Supplemental Digital Content is available in the text
Keywords: acute myocardial infarction, diabetes mellitus, major adverse cardiac events
Abstract
This study assessed the 2-year clinical outcomes of patients with diabetes mellitus (DM) after acute myocardial infarction (AMI) in a cohort of the DIAMOND (DIabetic Acute Myocardial infarctiON Disease) registry. Clinical outcomes were compared between 1088 diabetic AMI patients in the DIAMOND registry after stabilization of MI and 1088 nondiabetic AMI patients from the KORMI (Korean AMI) registry after 1 : 1 propensity score matching using traditional cardiovascular risk factors. Stabilized patients were defined as patients who did not have any clinical events within 1 month after AMI. Primary outcomes were the 2-year rate of major adverse cardiac events (MACEs), a composite of all-cause death, recurrent MI (re-MI), and target vessel revascularization (TVR). Matched comparisons revealed that diabetic patients exhibited significantly lower left ventricular ejection fraction (LVEF) and estimated glomerular filtration rate and smaller stent size. Diabetic patients exhibited significantly higher 2-year rates of MACE (8.0% vs 3.7%), all-cause death (3.9% vs 1.4%), re-MI (2.8% vs 1.2%), and TVR (3.5% vs 1.3%) than nondiabetic patients (all P < 0.01), and higher cumulative rates in Kaplan–Meier analyses of MACE, all-cause death, and TVR (all P < 0.05). A multivariate Cox regression analysis revealed that chronic kidney disease, LVEF < 35%, and long stent were independent predictors of MACE, and large stent diameter and the use of drug-eluting stents were protective factors against MACE. The 2-year MACE rate beyond 1 month after AMI was significantly higher in DM patients than non-DM patients, and this rate was associated with higher comorbidities, coronary lesions, and procedural characteristics in DM.
1. Introduction
Diabetes mellitus (DM) is strongly associated with adverse cardiovascular (CV) events.[1] DM affects the development of coronary artery disease (CAD) and clinical outcomes following the various manifestations of CAD. DM is a CAD risk-equivalent because the risk of acute myocardial infarction (AMI) in DM patients with no evidence of CAD matches the risk in patients with a previous history of AMI without DM.[2,3] Previous studies suggested that DM contributes to poor clinical outcomes after the event of AMI.[4–6] However, multiple confounding relationships between clinical factors may influence the early term events simultaneously.[7] Data on the long-term clinical outcomes according to DM are not well evaluated in stabilized patients with AMI. We compared 2-year clinical outcomes according to DM status in stabilized patients with AMI.
2. Methods
2.1. Study design and population
The DIabetic Acute Myocardial infarctiON Disease (DIAMOND) is a prospective, multicenter, observational study for the identification of clinical outcomes after an AMI event in type 2 DM patients in the Korean population. A total of 1192 consecutive DM patients presenting with ST-elevation myocardial infarction (STEMI) or non-STEMI (NSTEMI) were enrolled at 22 tertiary or university hospitals in Korea between April 2010 and June 2012. The following inclusion criteria were used: age ≥45 years; documented STEMI or NSTEMI as elevated cardiac troponin-I or T levels (exceeding upper normal limit) or creatine kinase-myocardial band fraction (CK-MB) levels (exceeding 3 times the upper limit of normal); angiographically confirmed ≥50% coronary luminal stenosis with or without intracoronary filling defect or haziness suggesting coronary thrombus, or coronary spasm-induced AMI without significant stenosis [<50% narrowing of the coronary luminal diameter measured by quantitative coronary angiography (QCA)]; and documented type 2 DM; and AMI patients were stabilized (i.e., did not have any clinical events within 1 month after the initial presentation of AMI). Sixty-seven patients who died during hospital admission or were lost to follow-up within 1 month after discharge were excluded from the present study. This study finally included 1125 diabetic patients with AMI from the DIAMOND registry.
Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Multivessel disease was defined as having other lesions with ≥50% stenosis in the noninfarct-related coronary artery. Transthoracic echocardiography assessed the left ventricular ejection fraction (LVEF) using the modified Simpson's biplanar method. Angiographic findings were collected when angiography was performed at any time during the follow-up period. All data were collected using an electronic case report form. DM patients with AMI in the DIAMOND study were matched 1 : 1 with 3178 non-DM patients with AMI in the Korea AMI (KORMI) registry to compare clinical outcomes according to DM status in stabilized AMI patients. KORMI was a prospective, multicenter, national registry from February 2008. The Institutional Review Board/Ethical Committees at each clinical site approved the study protocol, and written informed consent was obtained from all patients.
2.2. Study outcomes
The primary outcome in this study was rate of major adverse cardiac events (MACEs) including all-cause death, recurrent myocardial infarction (re-MI), and target vessel revascularization (TVR). The secondary outcomes were the rates of the individual components of the primary outcome and definite or probable stent thrombosis (ST) using the Academic Research Consortium criteria definition.[8] All death was considered cardiac unless there was a clear noncardiac cause. Re-MI was defined as the occurrence of characteristics of MI 28 days after the initial presentation of AMI.[9] TVR was defined as any repeat percutaneous or surgical revascularization of any segment of the target vessel.[8]
2.3. Statistical analysis
Continuous variables are expressed as the means ± standard deviation and compared using Student t test. Categorical variables are presented as absolute counts and percentages and compared using the χ2 test or Fisher exact tests, where appropriate. Statistical analyses were of an explorative and descriptive nature, and the study was primarily used for hypothesis generation. All issues concerning patient validity, data consistency checks, and permissible data modifications are described in detail in the Data Management Plan, and all statistical items, including calculated variables and proposed table format and content, were detailed in the Statistical Analysis Plan (SAP), which was finalized before the study database lock. All analyses were performed on the total study population (i.e., pooled analysis). Propensity score analysis was used to compensate for the single-arm, nonrandomized characteristics of this study. The pre-specified clinical variables were age, gender, and coronary risk factors, including hypertension, hyperlipidemia, and smoking. Kaplan–Meier analysis was used to calculate the cumulative incidences of primary and secondary clinical outcomes according to DM status. Comparisons between groups were performed using the log-rank test. A logistic regression model was used to identify independent determinants for the primary outcome. Variables with P < 0.05 in the univariate analysis were entered into multivariate logistic regression analysis. Analyses were performed using SPSS statistical software, version 20.0 (SPSS, Inc., Chicago, IL). A P value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline patient characteristics
Table 1 presents baseline clinical characteristics of the 1125 AMI patients with DM and 3178 AMI patients without DM. The mean duration of DM was 10.9 ± 8.5 years in the present study. DM patients compared with non-DM patients in unmatched populations were significantly older with higher BMI, high-sensitivity C-reactive protein (hs-CRP) levels, and incidences of hypertension, hyperlipidemia, history of previous MI, LVEF <35%, and CKD and lower CK-MB and troponin-I peak levels, incidence of smoking, and STEMI. Similar distributions of medication use, including glycoprotein IIb/IIIa inhibitors, aspirin, clopidogrel, beta blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins, were observed. Hs-CRP level and the incidence of history of previous MI, LVEF <35%, and CKD remained significantly higher in DM patients than non-DM patients after 1 : 1 propensity score matching (Fig. 1).
Table 1.
Baseline clinical characteristics.
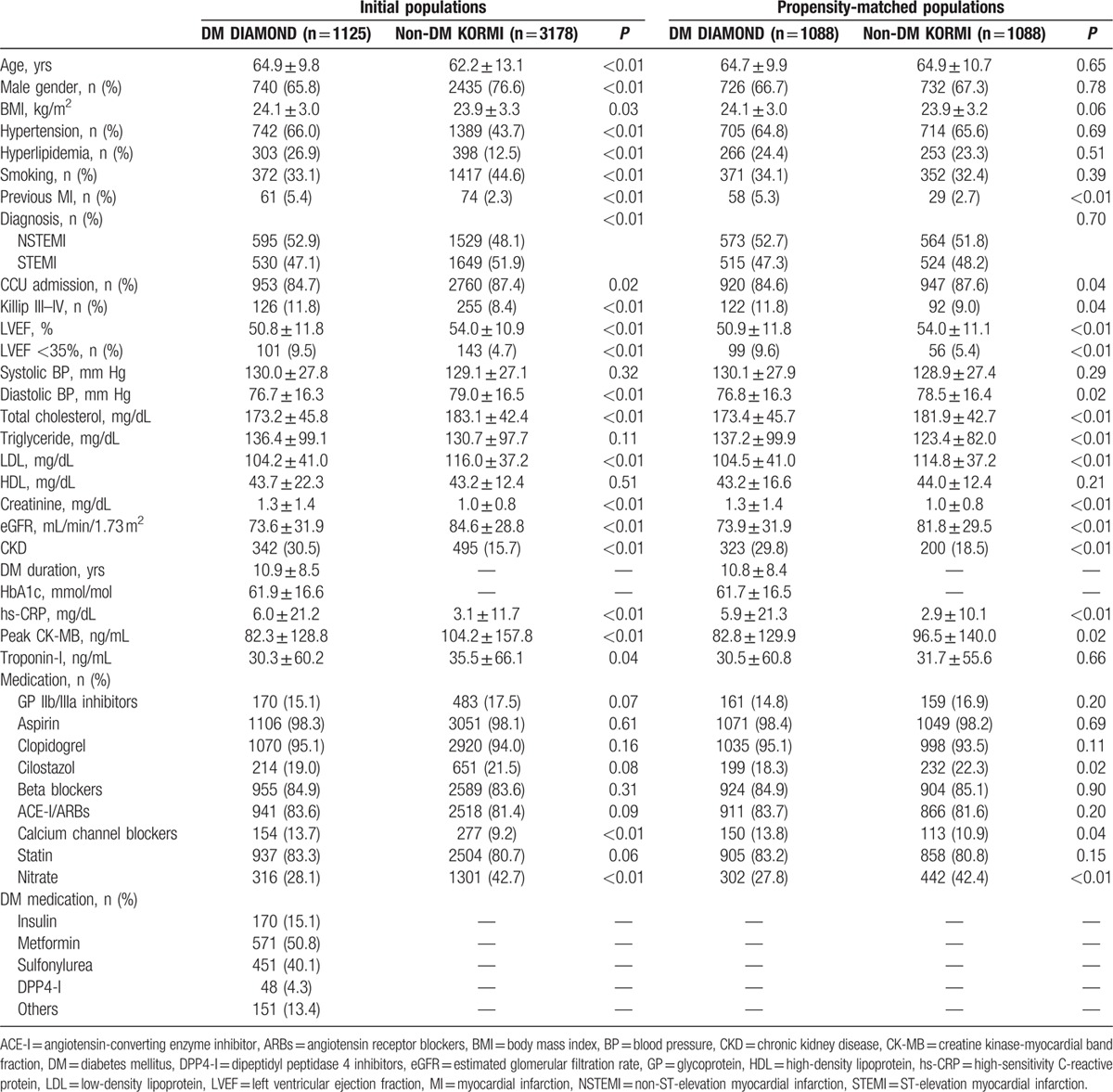
Figure 1.
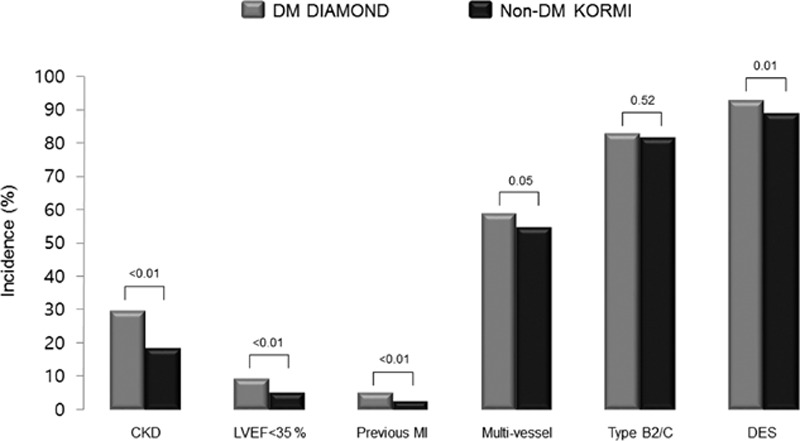
Comparison of clinical and procedural factors according to DM in the propensity score matched population.
3.2. Angiographic characteristics
Table 2 presents the angiographic characteristics. DM patients with AMI compared with non-DM patients with AMI in the initial populations exhibited a higher incidence of multivessel disease and treatment with DES, similar incidence of B2 or C type lesions, less frequent thrombolysis in myocardial infarction (TIMI) 0 flow before percutaneous coronary intervention (PCI), similar distributions of TIMI 2 or 3 flow after PCI, and more stents implanted with smaller diameter and greater length on average. DM patients with AMI compared with non-DM patients with AMI in the propensity score matched populations exhibited a lower frequency of TIMI 0 flow before PCI and a similar frequency of TIMI 2 or 3 flow after PCI, no significant differences in stent length or number but smaller stent diameter, more frequent DES implants, and similar incidences of multivessel disease and B2 or C type lesions (Fig. 1).
Table 2.
Angiographic and procedural characteristics.

3.3. Two-year clinical outcomes
3.3.1. Initial populations
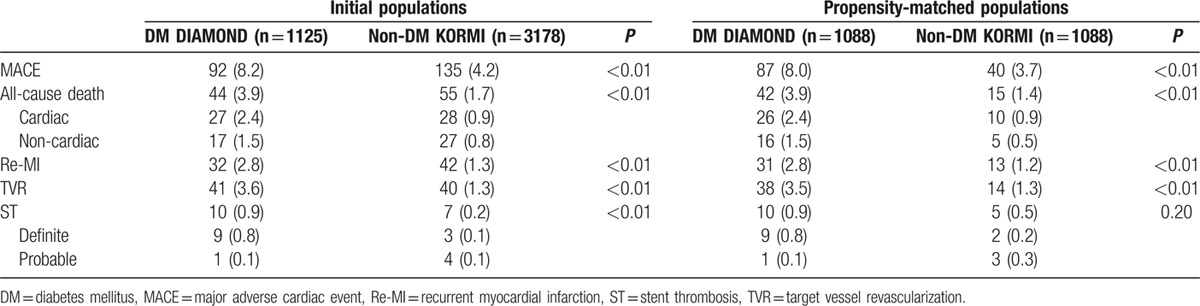
The rate of MACE (8.2% vs 4.2%), all-cause death (3.9 vs 1.7), re-MI (2.8% vs 1.3%), TVR (3.6% vs 1.3%), and ST (0.9 vs 0.2) was significantly higher in DM than non-DM patients with AMI (all P < 0.05). The rates of cardiac and noncardiac death were 2.5% and 1.4% in DM patients, respectively, and 0.9% and 0.8% in non-DM patients, respectively (Table 3).
Table 3.
Two-year clinical outcomes according to DM status.
3.3.2. Propensity score matched populations
The rates of MACE (8.0% vs 3.7%; P < 0.01), all-cause death (3.9 vs 1.4; P < 0.001), re-MI (2.8% vs 1.2%; P < 0.01), and TVR (3.5% vs 1.3%; P < 0.01) were also significantly higher in DM than non-DM patients with AMI, respectively. However, the rate of ST (0.9% vs 0.5%, P = 0.20) was comparable (Table 3). Kaplan–Meier survival analysis revealed that the cumulative incidences of MACE, all-cause death, and TVR were significantly higher in DM patients than non-DM patients (all P < 0.05). However, the cumulative incidences of re-MI and ST were not significantly different between DM and non-DM patients (Fig. 2, A–E).
Figure 2.
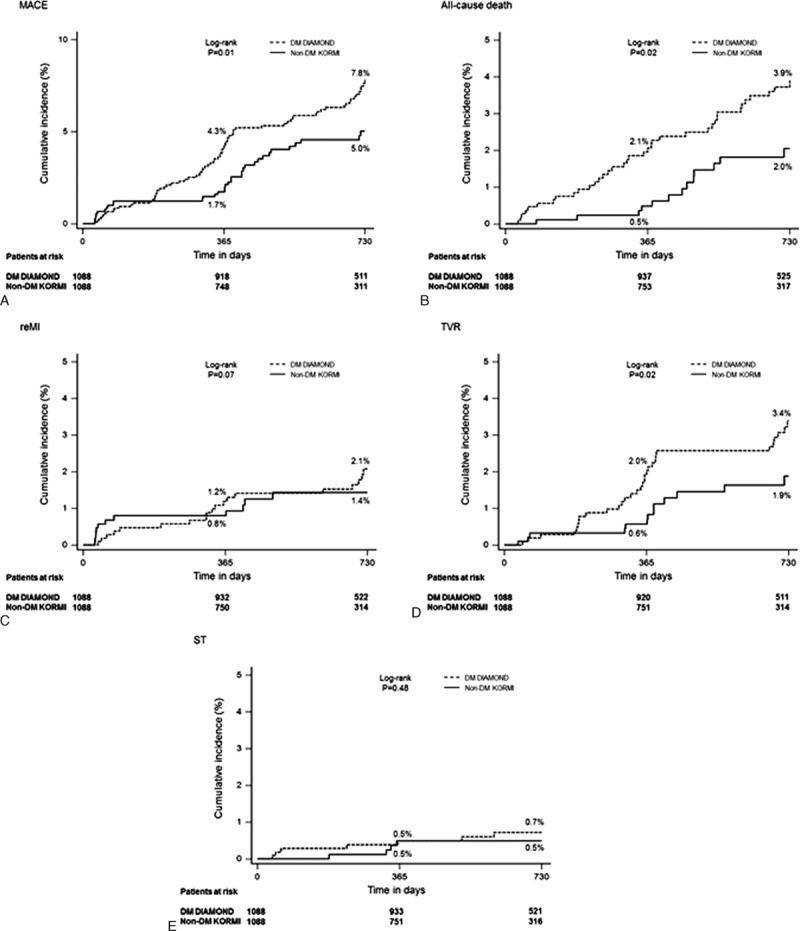
Kaplan–Meier curves for the primary and secondary outcomes according to DM in the propensity score matched population. Cumulative incidence curves are shown for (A) MACE, (B) all-cause death, (C) re-MI, (D) TVR, and (E) ST.
3.4. Independent determinants for MACE in initial populations
Univariate logistic regression analysis revealed that age >60 years [odds ratio, 1.90 (95% confidence interval, 1.41–2.56)], previous MI [1.97 (1.09–3.55)], hyperlipidemia [1.41 (1.01–1.95)], hypertension [1.65 (1.26–2.17)], CKD [2.49 (1.87–3.30)], Killip III-IV [1.83 (1.25–2.68)], LVEF <35% [2.43 (1.59–3.72)], DM [2.01 (1.53–2.64)], multivessel disease [1.36 (1.03–1.79)], stent diameter [0.59 (0.41–0.85)], stent length [1.02 (1.01–1.04)], and DES use [0.51 (0.34–0.75)] were significantly associated with 2-year MACE (all P < 0.05).
Multivariate logistic regression analysis revealed that CKD [1.46 (1.01–2.12)], LVEF < 35% [1.98 (1.17–3.34)], stent diameter [0.64 (0.43–0.96)], stent length [1.02 (1.01–1.04)], and DES use [0.44 (0.29–0.68)] were independent predictors for 2-year MACE (all Ps < 0.05). However, DM was not independently associated with 2-year MACE after adjusting for confounding risk factors [1.40 (0.99–1.99); P = 0.06] (Fig. 3) (Supplementary file: Table S1).
Figure 3.
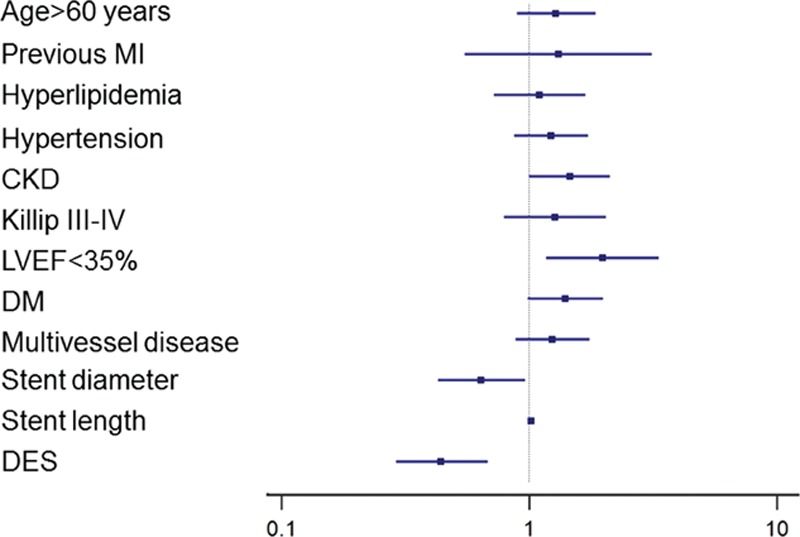
The estimated odds ratio of clinical risk factors for the primary outcome in the initial populations.
4. Discussion
The primary findings of this propensity score matching study is that stabilized AMI patients with DM have a higher incidence of comorbidities, such as CKD and systolic heart failure with LVEF <35%, and cumulative incidence of 2-year MACE than non-DM patients. The latter comorbidities, but not DM, were independent predictors of MACE. These results suggest that the higher incidence of comorbidities in DM patients is strongly associated with worse clinical outcomes in stabilized patients with AMI.
Diabetics exhibit multiple concomitant metabolic abnormalities, including hypertension, obesity, and hyperlipidaemia.[10,11] The present study performed propensity score matching between DM and non-DM patients to minimize the confounding metabolic impact on adverse clinical outcomes after an AMI event. Notably, the incidence of CKD and LVEF <35% was significantly higher in DM patients than non-DM patients after matching CV risk factors. Several previous studies reported that the presence of mild to moderate renal impairment after AMI increased the rate of adverse clinical outcomes.[12–14] Recently, Anavekar et al[15] emphasized that even mild renal impairment should be considered a potent and independent risk factor for CV complications in patients with AMI. The longstanding history of DM in DIAMOND registry participants suggests that the higher incidence of CKD in DM patients in the present study is associated with diabetic nephropathy.[16] A previous large cohort study identified that reduced LV function was significantly associated with increased 1-month and 1-year mortality risk in patients with AMI.[7] Ehl et al[17] reported that DM patients exhibited a lower LVEF than non-DM patients regardless of CAD extent and suggested that this difference was associated with worse CV mortality in DM patients. The present study found that the incidence of LVEF <35% was significantly higher in DM patients despite the absence of significant differences in the incidence of multivessel disease and type B2/C lesions between the propensity-matched populations. Therefore, the higher comorbidity of CKD and LVEF <35% in DM patients may contribute to the increased risk for the development of MACE during the 2-year follow-up in this study.
The incidences of multivessel and small vessel disease are significantly higher in DM patients than non-DM patients because of the greater burden of atherosclerosis associated with diabetes.[18] This association may translate into the need to implant a greater number of longer and smaller sized stents in DM patients, which was observed in the initial populations in the present study. DES use for the treatment of AMI was more frequently observed in DM patients than non-DM patients in the present study, which may reflect operator preference driven by the more favorable profile for DES in the prevention of restenosis and repeat revascularization.[19–21]
Previous studies investigating the impact of DM on clinical outcomes yielded inconsistent results, which likely reflects differences in clinical diagnosis,[22–24] and data on clinical outcomes after DES implantation according to DM status in patients with AMI are limited. Syed et al[25] reported that a 1-year composite of death, Q-wave MI, and TLR were not different between AMI patients with and without DM who underwent first-generation DES implantation after adjustment for baseline comorbidities. The cumulative incidence of 2-year MACE was significantly higher in DM patients than non-DM patients in the present study, but DM was not an independent predictor for the event of MACE. The higher use of DES in DM patients may explain the result that DM did not independently predict 2-year MACE in the present study because DES use was independently associated with a decreased risk for 2-year MACE in patients with AMI.
A subgroup analysis of patients with acute coronary syndrome (ACS) in 11 Thrombolysis in Myocardial Infarction Study Group trials revealed that the mortality rate at 30 days and 1-year follow-up was significantly higher in DM than in non-DM patients.[26] The mortality rate between 30 days and 1 year in their study was approximately 5% in 10,613 DM patients, which is higher than the present study (3.9% at 2 years). The latter discrepancy in mortality may be secondary to the higher incidence of PCI with DES and optimal medical therapy with statins and thienopyridine in the present study. The cumulative incidence of 2-year MACE was 8.2% in DM patients with AMI in the present study. This result may be acceptable in the setting of DM patients with AMI based on contemporary PCI with optimal medical treatment compared with previous results of the event rate for a composite of adverse clinical outcomes in DM patients.[27,28]
Coronary atherosclerosis involves a prolonged asymptomatic developmental phase, and the first manifestations often result in AMI or sudden cardiac death. The prognostic risk stratification of symptomatic or asymptomatic but at a high risk of CAD patients using noninvasive methods becomes more important with the development of noninvasive imaging modalities for CAD diagnosis.[29–32] Several studies emphasized the role of physical activity and nutrition in the prevention of the progression of subclinical atherosclerosis, metabolic abnormalities, and adverse CV events in the general population.[33–38] The prevalence of metabolic syndrome is rapidly increasing worldwide, and it affects approximately 31% of Korean adults.[39] Previous studies in Korea reported that abdominal obesity was associated with CAD risk regardless of the level of physical activity,[40] and high carbohydrate intake, which may be somewhat different in Asian than Western populations, was significantly associated with the risk of DM and low high-density lipoprotein-cholesterol (HDL-C) levels in Korea.[41] However, the impact of physical activity and nutrition on clinical outcomes was primarily evaluated on the primary prevention of CV events in the general population. A recent meta-analysis reported the efficacy of exercise-based cardiac rehabilitation in patients with coronary heart disease,[42] but further prospective studies of larger sample sizes are warranted because of the paucity of data on these issues beyond strict medical treatment for the secondary prevention after major adverse CV events.
A number of studies that were performed in Western countries strongly suggested that socioeconomic inequality in mortality was a substantial public health issue.[43–47] Especially, Bucholz et al[48] reported that life expectancy after AMI was significantly lower in patients with low socioeconomic status than in those with high socioeconomic status irrespective of race. In south Korea, a previous large cohort study reported that the contribution of the causes of death to socioeconomic inequality in life expectancy varied by age groups and differed by educational comparisons.[49] Recently, Lim et al[50] reported that the consistent increase in the attainment of education contributed to the reduction in the population attributable fractions of lower education for mortality, although the fact that mortality inequalities have not improved. However, there is a paucity of data on the impact of socioeconomic and educational inequality on the short- and long-term MACE in patients with AMI. Further investigation to identify this issue might be necessary in South Korea.
The present study has several potential limitations. First, it compared single-arm data from 2 different registries, which inherently introduces selection bias and uneven distribution of risk factors. However, propensity score matching analysis was performed to minimize this limitation. Second, the present study focused on stabilized AMI patients, that is, without clinical events within hospital stay or 1 month after discharge, and clinical events are likely to occur during the acute stage after AMI presentation, especially for high-risk patients. Therefore, the present study may underestimate the risk of MACE in diabetic AMI patients. Third, the DIAMOND study was prospectively designed to compile clinical data sets to extend our knowledge of AMI with DM. Therefore, detailed angiographic and interventional information was not available. Fourth, the DIAMOND study did not include assessments of glucose control or adherence to antiplatelet medications during the follow-up period. Therefore, the influence of these factors on long-term MACE was not evaluated. Fifth, we could not address the impact of noninvasive imaging modalities, physical activity, or nutrition on clinical outcomes in the present study. Finally, the incidence of ST was low in both groups, which rendered the study underpowered to evaluate the impact of DM on long-term safety after DES implantation.
5. Conclusion
The incidence of 2-year MACE was significantly higher in stabilized AMI patients with DM than without DM after matching traditional CV risk factors. The higher comorbidities in DM patients may contribute to worse clinical outcomes compared with non-DM patients.
Supplementary Material
Acknowledgments
The authors thank Hyo-Eun Kim, MS (Keimyung University Dongsan Medical Center), and Keon-Woong Moon, MD, PhD (Catholic University Hospital), for their contributions for the statistical analyses in this study. These persons gave permission to be named in the Acknowledgements of the present study.
Footnotes
Funding: This study was supported by a grant from Bayer Korea, Co., Ltd. and the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1277).
Supplemental Digital Content is available for this article.
The authors report no conflicts of interest.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999; 100:1134–1146. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234. [DOI] [PubMed] [Google Scholar]
- 3.NCEP: Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel, III). JAMA 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 4.McGuire DK, Granger CB. Diabetes and ischemic heart disease. Am Heart J 1999; 138:S366–S375. [DOI] [PubMed] [Google Scholar]
- 5.Malmberg K, Ryden L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26:650–661. [DOI] [PubMed] [Google Scholar]
- 6.Norhammar A, Malmberg K, Diderholm E, et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol 2004; 43:585–591. [DOI] [PubMed] [Google Scholar]
- 7.Park HW, Yoon CH, Kang SH, et al. Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Int J Cardiol 2013; 169:254–261. [DOI] [PubMed] [Google Scholar]
- 8.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 10.Alexander CM, Landsman PB, Teutsch SM, et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003; 52:1210–1214. [DOI] [PubMed] [Google Scholar]
- 11.Tong PC, Kong AP, So WY, et al. The usefulness of the international diabetes federation and the national cholesterol education Program's adult treatment panel III definitions of the metabolic syndrome in predicting coronary heart disease in subjects with type 2 diabetes. Diabetes Care 2007; 30:1206–1211. [DOI] [PubMed] [Google Scholar]
- 12.Januzzi JL, Cannon CP, DiBattiste PM, et al. Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes. Am J Cardiol 2002; 90:1246–1249. [DOI] [PubMed] [Google Scholar]
- 13.Gibson CM, Pinto DS, Murphy SA, et al. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol 2003; 42:1535–1543. [DOI] [PubMed] [Google Scholar]
- 14.Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med 2002; 137:563–570. [DOI] [PubMed] [Google Scholar]
- 15.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004; 351:1285–1295. [DOI] [PubMed] [Google Scholar]
- 16.Gall MA, Nielsen FS, Smidt UM, et al. The course of kidney function in type 2 (non-insulin-dependent) diabetic patients with diabetic nephropathy. Diabetologia 1993; 36:1071–1078. [DOI] [PubMed] [Google Scholar]
- 17.Ehl NF, Kuhne M, Brinkert M, et al. Diabetes reduces left ventricular ejection fraction: irrespective of presence and extent of coronary artery disease. Eur J Endocrinol 2011; 165:945–951. [DOI] [PubMed] [Google Scholar]
- 18.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol 2007; 49:643–656. [DOI] [PubMed] [Google Scholar]
- 19.Moussa I, Leon MB, Baim DS, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation 2004; 109:2273–2278. [DOI] [PubMed] [Google Scholar]
- 20.Hermiller JB, Raizner A, Cannon L, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol 2005; 45:1172–1179. [DOI] [PubMed] [Google Scholar]
- 21.Sabaté M, Jiménez-Quevedo P, Angiolillo DJ, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation 2005; 112:2175–2183. [DOI] [PubMed] [Google Scholar]
- 22.Park DW, Flaherty JD, Davidson CJ, et al. Prognostic influence of diabetes mellitus on long-term clinical outcomes and stent thrombosis after drug-eluting stent implantation in Asian patients. Am J Cardiol 2009; 103:646–652. [DOI] [PubMed] [Google Scholar]
- 23.Lee MS, Jurewitz D, Zimmer R, et al. Impact of diabetes and acute coronary syndrome on survival in patients treated with drug-eluting stents. Catheter Cardiovasc Interv 2008; 72:909–914. [DOI] [PubMed] [Google Scholar]
- 24.Kedhi E, Genereux P, Palmerini T, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus: analysis from 18 pooled randomized trials. J Am Coll Cardiol 2014; 63:2111–2118. [DOI] [PubMed] [Google Scholar]
- 25.Syed AI, Ben-Dor I, Li Y, et al. Outcomes in diabetic versus nondiabetic patients who present with acute myocardial infarction and are treated with drug-eluting stents. Am J Cardiol 2010; 105:819–825. [DOI] [PubMed] [Google Scholar]
- 26.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007; 298:765–775. [DOI] [PubMed] [Google Scholar]
- 27.Park KW, Lee JM, Kang SH, et al. Everolimus-eluting xience v/promus versus zotarolimus-eluting resolute stents in patients with diabetes mellitus. JACC Cardiovasc Interv 2014; 7:471–481. [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation 2011; 124:893–900. [DOI] [PubMed] [Google Scholar]
- 29.Rybicki FJ, Udelson JE, Peacock WF, et al. 2015 Acr/Acc/Aha/Aats/Acep/Asnc/Nasci/Saem/Scct/Scmr/Scpc/Snmmi/Str/StS Appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J Am Coll Cardiol 2016; 67:853–879. [DOI] [PubMed] [Google Scholar]
- 30.Leischik R, Dworrak B, Littwitz H, et al. Prognostic significance of exercise stress echocardiography in 3329 outpatients (5-year longitudinal study). Int J Cardiol 2007; 119:297–305. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez K, Kwan AC, Lai S, et al. Coronary plaque burden at coronary CT angiography in asymptomatic men and women. Radiology 2015; 277:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budoff MJ, Raggi P, Beller GA, et al. Noninvasive cardiovascular risk assessment of the asymptomatic diabetic patient: the imaging council of the American College of Cardiology. JACC Cardiovasc Imaging 2016; 9:176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leischik R, Foshag P, Strauß M, et al. Physical activity, cardiorespiratory fitness and carotid intima thickness: sedentary occupation as risk factor for atherosclerosis and obesity. Eur Rev Med Pharmacol Sci 2015; 19:3157–3168. [PubMed] [Google Scholar]
- 34.Edwardson CL, Gorely T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One 2012; 7:e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organisation. Global Strategy on Diet, Physical Activity and Health. 2004; 2015. Available at: http://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf. [Google Scholar]
- 36.World Health Organisation. The World Health Report. 2013. Available at: http://www.who.int/whr/2013/report/en/. [Google Scholar]
- 37.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 2009; 43:1–2. [PubMed] [Google Scholar]
- 38.Leischik R, Foshag P, Strauss M, et al. Aerobic capacity, physical activity and metabolic risk factors in firefighters compared with police officers and sedentary clerks. PLoS One 2015; 10:e0133113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim S, Shin H, Song JH, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care 2011; 34:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Han HR. Physical activity, abdominal obesity and the risk of coronary heart disease: a Korean national sample study. Public Health 2012; 126:410–416. [DOI] [PubMed] [Google Scholar]
- 41.Park SH, Lee KS, Park HY. Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). Int J Cardiol 2010; 139:234–240. [DOI] [PubMed] [Google Scholar]
- 42.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson RG. Socioeconomic determinants of health. Health inequalities: relative or absolute material standards? BMJ 1997; 314:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leischik R, Dworrak B, Strauss M, et al. Plasticity of health. German J Med 2016; 1:1–17. [Google Scholar]
- 45.Soler-Vila H, García-Esquinas E, León-Muñoz LM, et al. Contribution of health behaviours and clinical factors to socioeconomic differences in frailty among older adults. J Epidemiol Community Health 2016; 70:354–360. [DOI] [PubMed] [Google Scholar]
- 46.Demakakos P, Biddulph JP, Bobak M, et al. Wealth and mortality at older ages: a prospective cohort study. J Epidemiol Community Health 2016; 70:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bann D, Chen H, Bonell C, et al. Socioeconomic differences in the benefits of structured physical activity compared with health education on the prevention of major mobility disability in older adults: the LIFE study. J Epidemiol Community Health 2016; 207321.pii: jech-2016-207321. doi: 10.1136/jech-2016-207321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bucholz EM, Ma S, Normand SL, et al. Race, socioeconomic status, and life expectancy after acute myocardial infarction. Circulation 2015; 132:1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung-Choi K, Khang YH, Cho HJ, et al. Decomposition of educational differences in life expectancy by age and causes of death among South Korean adults. BMC Public Health 2014; 14:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim D, Kong KA, Lee HA, et al. The population attributable fraction of low education for mortality in South Korea with improvement in educational attainment and no improvement in mortality inequalities. BMC Public Health 2015; 15:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


