Whitney test in other patients. The results were reported as the odds ratio (OR) and 95% confidence interval (95% CI); P values less than 0.05 were considered significant in all performed tests.
Finally, as regards variables with P values <0.1, we further proceeded with multiple logistic regression, using backward stepwise selection, to calculate multivariate OR (95% CI); the used level of significance was P < 0.05.
Literature Review
In addition to the current study, we conducted a literature review of the Medline database (1966–2015) (National Library of Medicine, Bethesda, MD), reviewing articles of all languages, and we compared data from the current report with previously published data.
RESULTS
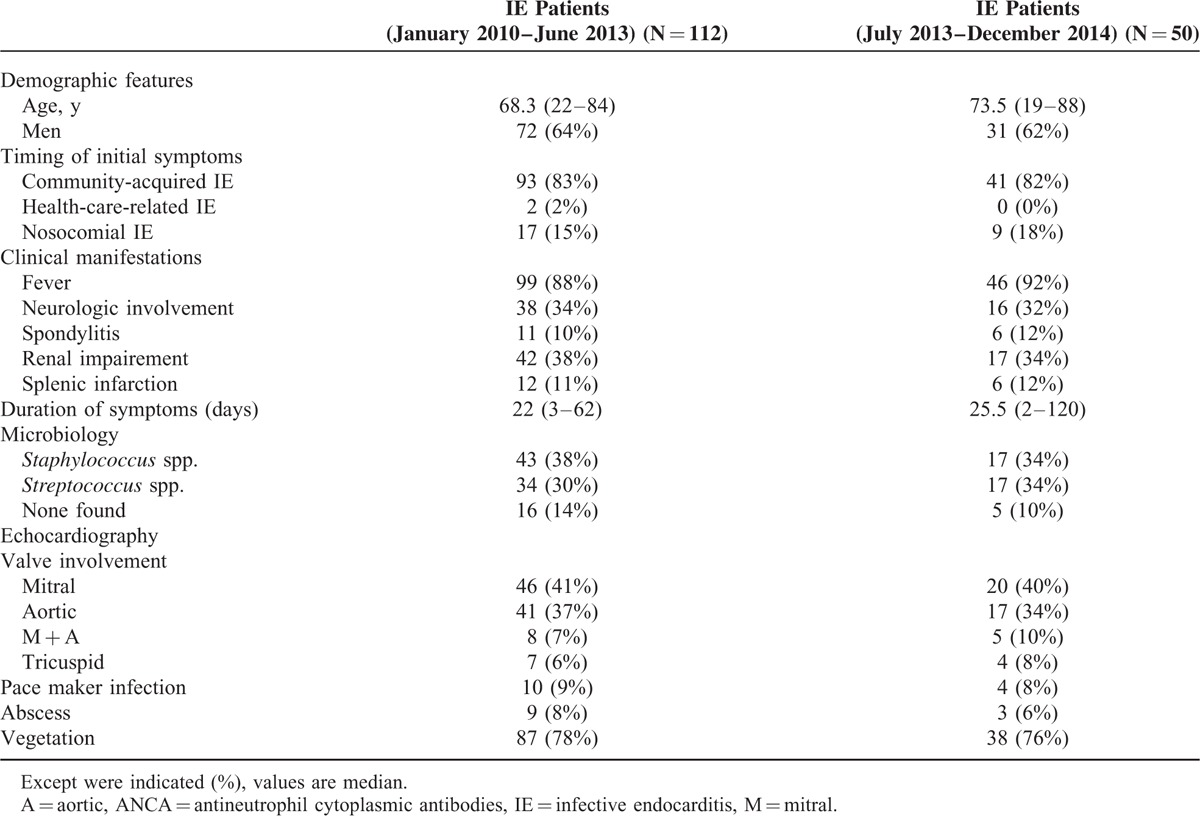
Among the 162 consecutive patients with IE, findings of serum samples for ANCA were available for 50 patients. Indeed, from July 2013 to December 2014, all patients with IE (N = 50) systematically underwent ANCA testing.
Altogether, these 50 latter patients with IE were included in the study. Nevertheless, the characteristics of patients who did not undergo ANCA testing (from January 2010 to June 2013) were not different from those of patients who had ANCA testing (Table 1).
TABLE 1.
Characteristics of the 162 patients with infective endocarditis
All 50 patients had definite IE. Indeed, 2 of our patients, without valve vegetation on echocardiography, had valvular abscess. Furthermore, 2 other patients, without valve vegetation on echocardiography, exhibited cardiac marked uptake on 18F-fluorodeoxyglucose-positon emission tomography scanning. These 2 latter patients underwent cardiac valvular surgery; bacterial analysis of cardiac valvular specimens was positive for both cultures and polymerase chain reaction.
Prevalence of Antineutrophil Cytoplasmic Antibodies in Patients With Infective Endocarditis
In our cohort of 50 patients with IE, ANCA were positive in 12 of them (24%).
Using ANCA staining pattern, patients exhibited c-ANCA (N = 6) and p-ANCA (N = 5).
Regarding ELISA test, patients had: PR3-ANCA (N = 4), MPO-ANCA (N = 1), PR3 + MPO-ANCA (N = 2). One of them exhibited high ELISA PR3 level without immunofluorescence positivity.
Comparison of Characteristics Between Infective Endocarditis Patients With and Without Antineutrophil Cytoplasmic Antibodies
General Features
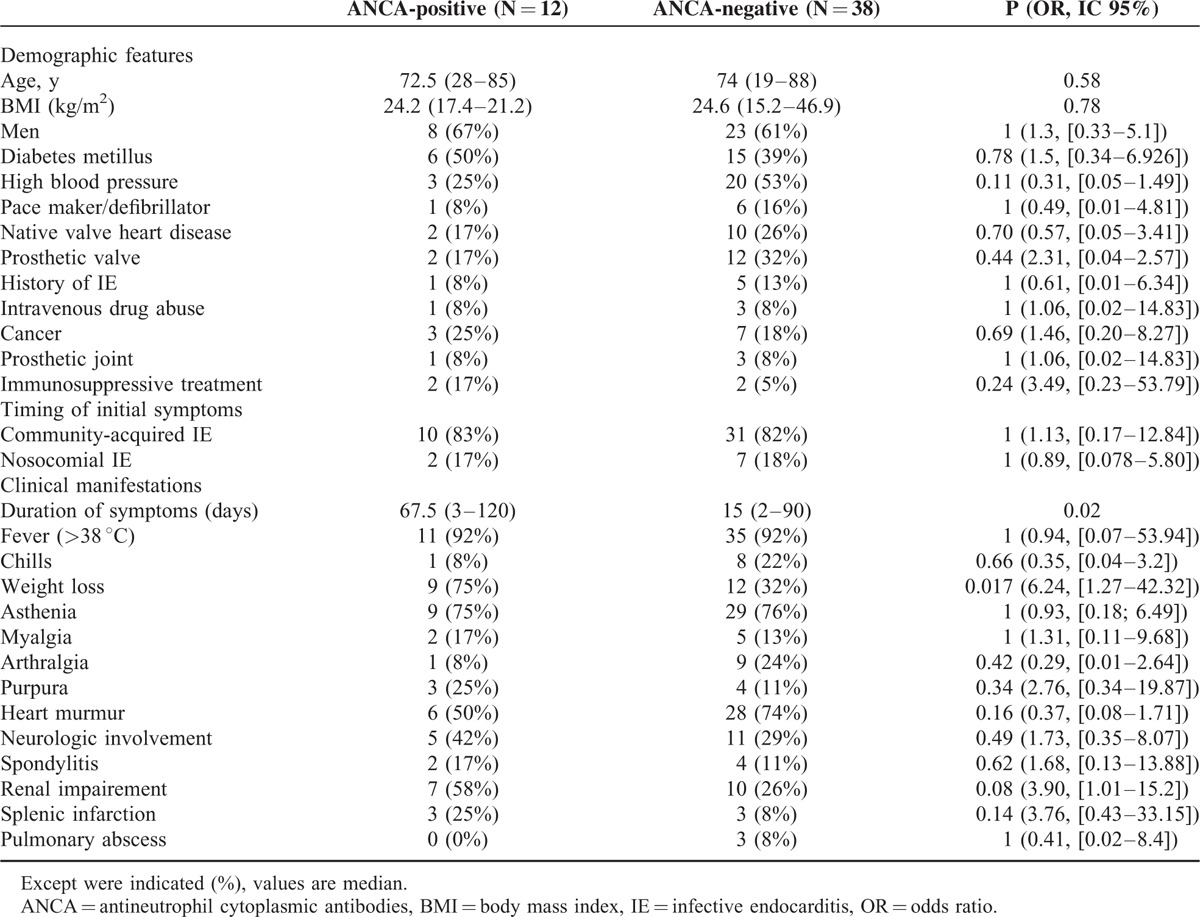
We failed to show any statistically significant differences between ANCA-positive and negative patients with IE regarding: age (P = 0.58), sex (P = 0.71); comorbidities: arterial hypertension (P = 0.11), diabetes mellitus (P = 0.74), and cancer (P = 1); previous history of: intravenous drug abuse (P = 1), predisposing condition on native valve (P = 0.70), IE (P = 1), pacemaker or cardioverter-defibrillator (P = 1), prosthetic heart valve (P = 0.47), and prosthetic joint (P = 1) (Table 2). Furthermore, we did not find any difference regarding previous immunosuppressive therapy (P = 0.24) between both groups of patients.
TABLE 2.
General and Clinical Characteristics in Infective Endocarditis Patients With and Without Antineutrophil Cytoplasmic Antibodies
Clinical Manifestations
The median duration between the onset of first symptoms and IE diagnosis was longer in ANCA-positive patients (67.5 versus 15 days; P = 0.02). Furthermore, ANCA-positive patients more often exhibited weight loss at IE diagnosis (P = 0.017).
Infective endocarditis patients with and without ANCA did not differ for: fever (P = 1), chills (P = 0.66), asthenia (P = 1); arterial emboli (P = 0.51), heart failure (P = 1), heart murmurs (P = 0.16); arthralgia (P = 0.42), myalgia (P = 0.65); purpura (P = 0.34), Janeway lesions (P = 1), Osler nodes (P = 0.43); spondylitis (P = 0.62); neurologic involvement (P = 0.49), including cerebral emboli (25% versus 18%), cerebral hemorrhage (4% versus 8%), cerebral mycotic aneurysms (8% versus 0%), and amyloid-like angiopathy (0% versus 3%); splenic infarction (0.14); and pulmonary abscess (P = 1) (Table 2).
Interestingly, renal impairment tended to be more frequent in patients with ANCA than in those without (58% versus 26%; P = 0.08), although not significantly so (Table 2). These 7 patients exhibited acute (N = 5) or rapidly progressive (N = 2) renal failure. Three of 7 patients who exhibited nephritic syndrome with hematuria (N = 1) underwent renal biopsy. In these 3 patients, histologic analysis of renal biopsy specimens showed: crescentic GN (N = 1). Glomerular inflammatory changes were diffuse in this patient, immunofluorescence studies showing presence of C3 and IgM. This patient progressed to end-stage renal disease, requiring renal dialysis; and diffuse proliferative GN with endocapillary proliferation without crescents or necrosis (N = 2), immunofluorescence studies being positive for C3 and IgG (N = 1) and IgM (N = 1). One of this patient exhibited complete renal resolution, whereas the other patient developed end-stage renal disease requiring renal dialysis. Regarding the 4 remaining patients who did not undergo renal biopsy, none exhibited nephrotic/nephritic syndrome; in these latter patients, the outcome of renal manifestations was as follows: complete renal resolution (N = 2) and persistent renal dysfunction (N = 2).
Laboratory Findings
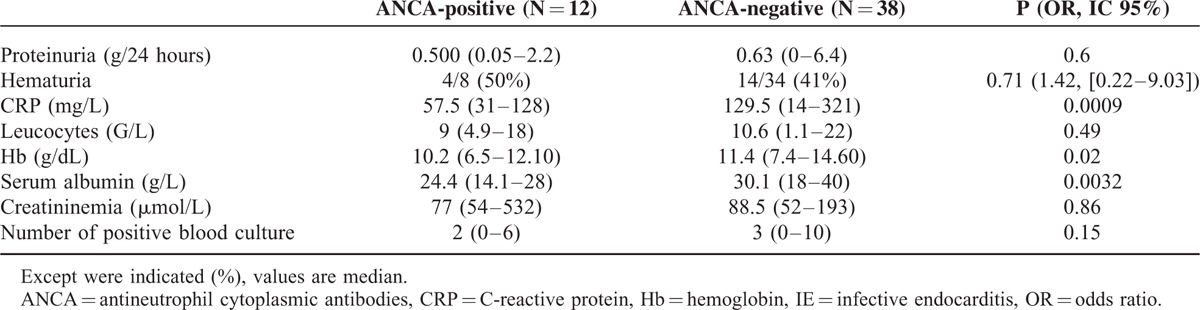
As shown in Table 3, regarding biochemical tests, ANCA-positive patients with IE had lower median values of: C-reactive protein (P = 0.0009), hemoglobin (P = 0.02), and serum albumin (P = 0.0032).
TABLE 3.
Biochemical Findings in Infective Endocarditis Patients With and Without Antineutrophil Cytoplasmic Antibodies
Characteristics of Infective Endocarditis
Patients with and without ANCA did not differ for prevalence of community-acquired IE, health care-associated IE or nosocomial IE (P = 1). The door-of-entry of IE was found in half of our ANCA-positive patients, as follows: colonic polyp (N = 2), intravenous drug abuse (N = 1), tooth extraction (N = 1), cardiac catheterization (N = 1), and chronic supra-pubic catheter (N = 1).
Antineutrophil cytoplasmic antibodies-positive patients with IE more frequently exhibited involvement of both aortic and mitral valves (P = 0.009). The prevalence of vegetations on echocardiography (P = 1) as well as the size of vegetations (P = 0.98) were not different between IE patients with and without ANCA (Table 4).
TABLE 4.
Microbiological and Echocardiographic Findings, Therapy, and Outcome of Infective Endocarditis Patients With and Without Antineutrophil Cytoplasmic Antibodies
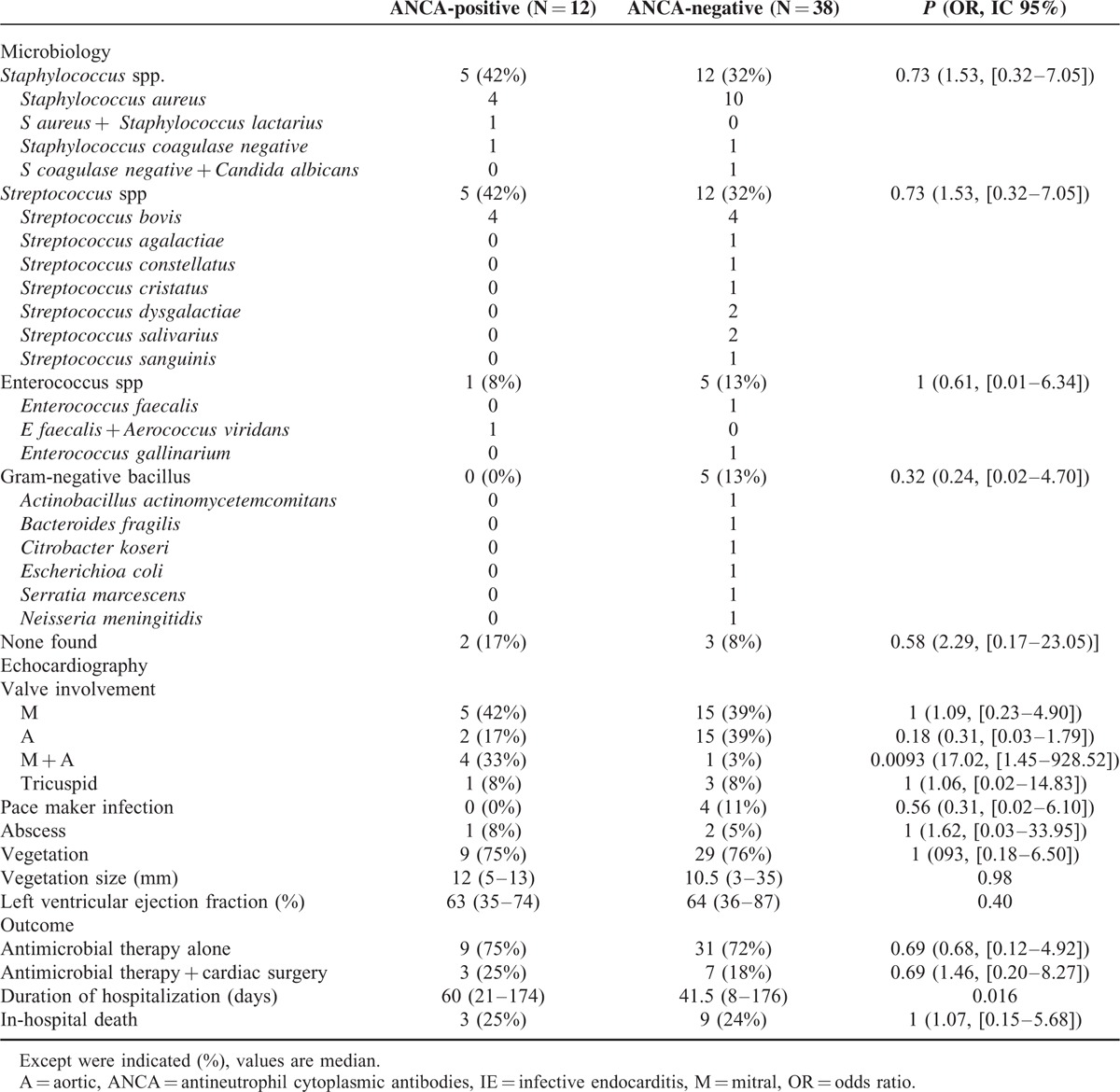
On echocardiography, there was also no significant difference between patients with and without ANCA regarding the following IE complications: paravalvular abscess (P = 1); valvular perforation (P = 1) and cardiac dysfunction, as shown by similar median value of left ventricular ejection fraction (63% versus 64%; P = 0.40).
In addition, we failed to find differences between patients with and without ANCA regarding the causative pathogen microorganism of IE, that is, Staphylococcus spp. (P = 0.73), Streptococcus spp. (P = 0.73), Enterococcus spp. (P = 1), Gram-negative bacillus (P = 0.32), or absence of micro-organism (P = 0.58) (Table 4). Finally, the median number of positive blood cultures tended to be lower in the group of ANCA-positive patients with IE (2 versus 3; P = 0.15), although not significantly so.
Outcome of Infective Endocarditis
Therapy of IE did not differ between patients with and without ANCA for: antimicrobial therapy alone (75% versus 82%; P = 0.69) and combined therapy of antimicrobial agents and surgery procedures (25% versus 18%; P = 0.69). Surgical therapy consisted of: debridement associated with valvuloplasty (33% versus 43%) and heart valve replacement (67% versus 57%). Furthermore, 2 patients in ANCA-positive group also received immunosuppressive therapy [bolus of intravenous corticosteroids: 750 mg (N = 1) and 1 g N = 1)] because of initial diagnosis of systemic vasculitis. By contrast, no misdiagnosis was made in ANCA-negative group (P = 0.054).
Furthermore, in-hospital mortality was similar between IE patients with and without ANCA (25% versus 24%; P = 1). Death was directly because of IE complications: in the 3 patients with ANCA (25%): multiple organ failure (N = 2), end-stage renal failure (N = 1); and in the 9 patients without ANCA (24%): cerebral hemorrhage (N = 3), heart failure (N = 2), respiratory distress (N = 1), cardiopulmonary failure (N = 1), multiple organ failure (N = 2), and sudden death (N = 1). Finally, the median duration of hospital stay was higher in ANCA-positive patients (60 versus 41.5 days; P = 0.016).
Results of Multiple Logistic Regression Analysis to Identify Infective Endocarditis Parameters Associated With Antineutrophil Cytoplasmic Antibodies
Longer duration of symptoms before diagnosis (OR: 1.47 [95% CI: 1.14–1.90]; P = 0.004) and multiple valve (mitral and aortic) involvement (OR: 1.31 [95% CI: 1.02–1.71]; P = 0.04) were significant factors for IE associated with ANCA.
DISCUSSION
Infective endocarditis still remains a serious condition. In previous series, the clinical features, etiology, complications, and outcome have been documented in patients with IE, which resulted in most appropriate therapies, although mortality remains high in these patients.16,21–30 Interestingly, few investigators have described ANCA-associated IE. These authors have thus reported ANCA in 18% to 33% of patients with IE.31,32
To the best of our knowledge, the current study is one of the largest to evaluate the prevalence of ANCA in patients with IE. Among 50 unselected patients with IE, up to 24% had ANCA. Because IE is not an uncommon disease, our findings thus underscore that IE is an important cause of ANCAs in internal medicine. Although, the current study has, in fact, the following limitations: our study was done at tertiary hospital. Thus, our cohort may not reflect the features of overall patients with IE in the general population. Accordingly, our conclusions are applicable to IE patients admitted in Departments of Internal Medicine of tertiary hospitals. Although, this bias cannot be avoided, it has to be known;27 the possibility that ANCA might have been positive before IE onset cannot be ruled out, although our ANCA-positive patients did not have previous history of connective-tissue disease and/or systemic vasculitis; and follow-up sera were not tested systematically in all 12 IE patients with ANCA. In 3 of these patients who underwent ANCA testing at 3-month follow-up, ANCA testing, however, proved negative after successful antibiotic therapy of IE. Our latter findings reinforce the relationship between IE and ANCA in these patients. Altogether, our findings suggest that the identification of ANCA may be useful in IE to improve such patient's management.
In our literature search using the Medline database, we identified 50 well documented cases of ANCA-associated IE; most of them were case reports.9–11,21,24,31–61 Interestingly, we observed that IE patients with ANCA more often exhibited: c-ANCA than p-ANCA (84% versus 14%); and PR3 ANCA compared with MPO-ANCA and PR3 + MPO-ANCA (63% versus 17% versus 10%) (Table 4). The current study also highlights that patients with IE more often developed c-ANCA.
Mechanisms of ANCA-induction are still unclear in patients with IE. Previous authors have postulated that ANCA formation in IE may result from: autoantigen complementarity has been suggested one of the mechanisms breaking tolerance to ANCA antigens.47,62 The complementary peptide immunogen could be a microbial exogenous peptide.47,62 Several microorganisms (Staphylococcus aureus, E histolytica) have peptides homologous to cPR3; and may trigger an autoantibody response.47,63 It has been speculated that complementary peptides to PR3 could be beneficial to pathogens expressing cPR3 by binding to/neutralizing the antimicrobial properties of PR3 and MPO64; molecular mimicry between epitopes of self-antigens and antibodies to microorganisms.47 Lysosome-associated membrane protein-2 (LAMP-2) is a glycosylated type 2 membrane protein expressed on the membrane of neutrophil intracellular vesicles, containing PR3 and MPO.47,65 Anti-LAMP-2 ANCA cross react with the adhesive bacterial fimbrian protein Fim-H, which is detected in many Gram-negative bacteria; thus, previous investigators have suggested that anti-LAMP-2 ANCA may be because of molecular mimicry following Gram-negative bacteria (with Fim-H)7,47; neutrophil extracellular traps formation may result in the synthesis, in neutrophils, of antimicrobial proteins such as PR3 and MPO.47 It seems to play an important role in bacterial infection (Staphylococcus, Streptococcus, Enterococcus) control;35,47,60 and Toll-like receptors. The bacterial DNA hypomethylated motifs (ligands for Toll-like receptor 9) have been found to lead to the in vitro production of ANCA by B-lymphocytes.11,47
From a practical point of view, the identification of features of ANCA-associated IE appears to be crucial, to improve both early diagnosis and management in these patients. Nevertheless, to our knowledge, no predictive factors for ANCA-associated IE have been clearly defined to date.
In our literature review, we observed that ANCA-associated IE tended to occur more commonly in men (80% of patients); in these patients, the median age at IE diagnosis was 53.5 years9–11,21,24,31–61 (Table 5). The current study confirms these data, as many of our patients (67%) were men, although our data do not permit us to determine whether there is an association between sex and ANCA-associated IE. Furthermore, the median age of our patients with ANCA-associated IE (72.5 years) reflects, in part, the demographic features in the north-western area in France.
TABLE 5.
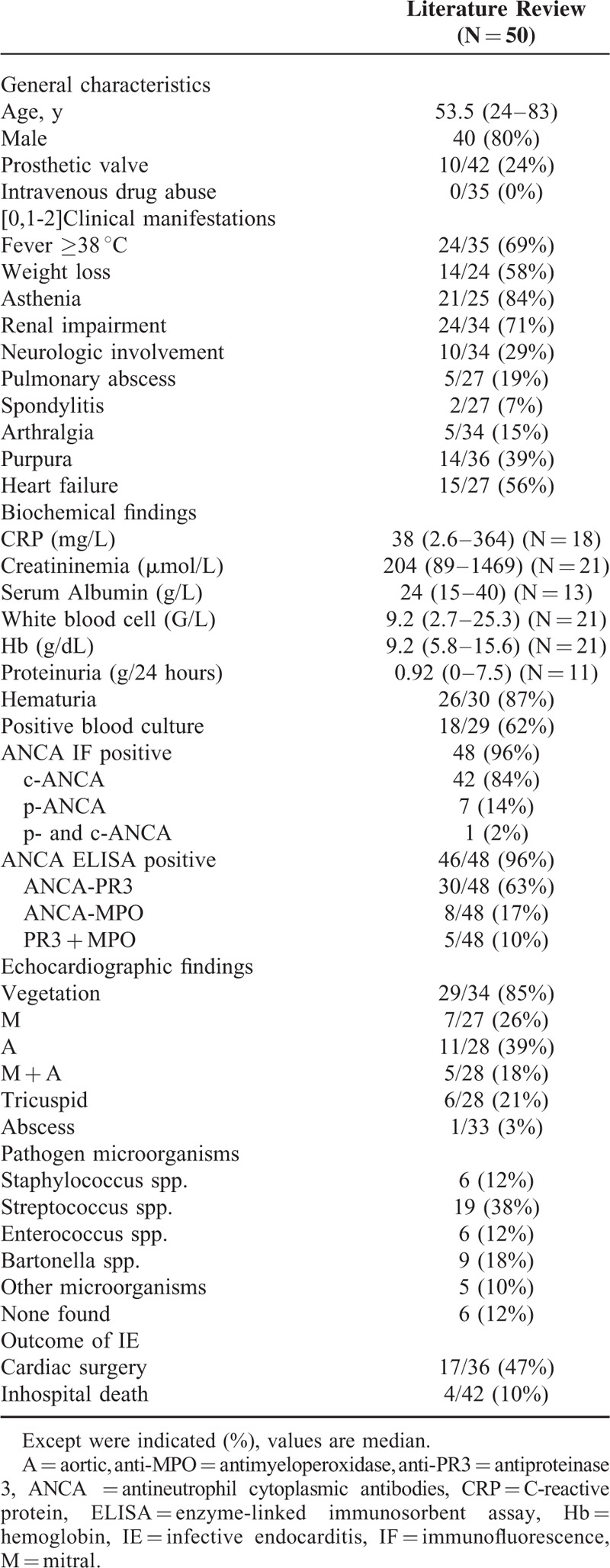
In our review, we further found that the main clinical manifestations of ANCA-associated IE were: asthenia (84%), fever (69%), weight loss (58%), and renal impairment (71%) (Table 5).9–11,21,24,31–61 In this instance, IE patients with ANCA, compared with those without, more frequently presented: deterioration of general status with weight loss; and renal impairment (58% versus 26%). In 29 IE-associated biopsy proven GN, 8 patients (28%) had ANCA. Unfortunately, these investigators did not compare the clinical and histologic features of GN between IE patients with and without ANCA.7 In our 3 ANCA-positive patients with biopsy proven GN, patients exhibited diffuse proliferative GN (N = 2) and necrotizing/crescentic GN (N = 1). Our findings suggest that IE patients with ANCA should undergo systematic investigations to detect underlying life-threatening renal complications, although no definite conclusion can be drawn from our data. Our literature review, in fact, identified 24 case reports of documented ANCA-positive patients with IE exhibiting renal impairment.11,24,37,39,40,42–45,47,48,50,51,54,57–59,61 Twenty-one of these latter patients underwent renal biopsy showing: extracapillary GN with immune deposits (N = 5), pauci-immune GN (N = 4), segmental and focal necrotizing GN (N = 4), endocapillary GN with immune deposits (N = 3), interstitial nephritis (N = 1), chronic sclerotic GN (N = 1), both pauci-immune GN and interstitial nephritis (N = 2), and both endocapillary GN and interstitial nephritis (N = 1).11,24,37,39,40,42–45,47,48,50,51,54,57–59,61 Altogether, our data suggest that whether patients with ANCA-associated IE, develop commonly renal impairment, renal damage are nonspecific in these patients. Some damage could mimic ANCA-associated vasculitis renal attempts (pauci immune GN). On the contrary, in ANCA-negative IE, other authors reported that the most common biopsy finding was crescentic GN followed by endocapillary proliferative GN.14,66,67 Interestingly, in our experience, most ANCA-positive patients with IE had favorable outcome of renal impairment (N = 4/7). Thus, we suggest that renal biopsy may be performed in the population of ANCA-positive patients with IE, who do not exhibit improvement of renal function tests after initiation of appropriate antibiotic therapy, to detect other underlying condition responsible for renal dysfunction.
Another interesting finding in our study was that diagnosis at an early stage of IE was less frequent in the group of ANCA-positive patients, as shown by longer median interval between onset of first symptoms and IE diagnosis. We suggest that the presence of constitutional symptoms (weight loss, asthenia), renal impairment, and ANCA has resulted in the diagnosis at later stage of IE. In our patients, weight loss, asthenia, and low fever (≤38.5 °C) were indeed the only symptoms of IE in up to 17% of patients. It is thus crucial to maintain a low threshold for investigation of IE in these ANCA-positive patients to avoid underdiagnosis. Also, the onset of a new heart murmur is an important clinical sign for the diagnosis of IE at earlier stage in these patients.
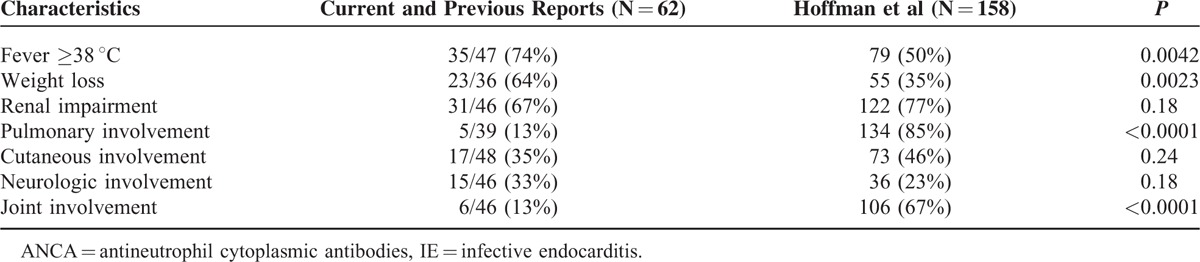
Furthermore, the main differential diagnoses of these insidious forms of ANCA-positive IE are ANCA-associated vasculitis.68,69 Comparing previous and current reports of ANCA-associated IE and granulomatosis with polyangiitis,69 we have interestingly observed the following distinct features between both groups of patients, that is: pulmonary (13% versus 85%; P < 0.0001) and articular signs (13% versus 67%; P < 0.0001) were less common in ANCA-associated IE; fever (≥38 °C) (74% versus 50%; P < 0.0042) and weight loss were more frequent (64% versus 35%; P = 0.0023) in ANCA-associated IE; and up to 25% of our ANCA-associated IE patients presented splenic infarction, whereas splenic infarction is an uncommon complication of ANCA-associated vasculitis.70–73 On the contrary, we failed to find difference regarding renal involvement, cutaneous symptoms, or neurologic involvement between ANCA-positive patients with IE and patients with granulomatosis and polyangiitis (Table 6).
TABLE 6.
Characteristics and Organ Involvements in a Population With Antineutrophil Cytoplasmic Antibodies-associated Infective Endocarditis (Current Report, Literature Data9–11,21,24,31–61) Versus Granulomatosis With Polyangiitis Cohort25,66
Regarding biochemical tests, IE patients with ANCA more often developed inflammatory process than those without, as shown by lower serum albumin and inflammatory anemia. Overall, these findings suggest that ANCA-positive patients with IE more often exhibit a subacute form of IE, with prolonged inflammation, resulting in diagnosis of IE at a later stage in this group of patients.
As with IE in the general population, the main causative pathogen microorganisms in ANCA-positive patients with IE were Staphylococci and Streptococci.11 Our study, however, shows a higher prevalence of IE related to Staphylococcus than has previously been reported (42% versus 12%) in ANCA-positive patients.37,42,45,49 This discrepancy may be explained by the predominance of S aureus, which has increasingly been described in America and Europe.74,75
A potential door-of-entry of IE was found in only 50% of our ANCA-positive patients; these data may be explained by the fact that most of ANCA-positive patients had community-acquired IE (83%); of note, no patient had health-care-associated IE and 17% of patients had nosocomial IE. In this instance, the prevalence of community-acquired IE and nosocomial IE was similar in both groups of patients.
Culture-negative IE accounted for as high as 17% of our ANCA-positive patients. Our findings confirm previous authors’ data, reporting culture-negative IE in 12% of such patients.31,43,48,58 In this instance, we did not experience cases of ANCA-positive patients with IE related to Bartonella spp. On the contrary, in our literature review, we have noted an overrepresentation of IE related to Bartonella (mainly Bartonella henselae and Bartonella quintana) in ANCA-positive patients31,44,50–55,59. Indeed, Aslangul et al34 reported ANCA positivity in up to 60% of patients with Bartonella IE. These authors have postulated that Bartonella effects in endothelium with recruitment of macrophages and leukocytes associated with delayed leukocytes apoptosis may lead to ANCA production.76 These data suggest that ANCA-positive patients with IE, exhibiting negative blood cultures, should undergo Bartonella serology and/or research by polymerase chain reaction in heart valves (in those requiring cardiac surgery).
On echocardiography, presence of vegetations was detected in 75% of our ANCA-positive and -negative patients with IE. This prevalence was lower than the rate of 85% reported in this subgroup of patients, in our literature review9–11,21,24,31–61 (Table 5); vegetation sizes were also similar between ANCA-positive and ANCA-negative IE patients. Our findings suggest that vegetations features may not be predictive factors of ANCA-associated IE.
Interestingly, Chirinos et al38 have reported that valvular heart involvement in ANCA-associated vasculitis systematically affect the aortic valve. Thus, another main finding in our study was that IE patients with ANCA, compared with those without, more commonly exhibited both aortic and mitral valvular involvement. In our literature review, ANCA-positive patients with IE exhibited: isolated mitral (39%) or aortic (39%) valvular vegetations, and both mitral and aortic vegetation (18%).9,51,52,57,59 Thus, in our ANCA-positive patients, we suggest that diagnosis of IE at later stage and immunosuppression (related to older age and marked weight loss) may have resulted into increased bacterial mitral and aortic colonization. Our data are relevant as they suggest that the presence of multiple valve involvement may be a predictive marker of IE rather than systemic vasculitis in ANCA-positive patients. Because of the small number of ANCA-positive patients with IE, further investigations, however, are warranted to confirm our findings.
In addition, another finding in our study was that hospital stay was longer in our ANCA-positive patients with IE. Our data may be, in part, explained by the fact that these patients had diagnosis of IE at later stage, resulting in more severe forms of IE, including multiple valve involvement, renal impairment, and deterioration of general status with marked weight loss. Furthermore, the overall in-hospital mortality rate was 25% in our ANCA-positive patients with IE, which is higher than the rate of 10% reported in the literature;37,43,52 it is possible that this discrepancy may be related to: older age and higher prevalence of Staphylococcus infection in our cohort; and low rate of surgery (25%) in our patient, as early surgery is believed to be an effective treatment to decrease mortality in patients with IE.77–79 In the current study, only in-hospital follow-up, however, was available for our patients. Thus, we cannot exclude that IE patients with ANCA have a decreased late survival; in fact, because of multiple valve involvement, probability of valvular surgery may be higher at long-term follow-up in these patients, leading to increased morbidity and mortality of valvular surgery.22
In conclusion, our study identifies a high prevalence of ANCA in unselected patients with IE in internal medicine (24%). Our findings further underscore that ANCA may be associated with a subacute form of IE leading to multiple valve involvement and more frequent renal impairment. Because death was because of IE complications in all patients, our data suggest that aggressive therapy, including valvular surgery, may be required to improve such patients’ outcome; although, only randomized trials would specify whether valvular surgery may improve the prognosis of such patients.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ANCA = antineutrophil cytoplasmic antibodies, ELISA = enzyme-linked immunosorbent assay, GN = glomerulonephritis, IE = infective endocarditis, LAMP-2 = lysosome-associated membrane protein-2, MPO = myeloperoxydase, OR = odds ratio, PR3 = proteinase 3.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53:743–753. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum 1998; 41:1521–1537. [DOI] [PubMed] [Google Scholar]
- 3.Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA). Autoimmunity 2005; 38:93–103. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinov KN, Ulff-Møller CJ, Tzamaloukas AH. Infections and antineutrophil cytoplasmic antibodies: triggering mechanisms. Autoimmun Rev 2015; 14:201–203. [DOI] [PubMed] [Google Scholar]
- 5.Husmann CA, Holle JU, Moosig F, et al. Genetics of toll like receptor 9 in ANCA associated vasculitides. Ann Rheum Dis 2014; 73:890–896. [DOI] [PubMed] [Google Scholar]
- 6.Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014; 10:463–473. [DOI] [PubMed] [Google Scholar]
- 7.Kain R, Rees AJ. What is the evidence for antibodies to LAMP-2 in the pathogenesis of ANCA associated small vessel vasculitis? Curr Opin Rheumatol 2013; 25:26–34. [DOI] [PubMed] [Google Scholar]
- 8.Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 2012; 18:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HK, Lamprecht P, Niles JL, et al. Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis Rheum 2000; 43:226–231. [DOI] [PubMed] [Google Scholar]
- 10.Soto A, Jorgensen C, Oksman F, et al. Endocarditis associated with ANCA. Clin Exp Rheumatol 1994; 12:203–204. [PubMed] [Google Scholar]
- 11.Wagner J, Andrassy K, Ritz E. Is vasculitis in subacute bacterial endocarditis associated with ANCA? Lancet 1991; 337:799–800. [DOI] [PubMed] [Google Scholar]
- 12.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis Off Publ Infect Dis Soc Am 2000; 30:633–638. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol 2013; 62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 14.Boils CL, Nasr SH, Walker PD, et al. Update on endocarditis-associated glomerulonephritis. Kidney Int 2015; 87:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch DR, Corey GR, Hoen B, et al. International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz P, Kestler M, De Alarcon A, et al. Spanish Collaboration on Endocarditis-Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España (GAMES). Current epidemiology and outcome of infective endocarditis: a multicenter, prospective, cohort study. Medicine (Baltimore) 2015; 94:e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdanos DP, Smyk DS, Invernizzi P, et al. Infectome: a platform to trace infectious triggers of autoimmunity. Autoimmun Rev 2013; 12:726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damoiseaux J, Dähnrich C, Rosemann A, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis 2009; 68:228–233. [DOI] [PubMed] [Google Scholar]
- 19.Dumoitier N, Terrier B, London J, et al. Implication of B lymphocytes in the pathogenesis of ANCA-associated vasculitides. Autoimmun Rev 2015; 14:996–1004. [DOI] [PubMed] [Google Scholar]
- 20.Selmi C, Cantarini L, Kivity S, et al. The 2014 ACR annual meeting: a bird's eye view of autoimmunity in 2015. Autoimmun Rev 2015; 14:622–632. [DOI] [PubMed] [Google Scholar]
- 21.Chen PC, Tung YC, Wu PW, et al. Dental procedures and the risk of infective endocarditis. Medicine (Baltimore) 2015; 94:e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Egea V, Muñoz P, Valerio M, et al. GAMES Study Group. Characteristics and outcome of streptococcus pneumoniae endocarditis in the XXI century: a systematic review of 111 cases (2000-2013). Medicine (Baltimore) 2015; 94:e1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuchi T, Iwata K, Ohji G. Failure of early diagnosis of infective endocarditis in Japan: a retrospective descriptive analysis. Medicine (Baltimore) 2014; 93:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh GC, Sharma B, Katageri B, et al. ANCA positivity in a patient with infective endocarditis-associated glomerulonephritis: a diagnostic dilemma. Yale J Biol Med 2014; 87:373–377. [PMC free article] [PubMed] [Google Scholar]
- 25.González I, Sarriá C, López J, et al. Symptomatic peripheral mycotic aneurysms due to infective endocarditis: a contemporary profile. Medicine (Baltimore) 2014; 93:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatib R, Sharma M. Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine (Baltimore) 2013; 92:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López J, Revilla A, Vilacosta I, et al. Multiple-valve infective endocarditis: clinical, microbiologic, echocardiographic, and prognostic profile. Medicine (Baltimore) 2011; 90:231–236. [DOI] [PubMed] [Google Scholar]
- 28.Olmos C, Vilacosta I, Pozo E, et al. Prognostic implications of diabetes in patients with left-sided endocarditis: findings from a large cohort study. Medicine (Baltimore) 2014; 93:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortiz C, López J, García H, et al. Clinical classification and prognosis of isolated right-sided infective endocarditis. Medicine (Baltimore) 2014; 93:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riddell J, 4th, Kauffman CA, Smith JA, et al. Histoplasma capsulatum endocarditis: multicenter case series with review of current diagnostic techniques and treatment. Medicine (Baltimore) 2014; 93:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahr A, Batteux F, Tubiana S, et al. IMAGE Study Group. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol 2014; 66:1672–1677. [DOI] [PubMed] [Google Scholar]
- 32.Ying C-M, Yao D-T, Ding H-H, et al. Infective endocarditis with antineutrophil cytoplasmic antibody: report of 13 cases and literature review. PLoS One 2014; 9:e89777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agard C, Brisseau J-M, Grossi O, et al. Two cases of atypical Whipple's disease associated with cytoplasmic ANCA of undefined specificity. Scand J Rheumatol 2012; 41:246–248. [DOI] [PubMed] [Google Scholar]
- 34.Aslangul E, Goulvestre C, Mallat Z, et al. Human bartonella infective endocarditis is associated with high frequency of antiproteinase 3 antibodies. J Rheumatol 2014; 41:408–410. [DOI] [PubMed] [Google Scholar]
- 35.Bauer A, Jabs WJ, Süfke S, et al. Vasculitic purpura with antineutrophil cytoplasmic antibody-positive acute renal failure in a patient with Streptococcus bovis case and Neisseria subflava bacteremia and subacute endocarditis. Clin Nephrol 2004; 62:144–148. [DOI] [PubMed] [Google Scholar]
- 36.Bielefeld P, Lévêque L, Olsson NO, et al. c-ANCA and infective endocarditis. Rev Med Interne 2000; 21:543. [Google Scholar]
- 37.Bonaci-Nikolic B, Andrejevic S, Pavlovic M, et al. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol 2010; 29:893–904. [DOI] [PubMed] [Google Scholar]
- 38.Chirinos JA, Corrales-Medina VF, Garcia S, et al. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol 2007; 26:590–595. [DOI] [PubMed] [Google Scholar]
- 39.De Corla-Souza A, Cunha BA. Streptococcal viridans subacute bacterial endocarditis associated with antineutrophil cytoplasmic autoantibodies (ANCA). Heart Lung 2003; 32:140–143. [DOI] [PubMed] [Google Scholar]
- 40.Fukasawa H, Hayashi M, Kinoshita N, et al. Rapidly progressive glomerulonephritis associated with PR3-ANCA positive subacute bacterial endocarditis. Intern Med 2012; 51:2587–2590. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda M, Motokawa M, Usami T, et al. PR3-ANCA-positive crescentic necrotizing glomerulonephritis accompanied by isolated pulmonic valve infective endocarditis, with reference to previous reports of renal pathology. Clin Nephrol 2006; 66:202–209. [DOI] [PubMed] [Google Scholar]
- 42.Hanf W, Serre JE, Salmon JH, et al. Rapidly progressive ANCA positive glomerulonephritis as the presenting feature of infectious endocarditis. Rev Med Interne 2011; 32:e116–e118. [DOI] [PubMed] [Google Scholar]
- 43.Haseyama T, Imai H, Komatsuda A, et al. Proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) positive crescentic glomerulonephritis in a patient with Down's syndrome and infectious endocarditis. Nephrol Dial Transplant 1998; 13:2142–2146. [DOI] [PubMed] [Google Scholar]
- 44.Holmes AH, Greenough TC, Balady GJ, et al. Bartonella henselae endocarditis in an immunocompetent adult. Clin Infect Dis 1995; 21:1004–1007. [DOI] [PubMed] [Google Scholar]
- 45.Kawamorita Y, Fujigaki Y, Imase A, et al. Successful treatment of infectious endocarditis associated glomerulonephritis mimicking c3 glomerulonephritis in a case with no previous cardiac disease. Case Rep Nephrol 2014; 2014:569047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kishimoto N, Mori Y, Yamahara H, et al. Cytoplasmic antineutrophil cytoplasmic antibody positive pauci-immune glomerulonephritis associated with infectious endocarditis. Clin Nephrol 2006; 66:447–454. [DOI] [PubMed] [Google Scholar]
- 47.Konstantinov KN, Harris AA, Hartshorne MF, et al. Symptomatic anti-neutrophil cytoplasmic antibody-positive disease complicating subacute bacterial endocarditis: to treat or not to treat? Case Rep Nephrol Urol 2012; 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng H, Chen WF, Wu C, et al. Culture-negative subacute bacterial endocarditis masquerades as granulomatosis with polyangiitis (Wegener's granulomatosis) involving both the kidney and lung. BMC Nephrol 2012; 13:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riding AM, D’Cruz DP. A case of mistaken identity: subacute bacterial endocarditis associated with p-antineutrophil cytoplasmic antibody. BMJ Case Rep 2010; 2010: bcr0920103299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert SC, Forbes SH, Soleimanian S, et al. Complements do not lie. BMJ Case Rep 2011; 2011: bcr0820114705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvado C, Mekinian A, Rouvier P, et al. Rapidly progressive crescentic glomerulonephritis and aneurism with antineutrophil cytoplasmic antibody: Bartonella henselae endocarditis. Press Med 2013; 42:1060–1061. [DOI] [PubMed] [Google Scholar]
- 52.Satake K, Ohsawa I, Kobayashi N, et al. Three cases of PR3-ANCA positive subacute endocarditis caused by attenuated bacteria (Propionibacterium, Gemella, and Bartonella) complicated with kidney injury. Mod Rheumatol Jpn Rheum 2011; 21:536–541. [DOI] [PubMed] [Google Scholar]
- 53.Subra JF, Michelet C, Laporte J, et al. The presence of cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA) in the course of subacute bacterial endocarditis with glomerular involvement, coincidence or association? Clin Nephrol 1998; 49:15–18. [PubMed] [Google Scholar]
- 54.Sugiyama H, Sahara M, Imai Y, et al. Infective endocarditis by Bartonella quintana masquerading as antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Cardiology 2009; 114:208–211. [DOI] [PubMed] [Google Scholar]
- 55.Teoh LSG, Hart HH, Soh MC, et al. Bartonella henselae aortic valve endocarditis mimicking systemic vasculitis. BMJ Case Rep 2010; 2010:bcr0420102945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiliakos AM, Tiliakos NA. Dual ANCA positivity in subacute bacterial endocarditis. J Clin Rheumatol 2008; 14:38–40. [DOI] [PubMed] [Google Scholar]
- 57.Uh M, McCormick IA, Kelsall JT. Positive cytoplasmic antineutrophil cytoplasmic antigen with PR3 specificity glomerulonephritis in a patient with subacute bacterial endocarditis. J Rheumatol 2011; 38:1527–1528. [DOI] [PubMed] [Google Scholar]
- 58.Veerappan I, Prabitha EN, Abraham A, et al. Double ANCA-positive vasculitis in a patient with infective endocarditis. Indian J Nephrol 2012; 22:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vikram HR, Bacani AK, DeValeria PA, et al. Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol 2007; 45:4081–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilczynska M, Khoo JP, McCann GP. Unusual extracardiac manifestations of isolated native tricuspid valve endocarditis. BMJ Case Rep 2010; 2010:bcr1120092502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeledon JI, McKelvey RL, Servilla KS, et al. Glomerulonephritis causing acute renal failure during the course of bacterial infections. Histological varieties, potential pathogenetic pathways and treatment. Int Urol Nephrol 2008; 40:461–470. [DOI] [PubMed] [Google Scholar]
- 62.Preston GA, Pendergraft WF, Falk RJ. New insights that link microbes with the generation of antineutrophil cytoplasmic autoantibodies: the theory of autoantigen complementarity. Curr Opin Nephrol Hypertens 2005; 14:217–222. [DOI] [PubMed] [Google Scholar]
- 63.Pendergraft WF, 3rd, Preston GA, Shah RR, et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med 2004; 10:72–79. [DOI] [PubMed] [Google Scholar]
- 64.Jennette JC, Falk RJ, Hu P, et al. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol 2013; 8:139–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 2009; 10:623–635. [DOI] [PubMed] [Google Scholar]
- 66.Habib G, Hoen B, Tornos P, et al. ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009; 30:2369–2413. [DOI] [PubMed] [Google Scholar]
- 67.Tadema H, Heeringa P, Kallenberg CGM. Bacterial infections in Wegener's granulomatosis: mechanisms potentially involved in autoimmune pathogenesis. Curr Opin Rheumatol 2011; 23:366–371. [DOI] [PubMed] [Google Scholar]
- 68.Damoiseaux J, Andrade LE, Fritzler MJ, et al. Autoantibodies 2015: from diagnostic biomarkers toward prediction, prognosis and prevention. Autoimmun Rev 2015; 14:555–563. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498. [DOI] [PubMed] [Google Scholar]
- 70.Ghinoi A, Pipitone N, Cavazza A, et al. Wegener granulomatosis with spleen infarction: case report and review of the literature. Semin Arthritis Rheum 2008; 37:328–333. [DOI] [PubMed] [Google Scholar]
- 71.Jamoussi SK, Ben Dhaou B, Boussema F, et al. Unusual presentation of Wegener's granulomatosis. Rev Med Interne 2010; 31:e16–e18. [DOI] [PubMed] [Google Scholar]
- 72.Rentsch J, McColl G. Splenic infarction in Wegener's granulomatosis. J Rheumatol 2000; 27:1554–1555. [PubMed] [Google Scholar]
- 73.Revest M, Doco-Lecompte T, Hoen B. Epidemiology of infective endocarditis in France. BEH 2013; 10:89–94. [Google Scholar]
- 74.Athan E, Chu VH, Tattevin P, et al. ICE-PCS Investigators. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. J Am Med Assoc 2012; 307:1727–1735. [DOI] [PubMed] [Google Scholar]
- 75.Wang P, Lu J, Wang H, et al. Clinical characteristics of infective endocarditis: analysis of 368 cases. Zhonghua Xin Xue Guan Bing Za Zhi 2014; 42:140–144. [PubMed] [Google Scholar]
- 76.Matera G, Liberto MC, Quirino A, et al. Bartonella quintana lipopolysaccharide effects on leukocytes, CXC chemokines and apoptosis: a study on the human whole blood and a rat model. Int Immunopharmacol 2003; 3:853–864. [DOI] [PubMed] [Google Scholar]
- 77.Nakatani S, Mitsutake K, Ohara T, et al. Recent picture of infective endocarditis in Japan: lessons from Cardiac Disease Registration (CADRE-IE). Circ J 2013; 77:1558–1564. [DOI] [PubMed] [Google Scholar]
- 78.Thuny F, Grisoli D, Collart F, et al. Management of infective endocarditis: challenges and perspectives. Lancet 2012; 379:965–975. [DOI] [PubMed] [Google Scholar]
- 79.Wang TK. Mortality and neurological complications after early or late surgery for infective endocarditis and stroke. Clin Infect Dis 2013; 57:1662–1663. [DOI] [PubMed] [Google Scholar]


