Abstract
Normal pulmonary artery pressure and pulmonary hypertension assessment of newborns is rarely reported. The aim of the study is to explore dynamic changes of pulmonary arterial pressure and ductus arteriosus in human newborns from birth to 72 h of age with echocardiography.
A total of 76 cases of normal newborns were prospectively detected by echocardiography after birth of 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h, respectively. Ductus arteriosus diameter, blood shunt direction, blood flow velocity, and pressure gradient were recorded. The brachial artery blood pressure were measured to estimate the pulmonary artery systolic pressure (PASP) and pulmonary artery diastolic pressure (PADP) using patent ductus arteriosus pressure gradient method. The mean pulmonary artery pressure (PAMP) were calculated by equation of PAMP = PADP + 1/3(PASP-PADP).
(1) There were 76 cases of normal newborns. Among them, 29 cases (38%) ductus arteriosus closed within 24 h, 59 cases (78%) closed within 48 h, 72 cases (95%) closed within 72 h, and 4 cases (5%) ductus arteriosus not closed within 72 h. (2) The ductus arteriosus diameter of 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h after birth was 4.60 ± 0.59 mm, 3.37 ± 0.59 mm, 2.47 ± 0.49 mm, 1.89 ± 0.41 mm, 1.61 ± 0.35 mm, and 1.20 ± 0.24 mm, respectively. Compared all of the ductus arteriosus diameter of the above time periods, there were statistically differences with P < 0.05, respectively. (3) The mean PASP in 2 h, 6 h, 12 h, 24 h, 48 h, 72 h after birth were 76.58 ± 7.28 mm Hg, 65.53 ± 9.25mm Hg, 52.51 ± 9.07 mm Hg, 43.83 ± 7.90 mm Hg, 38.07 ± 8.26 mm Hg, and 36 ± 6.48 mm Hg, respectively. The PADP of the above time period were 37.88 ± 5.56 mm Hg, 29.93 ± 7.91 mm Hg, 23.43 ± 7.37 mm Hg, 19.70 ± 8.51 mm Hg, 13.85 ± 5.58 mm Hg, 13.25 ± 6.18 mm Hg, respectively. The PAMP of the above time period were 63.41 ± 7.03 mm Hg, 51.78 ± 9.82 mm Hg, 40.94 ± 9.32 mm Hg, 34.39 ± 9.89 mm Hg, 26.23 ± 7.49 mm Hg, 25.25 ± 8.29 mm Hg, respectively. There were statistically differences with P < 0.05 between each time periods of PASP, PADP, and PAMP. (4) The upper 95% limit reference range of PASP of normal newborns of 72 h after birth were 39.97 mm Hg.
(1) Normal newborns ductus arteriosus diameter gradually decreased after birth, and 95% of them spontaneous closed within 24 to 72 h. (2) Normal newborns pulmonary artery pressure showed a gradually decline after birth, the upper 95% limit reference range for PASP measured in normal newborns <72 h of age was 39.97 mm Hg. Therefore, the diagnostic criteria of newborns pulmonary hypertension may be >40.00 mm Hg according to our limited study.
INTRODUCTION
In the fetus, pulmonary circulation was filled with amniotic liquid and compressed. The pulmonary circulation was a high resistance circuit whereas the systemic circulation included a low resistance, placenta, and high flow component. Most of the right ventricular output entered the descending aorta across the ductus arteriosus and only 10% entered the pulmonary circulation.1 After birth, lung aeration open, lung fluid elimination, pulmonary flow and oxygen partial pressure increased, and alveolar surface active substance secretion increased made PVR declined continuously. If due to various reasons after birth, the newborns cannot adapt to the outside environment commendably, circulation conversion failure, PVR failure to decline continuously, so all these negative factors can lead to pulmonary hypertension of the newborn (PHN). Pulmonary artery pressure from fetal, newborn to infant period is constantly changed, especially in the neonatal period, pulmonary artery pressure is decreased sharply in 72 h after birth, whereas it is still higher than that of normal adults. However, So far, newborns pulmonary hypertension diagnosis standard is still not clearly established, and it is still use adult pulmonary artery systolic pressure (PASP) >30 mm Hg as a reference.2 Even previous study reported that newborns pulmonary artery pressure within 2 weeks was higher than the above value,1,3 newborns pulmonary artery pressure assessment, especially early pulmonary artery pressure, is rarely reported. As we all known that the gold standard of determination the pulmonary artery pressure is right heart floating catheter method, however, owing to the invasiveness and poor tolerance, it cannot be performed in the newborns. Echocardiography is the first choice for noninvasive quantitative estimates of newborns pulmonary artery. And the most commonly used is the tricuspid regurgitation (TR) method and open artery catheter (Patent ductus arteriosus, PDA) method. Echocardiographic measurements of tricuspid regurgitant jet velocity and the Doppler flow velocity across the PDA to assess PASP had been proved to yield a favorable accuracy.4,5 With the development of echocardiography and Doppler ultrasound technology, it has been proved that echocardiography estimation of pulmonary artery pressure had a good correlation with right cardiac catheterization method.6,7 So, the aim of this study was to determine pulmonary artery systolic pressure of normal newborns after birth of 2 h,6 h,12 h,48 h, and 72 h, respectively, and to explore the spontaneous closure rate of ductus arteriosus in normal newborns within 72 h with echocardiography.
PARTICIPANTS AND METHODS
Study Population
We prospective studied 76 cases of normal newborns born in Second Affiliated Hospital of Xi’an Jiao Tong University in 3 months from January 2014 and March 2014. Inclusion criteria included the following: (1) 37 weeks≤gestational age < 42 weeks; (2) 2500 g≤birth weight < 4000 g; (3) no perinatal asphyxia or hypoxia (1 min Apgar scoring > 7 points); (4) echocardiography excluded cardiac structural abnormalities except patent foramen oale and patent ductus arteriosus; (5) pregnant women in good health during pregnancy and without pregnancy hypertension, pregnancy diabetes, hyperthyroidism, hypothyroidism, and autoimmune disease. The study protocol was approved by the ethics committee of the Second Affiliated hospital of Xi’an Jiao Tong University and written informed consent was obtained from each parents.
METHODS
Mindray M7series portable color Doper ultrasound system fitted with P7-3 s transducer were used to conduct echocardiography. The procedure was performed under the written consent of each case and the newborns should kept supine or in the left lateral position and kept in a quiet and relatively steady state throughout the procedure. Echocardiography was first routinely performed to rule out cardiac structural abnormalities. Parasternal short axis views of great vessels were obtained to display pulmonary artery, we rotated probe counterclockwise to display ductus arteriosus, a channel between pulmonary artery and the descending aorta. Ductus arteriosus diameter was measured using 2-dimensional ultrasound. Color Doppler was used to observe the presence or absence of shunt. Sampling volume of pulsed or continuous wave Doppler were placed on ductus arteriosus to obtain the ductus blood flow spectrum. Direction of blood flow shunt, velocity and pressure gradient were also recorded. All the above parameters were measured in 3 consecutive cardiac cycles to obtain the mean value of them. All operations were performed by the same sonographer, CONTEC08A electronic sphygmomanometer fitted with neonatal sphygmomanometer cuff was used to measure brachial artery blood pressure. All the newborns were dynamic monitored after the birth of 2 h, 6 h, 12 h, 24 h, 48 h, 72 h, respectively. After echocardiography was completed, the brachial artery blood pressure was measured 3 times immediately and the mean value was calculated.
In the absence of left ventricular outflow tract and aortic stenosis, brachial artery systolic pressure (BASP) could represent the aortic systolic pressure (AOSP) and brachial artery diastolic pressure (BADP) could represent the aorta diastolic pressure (AODP). The Bernoulli equation was used to estimate PASP (PASP = 4 V2 + right atrial pressure, where V indicates peak systolic velocity of the regurgitant jet) if there were no pulmonary stenosis. According to the great vessels pressure gradient method, when a bidirectional shunt of ductus arteriosus was obtained, pulmonary artery systolic pressure (PASP) = AOSP + 4 V2 (V indicates the peak velocity of right to left shunt of ductus arteriosus). When bidirectional shunt occurred, end-diastolic ductus arteriosus flow direction was left to right shunt, so pulmonary artery diastolic pressure (PADP) = AODP−4 V2 (V indicates end-diastolic peak velocity of right to left shunt of ductus arteriosus). When only left to right shunt occurred, PASP = AOSP−4 V2 (V indicates the peak velocity of left to right shunt of ductus arteriosus), PADP = AODP−4 V2 (V indicates end-diastolic peak velocity of left to right shunt of ductus arteriosus)8 (Figure 1). Pulmonary arterial mean pressure (PAMP) = PADP + (PASP−PADP)/3.
FIGURE 1.
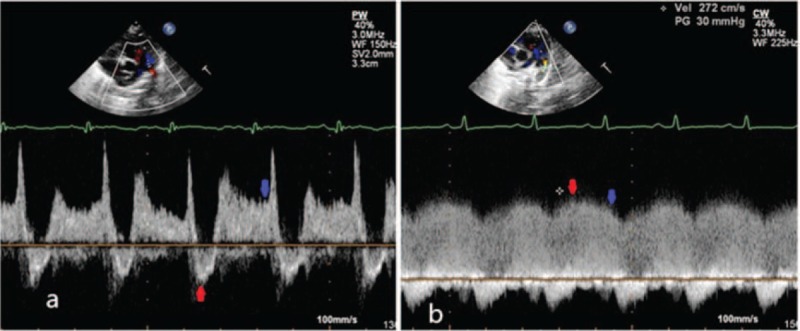
(A)When a bidirectional shunt of ductus arteriosus was obtained, the red arrow indicated the peak velocity of right to left shunt, which was used to estimate PASP. The blue arrow indicated the velocity of end-diastolic and was used to estimate PADP. (B) When a left to right shunt of ductus arteriosus was obtained, the red arrow indicated the peak velocity of left to right shunt, which was used to estimate PASP. Blue arrow indicated the velocity of end-diastolic and was used to estimate PADP. PASP = pulmonary artery systolic pressure, PADP = pulmonary artery diastolic pressure, PAMP = mean pulmonary artery pressure.
Statistical Analysis
Statistical analyses were performed using SPSS Version 18.0 (SPSS, Chicago, IL). All measurement data are expressed as mean ± standard deviation (x ± s), and the count data is expressed as cases or a percentage (%). Paired samples t test was performed to compare the change of ductus arteriosus diameter, PASP, PADP, and PAMP for each time period. χ2 test was conducted to compare the influence of gender, delivery mode, birth weight, and gestational age on the ductus arteriosus closure rate, and P < 0.05 was statistically different. The multifactor linear regression analysis method was used to analysis the relationship between PASP, PADP, PAMP and gender, gestational age, delivery mode, and birth weight.
RESULTS
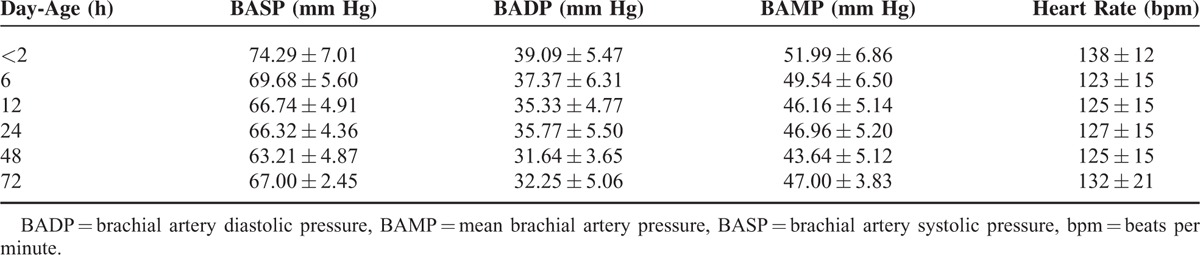
A total of 76 cases of normal newborns were included, 46% (35/76) were men. Mean gestational age were 39.58 ± 0.99 weeks and mean birth weight were 3406.6 ± 387.8 g. One-min Apgar scoring was 9 points and 5 min Apgar scoring was 10 points. The brachial artery pressure and heart rate of patent ductus arteriosus newborns after birth of 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h were showed in Table 1.
TABLE 1.
The Brachial Artery Pressure and Heart Rate of Patent Ductus Arteriosus Newborns Within Each Time Periods (x ± s)
The changes of ductus arteriosus diameter and closure were as follows: there were 76 cases of normal newborns. Among them, 29 cases (38%) ductus arteriosus closed within 24 h, 59 cases (78%) closed within 48 h, 72 cases (95%) closed within 72 h, 4 cases (5%) ductus arteriosus not closed within 72 h. Compared the ductus arteriosus diameter before and after the above each time period, there were statistically differences in the changes of ductus arteriosus diameter with P < 0.05 (Table 2). Ductus arteriosus diameter decreased gradually with the increasing of newborns day-age (Figure 2).
TABLE 2.
Newborns Ductus Arteriosus Diameter (x ± s) and Closure Rate Within Each Time Period
FIGURE 2.
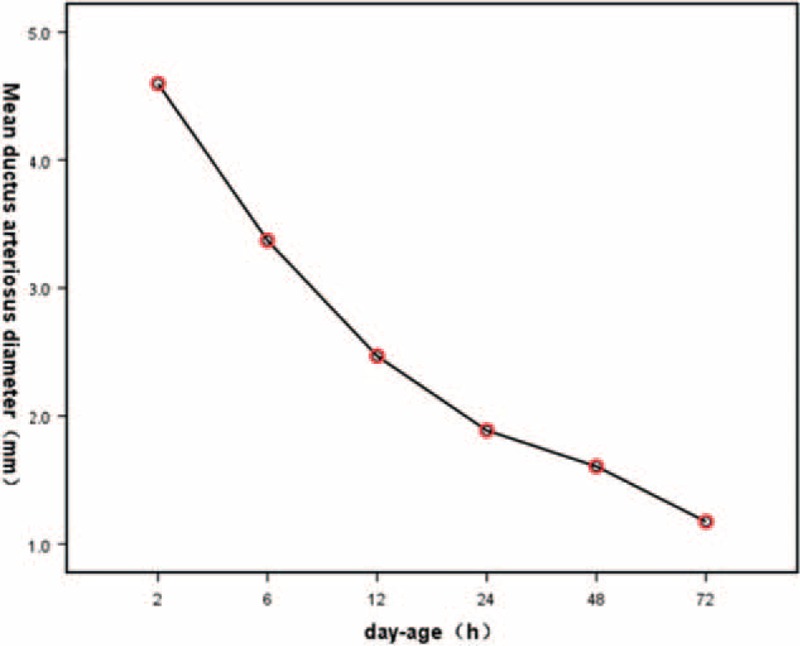
The line graph between day-age and mean value of ductus arteriosus diameter indicated that with the increasing of neonatal day-age, ductus arteriosus diameter decreased gradually.
To evaluate the influence factors of ductus arteriosus closure rate, we divided 76 cases of normal newborns into 2 groups respectively according to gender, delivery mode, gestational age (<40 weeks or ≥40 weeks), and birth weight (≤3000 g or > 3000 g). The χ2 test was performed to assess whether there were difference in 2 groups when compared the ductus arteriosus closure rate of 24 h, 48 h, and 72 h after birth. The χ2 test was used to compare the relationship of ductus arteriosus closure rate and delivery mode, gestational age, birth weight, and gender. The results showed no statistical significance when the χ2 test was used to compare the relationship between delivery mode, gestational age, birth weight, gender, and ductus arteriosus closure rate (P > 0.05).
We also recorded the ductus arteriosus flow spectrum. All of the 76 cases of normal newborns had a bidirectional shunt 2 h after birth, 42 cases had a bidirectional shunt and 34 cases had a unidirectional left to right shunt 6 h after birth, 9 cases had a bidirectional shunt and 67 cases had a unidirectional left to right shunt 12 h after birth, all the newborn infants had a bidirectional shunt 24 h after birth. Only 5 cases had a very small or transient shunt (Table 3). Pulmonary arterial pressure was obtained based on the Doppler spectrum. Compared the PASP, PADP, PAMP before and after the above each time period, there were statistically differences with all P < 0.05. With the increasing of neonatal day-age, PASP, PADP, PAMP decreased gradually (Figure 3). And 95% confidence interval upper limit of newborn infants PASP within each time period was calculated as follows: within 2 h ≤79.66 mm Hg, within 6 h≤74.23 mm Hg, within 12 h≤67.17 mm Hg, within 24 h≤57.13 mm Hg, within 48 h≤47.27 mm Hg, within 72 h≤39.97 mm Hg, respectively.
TABLE 3.
The Cases and Proportion of Different Shunt Directions of Ductus Arteriosus Blood Flow Within Each Time Period
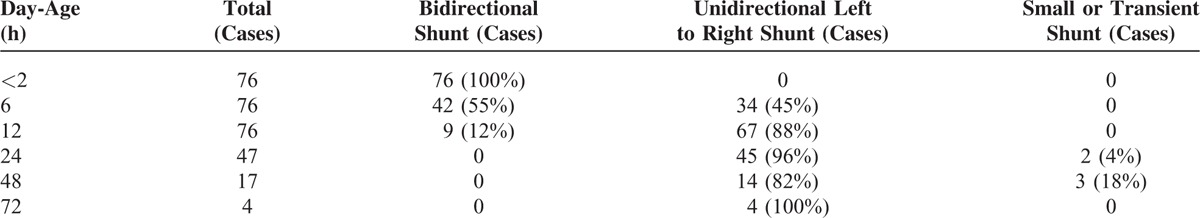
FIGURE 3.
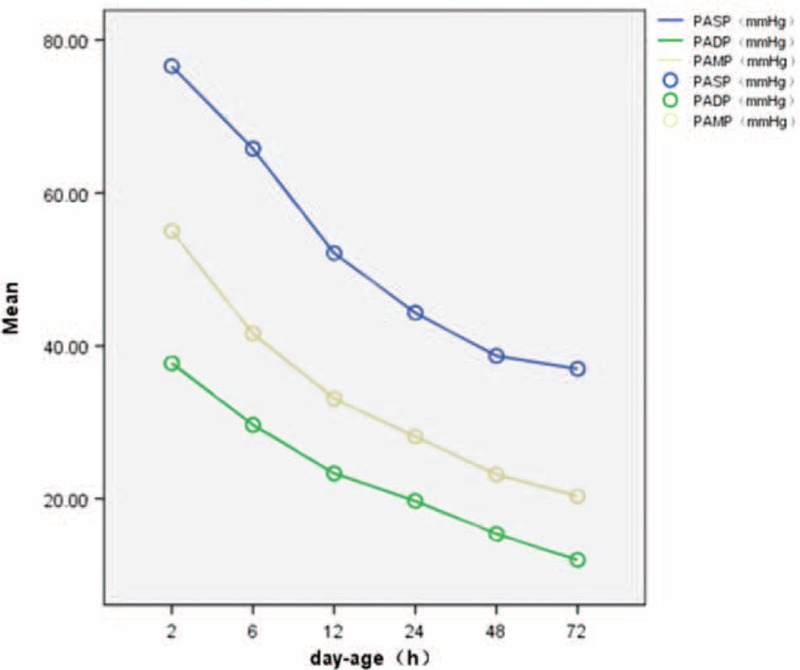
The line graph between day-age and PASP, PADP, PAMP indicated that with the increasing of neonatal day-age, PASP, PADP, PAMP decreased gradually. PASP = pulmonary artery systolic pressure, PADP = pulmonary artery diastolic pressure, PAMP = mean pulmonary artery pressure.
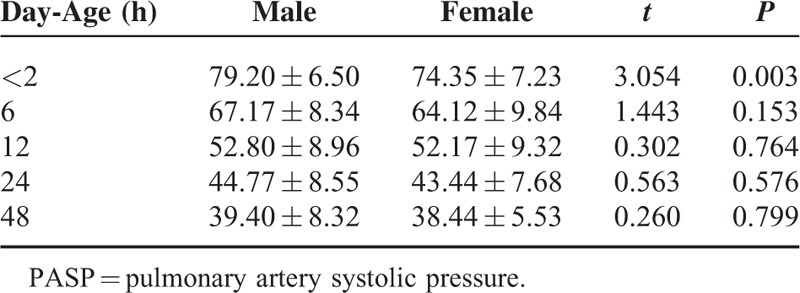
Multifactor linear regression analysis method was used to analysis the relationship between PASP, PADP, PAMP and gender, gestational age, delivery mode and birth weight. A total of 76 cases of normal newborns were divided into 2 groups respectively according to gender, delivery mode, gestational age (<40 weeks or ≥40 weeks) and birth weight (≤3000 g or > 3000 g). Independent sample t test was performed to compare whether there were difference of PASP, PADP, PAMP within each time period between 2 groups (data was not available in 72 h). The results showed that there were statistical significance of newborns PASP within 2 h after birth in gender and delivery mode (all P < 0.05)(Table 4, Table 5),which suggesting that PASP of male babies within 2 h after birth was slightly higher than that of female babies, and that newborns delivered by caesarean section within 2 h after birth was higher than that of newborns delivered by vaginal. There were no statistical significance of newborns PADP, PAMP within 2 h after birth and PASP, PADP, PAMP within the rest of the time period when gender, delivery mode, gestational age and birth weight were compared in the 2 groups (all P > 0.05).
TABLE 4.
Newborns PASP Within Each Time Period With Different Delivery Mode (x ± s) (mm Hg)
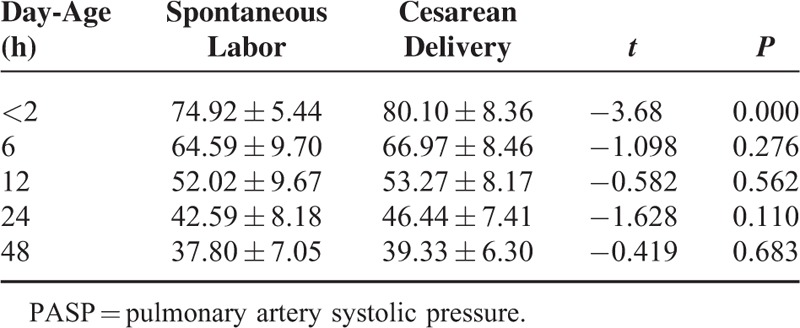
TABLE 5.
Newborns PASP Within Each Time Period With Different Genders (x ± s) (mm Hg)
DISCUSSION
Our study results indicated that a gradual decrease in normal newborns ductus arteriosus diameter after birth, and 95% of them spontaneous closed within 24 to 72 h. In fetal stages, PDA is an open channel between pulmonary artery and the descending aorta; however, patent ductus closed closure time differed owing to individual differences. We find that the earliest time of ductus closure was within 12 to 24 h, none of closed within 12 h, closure rates within 24 h were 38%, closure rates within 48 h were 78%, and closure rates within 72 h were 95%. Previous study found that earliest time of ductal closure were within 8 h, closure rates within 24 h were 42%, closure rates within 48 h were 90%, and closed all by 96 h of age.9 Because of the difference of region and race, our study had small differences on the closure rates compared with previous study.9 Even the presence of differences on the closure rates, most of the newborns patent ductus closed within 72 h. Some studies10,11 reported that normal newborns patent ductus functional closed within 10 to 15 h. These studies were based on invasive methods and depended on the oxygen saturation detection ductus shunt to estimate ductus closure time, while the oxygen saturation failing to detect a small shunt. As a result, the estimated ductus closure time was earlier than really closure time. Besides, some newborns suffered mild respiratory disease or congenital heart disease, it was not appropriate to apply the results of those newborns to the normal ones. When it come to the factors influencing the normal newborns ductus closure, we found that gender, gestational age, delivery mode, and birth weight had no obvious effect on it, which was consistent with the Gentile’ results.9
Ductus arteriosus blood flow direction was determined by the pressure difference between the pulmonary artery and aorta.12 During fetal stage, pulmonary vascular resistance (PVR) was higher than systemic circulation, and blood flow from the right ventricle could pass through the patent ductus and into the systemic circulation easily (right-to-left shunt). It was reported that ∼90% of right ventricular output, during fetal stage, bypassed the lungs and flowed across the patent ductus.12 After birth, lung aeration opened up and PVR decreased suddenly, which resulted in pulmonary blood flow increased greatly. The pressure gradient across the patent ductus reversed, which facilitated blood flow from the systemic circulation to the pulmonary circulation (left-to-right shunt). The study performed by Van Vonderen13 reported that patent ductus shunting changed rapidly from predominantly right-to-left shun to predominantly left-to-right shunt at as early as 10 min after birth. Echocardiography was conducted 6 times in all 76 cases of normal newborns within 72 h after birth, we found that all newborns had a bidirectional ductal shunt within 2 h after birth, 55% had a bidirectional shunt within 6 h after birth, 12% had a bidirectional shunt within 12 h after birth, and all newborns had a left-to-right shunt after 24 h of birth, which indicated that the newborn infants pulmonary artery systolic pressure remains higher than the aortic systolic pressure within 2 h after birth. Owing to individual differences, newborns pulmonary artery pressure decreased in different degrees. Newborns pulmonary artery systolic pressure was equal to or even lower than the aortic systolic blood pressure within 2 to 6 h, about half of the newborns pulmonary artery systolic pressure was lower than the aortic systolic blood pressure to 6 h after birth. To 12 h after birth, most of the newborns pulmonary artery systolic pressure was lower than the aortic systolic blood pressure, to 24 h after birth, all newborns pulmonary artery systolic pressure was lower than the aortic systolic blood pressure. This was in accordance with previous studies.14,15 Skinner14 et al reported that 56% had a bidirectional shunt within 0 to 12 h among 34 healthy full-term newborns, 3% newborns had a bidirectional shunt within 13 to 36 h, and all had a right-to-left shunt within 36 to 72 h. Hu15 et al found that among the 296 healthy full-term newborns, 98% had a bidirectional shunt within 0 to 6 h, 26% had a bidirectional shunt within 6 to 10 h, and 3% had a bidirectional shunt within 10 to 24 h. The differences of our research results in the data could be attributed to the research methods and different sample size.
Normal newborns pulmonary arterial pressure gradually decreased with increasing age, especially decreased sharply within 24 h, and then decreased gradually. And to 72 h after birth, newborns pulmonary artery systolic pressure (PASP: 37 ± 4.97 mm Hg) was still higher than that of adult (PASP < 30 mm Hg). We also found that newborns pulmonary artery systolic pressure was higher than the aortic systolic blood pressure and pulmonary artery diastolic pressure was lower than the aorta diastolic blood pressure within 2 h after birth, which indicated that pulmonary artery diastolic pressure, reflected peripheral resistance, decreased swiftly than the pulmonary artery systolic pressure after birth. These findings were in accordance with normal newborns pulmonary arterial pressure obtained from retrograde catheterization of the umbilical arteries.16 The upper 95% limit reference range of PASP of normal newborns of 2 h, 6 h, 12 h, 24 h, 48 h, 72 h was estimated through ductal blood flow velocity. The specific value was as follows: within 2 h ≤79.66 mm Hg, within 6 h≤74.23 mm Hg, within 12 h≤67.17 mm Hg, within 24 h≤57.13 mm Hg, within 48 h≤47.27 mm Hg, within 72 h≤39.97 mm Hg, respectively. Some authors also had studied a normal range of newborn pulmonary artery pressure in previous works.15,17,18 Hu15 et al estimated PASP of 200 healthy full-term newborns, 32 min to 14 days after birth, using tricuspid regurgitant jet velocity. They concluded that 95% confidence interval upper limit of newborns PASP within 6 h, 0 to 1 day, 1 to 3 days, 3 to 7 days, 7 to 14 days was ≤75.30 mm Hg, ≤57.12 mm Hg, ≤46.51 mm Hg, ≤40.97 mm Hg, ≤39.14 mm Hg, respectively. However, their study was performed on 200 cases of different day-age newborns, whereas we followed up all of the 76 newborns from birth to 72 h. Besides, our estimation method of PASP was different from theirs. Schmitz17 et al estimated PASP of 51 newborns from birth to 4 days using tricuspid regurgitant jet velocity, they found PASP of 4 days of newborns < 30 mm Hg. Aldudak18 et al estimated PASP of 20 newborns from birth to 5 days using tricuspid regurgitant jet velocity, they found PASP of 5 days of newborns < 24.9 mm Hg. Our limited research had shown that newborns pulmonary artery pressure gradually declined within 72 h after birth, changed from brachial pressure higher than systemic pressure to brachial pressure lower than systemic pressure, but at 72 h time points, it remained in the higher level of 40 mm Hg. That was to say, newborns pulmonary arterial pressure was higher than adults. Therefore, we hold that the newborns pulmonary hypertension diagnostic criteria may be higher than 40.00 mm Hg. When newborns pulmonary arterial pressure declined to the normal adult levels, it remained to be further research.
We also found that PASP of newborns delivered by the caesarean section was higher than that of newborns delivered by vaginal within 2 h after birth, which indicated that spontaneous labor newborns were more easier to adapt to the environment outside the uterus. Research had shown that the newborns delivered by cesarean section had a higher incidence of respiratory diseases.19 It was reported that spontaneous labor could increased the release of catecholamine and adrenaline could triggered absorption of neonatal lungs liquid.20 By comparing the NO and endothelin level in the blood of newborns delivered by vaginal and cesarean section on the first day and fifth day, Endo et al21 found that the NO level of newborns delivered by vaginal was increased and the endothelin level was decreased, although there was no statistically significant difference. By compared pulmonary arterial pressure using tricuspid regurgitant jet velocity of newborns delivered by vaginal and cesarean section on the first day, the third day and fifth day, Aldudak18 et al found that PASP of newborns delivered by cesarean section was 5 mm Hg than that of newborns delivered by vaginal and the PASP declining lasted for the fifth day, while PASP declining of newborns delivered by vaginal only lasted for the third day. All the above studies suggested that delivery mode could influence newborns postpartum adaptation. In our study, we also found that PASP of male babies within 2 h after birth was slightly higher than that of female babies, and the possible reasons were still unclear, large sample, multicenter study further were needed to further confirmed. Besides, our study also had some limitations. The major problem of our study was the small number of cases with only 76 cases of normal newborns included. In ethics, normal newborns pulmonary arterial pressure cannot be measured with the gold standard of cardiac catheterization. This study only estimated normal newborns pulmonary arterial pressure within 72 h after birth, as to when newborns pulmonary arterial pressure declined to the normal adult levels, it remained to be further research.
CONCLUSION
Ductus arteriosus closure rate increased along with the age increasing and the closure rate was 95% at 72 h time points. The PASP within 2 h after birth of newborns delivered by vaginal was lower than that of delivered by the caesarean section. Normal newborns pulmonary artery pressure showed a gradually decline trend after birth, and pulmonary artery pressure of 72 h after birth was still higher than that of normal adults. The upper 95% limit for PASP measured in normal newborns <72 h of age was 39.97 mm Hg. Therefore, we hold that the newborns pulmonary hypertension diagnostic criteria may be >40.00 mm Hg.
Footnotes
Abbreviations: PADP = pulmonary artery diastolic pressure, PAMP = mean pulmonary artery pressure, PASP = pulmonary artery systolic pressure.
C-MK and E-FZ contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Greenough A, Khetriwal B. Pulmonary hypertension in the newborn. Paediatr Respir Rev 2005; 6:111–116. [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54 (1 Suppl):S55–66. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Gatzoulis MA, Adatia I, et al. [Updated clinical classification of pulmonary hypertension]. Turk Kardiyoloji Dernegi Arsivi: Turk Kardiyoloji Derneginin yayin organidir 2014; 42 suppl 1:45–54. [PubMed] [Google Scholar]
- 4.Lam CS, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009; 119:2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman A, Prokupetz A, Benderly M, et al. Pulmonary artery pressure in young healthy subjects. J Am Soc Echocardiogr 2012; 25:357–360. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed CM, Arafat SM, Hasan MK, et al. Validity of estimation of pulmonary artery pressure using continuous wave Doppler echocardiography in patient with patent ductus arteriosus (PDA). Univ Heart J 2012; 7:10–12. [Google Scholar]
- 7.Ge Z, Zhang Y, Fan D, et al. Simultaneous measurement of pulmonary artery diastolic pressure by Doppler echocardiography and catheterization in patients with patent ductus arteriosus. Am Heart J 1993; 125:263–266. [DOI] [PubMed] [Google Scholar]
- 8.Skinner JR, Boys RJ, Heads A, et al. Estimation of pulmonary arterial pressure in the newborn: study of the repeatability of four Doppler echocardiographic techniques. Pediatr Cardiol 1996; 17:360–369. [DOI] [PubMed] [Google Scholar]
- 9.Gentile R, Stevenson G, Dooley T, et al. Pulsed Doppler echocardiographic determination of time of ductal closure in normal newborn infants. J Pediatr 1981; 98:443–448. [DOI] [PubMed] [Google Scholar]
- 10.Koch J, Hensley G, Roy L, et al. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 2006; 117:1113–1121. [DOI] [PubMed] [Google Scholar]
- 11.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics 2010; 125:1020–1030. [DOI] [PubMed] [Google Scholar]
- 12.Crossley KJ, Allison BJ, Polglase GR, et al. Dynamic changes in the direction of blood flow through the ductus arteriosus at birth. J Physiol 2009; 587 (Pt 19):4695–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Vonderen JJ, te Pas AB, Kolster-Bijdevaate C, et al. Non-invasive measurements of ductus arteriosus flow directly after birth. Arch Dis Child Fetal Neonatal Ed 2014; 99:F408–412. [DOI] [PubMed] [Google Scholar]
- 14.Skinner JR, Boys RJ, Hunter S, et al. Non-invasive assessment of pulmonary arterial pressure in healthy neonates. Arch Dis Child 1991; 66 (4 Spec No):386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Q, Ren WD, Mao J, et al. Changes in pulmonary artery pressure during early transitional circulation in healthy full-term newborns. Ultrasonics 2015; 56:524–529. [DOI] [PubMed] [Google Scholar]
- 16.Gidding SS. 50 years ago in the Journal of Pediatrics: pulmonary arterial pressure changes in human newborn infants from birth to 3 days of age. J Pediatr 2014; 165:484. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz AJ, Weinzheimer HR, Fahnenstich H, et al. Color Doppler echocardiographic evaluation of tricuspid regurgitation and systolic pulmonary artery pressure in the full-term and preterm newborn. Angiology 1997; 48:725–734. [DOI] [PubMed] [Google Scholar]
- 18.Aldudak B, Kervancioglu M. Effect of mode of delivery on postnatal decline in pulmonary artery pressure. Saudi Med J 2011; 32:579–583. [PubMed] [Google Scholar]
- 19.Hansen AK, Wisborg K, Uldbjerg N, et al. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstetricia et Gynecologica Scandinavica 2007; 86:389–394. [DOI] [PubMed] [Google Scholar]
- 20.Vogl SE, Worda C, Egarter C, et al. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG 2006; 113:441–445. [DOI] [PubMed] [Google Scholar]
- 21.Endo A, Izumi H, Ayusawa M, et al. Spontaneous labor increases nitric oxide synthesis during the early neonatal period. Pediatr Int 2001; 43:340–342. [DOI] [PubMed] [Google Scholar]


