Abstract
Alcohol-induced osteonecrosis of the femoral head (ONFH) is an important pathogenesis of nontraumatic ONFH. However, the mechanisms of the pathogenesis are still unknown. Osteoprotegerin (OPG) and receptor activator of nuclear factor-kappa B ligand (RANKL) have been implicated in multiple functions including blocking osteoclast maturation, controlling vascular calcifications, and promoting tumor growth and metastasis. The purpose of this article was to explore the association between OPG and RANKL gene variants and alcohol-induced ONFH. Six hundred seventy male subjects (335 patients and 335 normal individuals) were enrolled in our study. We selected 24 single-nucleotide polymorphisms (SNPs) to evaluate the association between genetic susceptibility variants and alcohol-induced ONFH using the chi-square test and gene model analysis. Overall, the OPG SNPs (rs1032128 and rs11573828) were associated with the strongest increased risk of alcohol-induced ONFH in the recessive model (rs1032128: odds ratio [OR] 1.49, 95% confidence interval [CI] 1.00–2.22, P = 0.04 for G/A; rs11573828: OR 3.32, 95% CI 1.07–10.30, P = 0.03 for T/C). The RANKL SNP rs2200287 was also an increased risk factor (OR 3.65, 95% CI 1.53–8.47, P = 0.003 for T/C) in the recessive model. The rs11573856, rs3134056, and rs1564861 SNPs were considered protective factors for alcohol-induced ONFH. We concluded that OPG and RANKL polymorphisms were associated with the occurrence of alcohol-induced ONFH.
Keywords: alcohol-induced osteonecrosis of the femoral head, OPG, polymorphism, RANKL, single-nucleotide polymorphism
1. Introduction
Osteonecrosis of the femoral head (ONFH) involves the death of the cellular portion of the femoral head, with subsequent bone structure changes and the collapse of the femoral head that leads to bone fracture and dysfunction of the hip joint.[1] Multiple risk and pathogenic factors have been implicated in the development of nontraumatic ONFH. However, the concrete pathogenesis of nontraumatic ONFH is still widely unknown, although some macroscopic risk factors have been associated with nontraumatic ONFH including corticosteroid usage, alcohol intake, infections, marrow infiltrative diseases, and coagulation defects.[2]
Previous studies have indicated that alcoholism is potentially a primary cause of osteoporotic fractures and low bone density, and might eventually lead to alcohol-induced ONFH.[3] It was suggested that alcohol and steroid-induced ONFH are both due to intraosseous hypertension caused by adipose cell hypertrophy and proliferation, which may result in decreased blood supply to the femoral head.[4] Some studies have also suggested that chronic alcohol consumption may decrease the release of osteoprotegerin (OPG) by inhibition of osteoblasts, hormones, and cytokines that might induce the activation of osteoclasts and the OPG receptor activator of nuclear factor kappa B-ligand (RANKL) pathway, and also bone resorption.[5] Fine osseous blood supply to the femoral head can induce benign bone metabolism and maintain the balance between osteoclasts and osteoblasts. Chronic alcohol consumption may lead to the interruption of the osseous blood supply and osteonecrosis.[6] Based on our findings, we suggested that ethanol consumption can increase the risk of ONFH occurrence and that the OPG-RANKL pathway might be the most important mediator of alcohol-induced ONFH.
It has been suggested that the OPG/RANK/RANKL pathway impairs angiogenesis as a mechanism of nontraumatic ONFH.[5,7] OPG, which is expressed by bone marrow-derived mesenchymal stem cells, is a member of the tumor necrosis factor (TNF) receptor family and is a soluble receptor that inhibits osteoclastogenesis by binding to RANKL.[8,9] An abnormal OPG/RANKL ratio was found in a number of skeletal diseases that are characterized by extensive osteoclast activity including rheumatoid arthritis and osteonecrosis.[10]
Genetic variations that are considered biologically normal could influence protein transcription, expression of related factors, immunoreaction, and contribute to individual susceptibilities to certain pathological diseases.[11] Over the past few decades, a number of candidate genes have been investigated and linked with nontraumatic ONFH, such as OPG, receptor activator of nuclear factor-kappa B (NF-κB), RANK, and RANKL, which regulate the balance between osteoclasts and osteoblasts.[12,13] Also, previous studies have identified OPG polymorphisms that were associated with multiple cancers, vertebral fractures, and bone mineral density (BMD).[14,15]
In summary, prior findings led us to investigate the association between OPG and RANKL polymorphisms and alcohol-induced ONFH.
2. Materials and methods
2.1. Study population
All of the cases and control individuals were members of the north area of China population in men, and case group conducted in our study comprised of only alcohol-drinking male individuals who were all long-term alcohol users for more than 10 years, having a dose more than 400 mg per week; however, control group consisted of normal male individuals. Alcohol-induced ONFH cases were recruited between January 2013 and May 2015 from the Zhengzhou Traditional Chinese Medicine (TCM) Traumatology Hospital in Zhengzhou, and the control participants were enrolled from the Zhengzhou Medical Center in Henan.
2.2. Inclusion and exclusion criteria
The diagnosis of ONFH was made according to the following criteria proposed by the Research Committee[16]: (1) collapse of the femoral head without join space narrowing or acetabular abnormality on radiographs, including the crescent sign; (2) demarcating sclerosis in the femoral head without joint space narrowing or acetabular abnormality; (3) “cold in hot” on bone scans; (4) low-intensity band on T1-weighted magnetic resonance imaging (MRI; band-like pattern); and (5) trabecular and marrow necrosis on histology. Nontraumatic femoral head osteonecrosis was diagnosed in any patient meeting 2 or more of the 5 criteria. Subjects who were diagnosed with ONFH before alcohol intake, showed nontypical MR images that did not satisfy the diagnostic criteria including low band-like signals in the femoral head on T1-weighted images, patients who suffered from a hip joint disease or direct trauma during the alcohol intake period, and patients who did not agree to be enrolled in this study were excluded.[17]
The control male subjects were defined by the following criteria: those having no hip pain and anteroposterior and frog-leg lateral pelvic radiographs that did not show any lesions. All persons related to the enrolled patients were excluded from the control group.
2.3. Clinical data and demographic information
We used a standard epidemiological questionnaire and in-person interviews to collect personal data, including residential region, age, and education status, and also the history of medication use (including oral corticosteroids), alcohol consumption, osteopathic diseases, and underlying medical conditions (hyperlipidemia). Regarding alcohol consumption, we collected information regarding age at the start and end, typical frequency of drinking, and the usual volume of alcohol intake by beverage type. The case information was collected through a consultation with the treating physicians or from a medical chart review. All of the participants signed an informed consent agreement. The Zhengzhou TCM Traumatology Hospital Human Research Committee for Approval of Research Involving Human Subjects approved the use of humans in this study.
2.4. Selection of single-nucleotide polymorphisms and genotyping methods
The majority of the single-nucleotide polymorphisms (SNPs) selected was not previously reported; however, some SNPs were associated with other diseases such as BMD, rheumatoid arthritis (RA), and Paget disease of bone. The minor allele frequencies of all of the SNPs were >5% in the Hap Map of the Chinese Han Beijing population. Extraction of DNA from whole blood samples was performed using the Gold Mag-Mini Whole Blood Genomic DNA Purification Kit (Gold Mag Co., Ltd., Hainan City, China), and the DNA concentration was measured using a NanoDrop 2000 spectrophotometer.
We designed primers for amplification and extension reactions using Sequenom MassARRAY Assay Design 3.0 Software (Sequenom Inc., San Diego, CA)[18] (Table 1). Genotyping was performed using the Sequenom MassARRAY RS1000 system according to the manufacturer's protocol. After the experimentation progress mentioned above, data management and analysis were conducted using Sequenom Typer 4.0 software.[18,19]
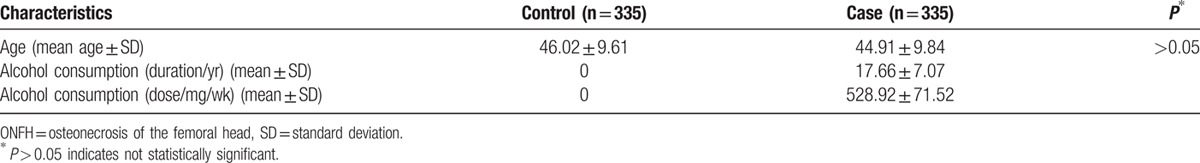
Table 1.
Characteristics of controls and alcohol-induced ONFH cases in male individuals.
2.5. Statistical analysis
Microsoft Excel and the SPSS 18.0 statistical package (SPSS, Chicago, IL) were utilized to execute the statistical analyses. A P value of less than 0.05 indicated statistically significant differences. We performed the 2-side chi-square tests to compute the genotype frequencies of case and control individuals.[20] Unconditional logistic regression analysis with an adjustment for age was used to test odds ratios (ORs) and 95% confidence intervals (CIs)[21] through that the results were more credible. The association of OPG and RANKL SNPs with the risk of ONFH was tested in dominant and recessive models, and also codominant and log-additive effects.
3. Results
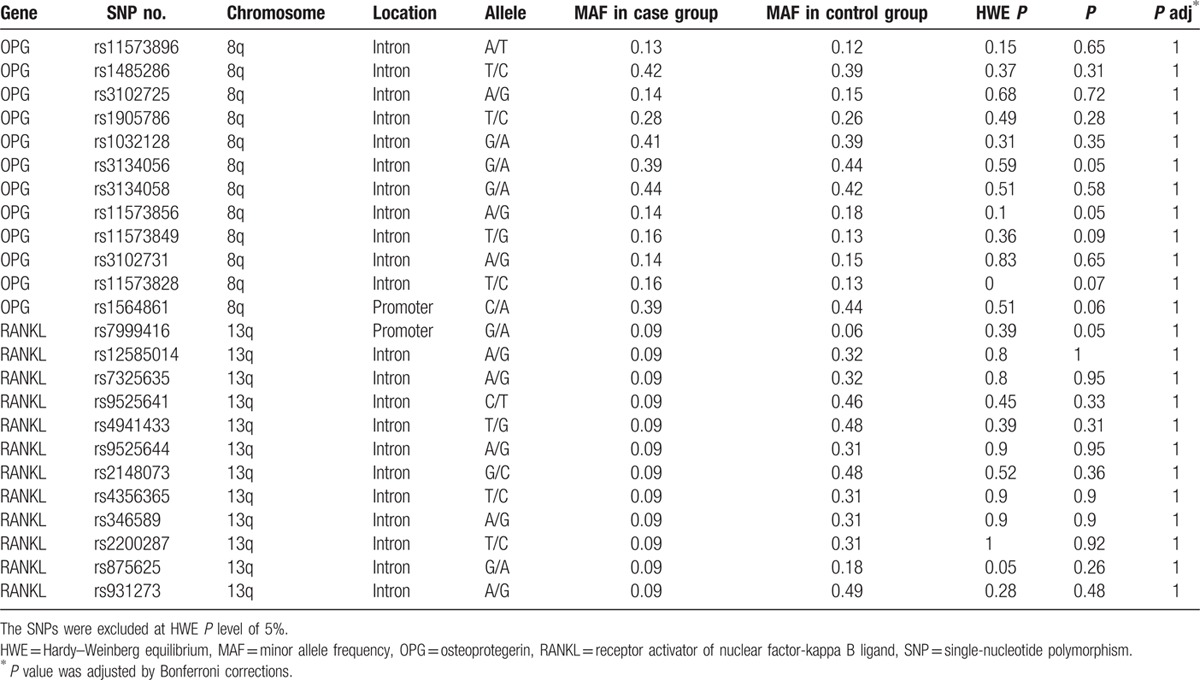
We conducted a case-control study involving 335 patients and 335 normal individuals to identify a potential association between OPG and RANKL polymorphisms and alcohol-induced ONFH. Twenty-four SNPs in these 2 genes were all in Hardy–Weinberg equilibrium (P ≤ 0.05). The associations between SNP genotypes and the susceptibility of alleles to alcohol-induced ONFH were all evaluated by chi-square analysis, the results of which are shown in Table 2.
Table 2.
Basic information on OPG and RANKL SNPs.
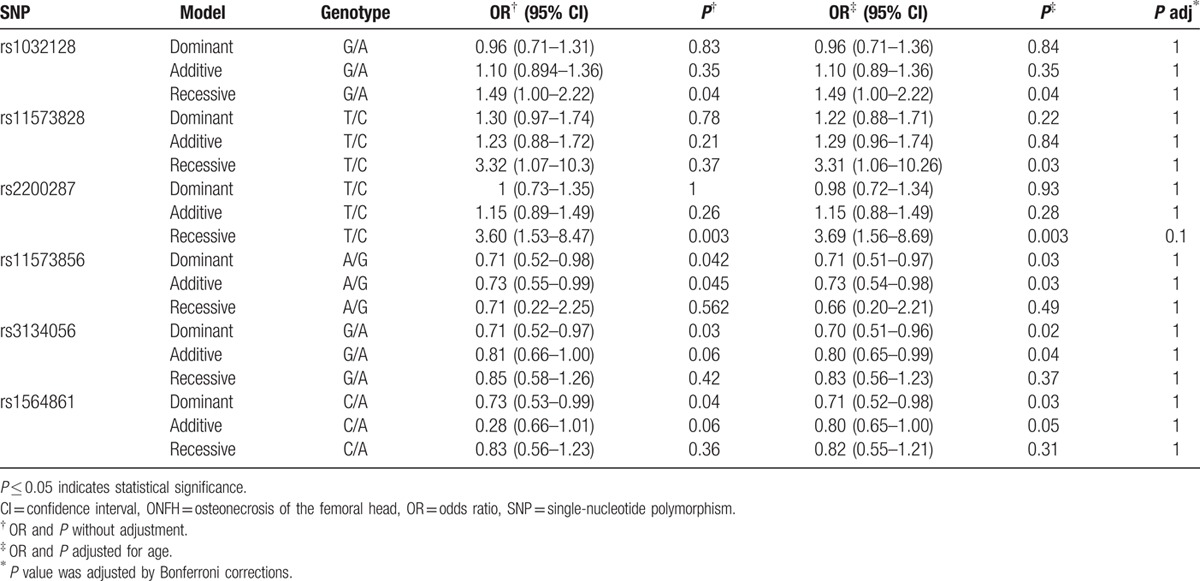
We assumed that the minor allele of each SNP was a risk factor compared with the wild-type allele. Different genetic analysis models were applied to analyze the associations between SNPs and alcohol-induced ONFH risks using unconditional logistic regression. The results indicated that 3 SNPs (rs1032128, rs11573828, and rs2200287) were associated with an increased risk of alcohol-induced ONFH. Additionally, the rs11573856, rs3134056, and rs1564861 SNPs were thought to protect against the occurrence of alcohol-induced ONFH. We discovered notable associations conferring increased risks between 3 SNPs (rs1032128, rs11573828, and rs2200287) and alcohol-induced ONFH that had different genotype distributions in the recessive model (rs1032128: OR 1.49, 95% CI 1.00–2.22, P = 0.04 for G/A; rs11573828: OR 3.32, 95% CI 1.07–10.30, P = 0.03 for T/C; rs2200287: OR 3.65, 95% CI 1.53–8.47, P = 0.003 for T/C). Furthermore, the results were still remarkable when calculated using the unconditional logistic regression analyses adjusted for age (rs1032128: adjusted OR 1.49, 95% CI 1.00–2.22, P = 0.04 for G/A; rs11573828: adjusted OR 3.31, 95% CI 1.06–10.26, P = 0.03 for T/C; rs2200287: adjusted OR 3.69, 95% CI 1.56–8.69, P = 0.002 for T/C). Three SNPs rs11573856, rs3134056, and rs1564861 all conferred protective effects against the occurrence of alcohol-induced ONFH in the dominant model (rs11573856: OR 0.71, 95% CI 0.52–0.98, P = 0.04; rs3134056: OR 0.71, 95% CI 0.52–0.97, P = 0.03; rs1564861: OR 0.72, 95% CI 0.53–0.99, P = 0.04) and when adjusted for age in the dominant model (rs11573856: OR 0.71, 95% CI 0.51–0.97, P = 0.03; rs3134056: adjusted OR 0.70, 95% CI 0.51–0.96, P = 0.02; rs1564861: OR 0.71, 95% CI 0.52–0.98, P = 0.03) (Table 3).
Table 3.
Logistic regression analysis of the association between SNPs and risk of alcohol-induced ONFH.
Pair-wise linkage disequilibrium (LD) analysis was performed for OPG using the polymorphisms detected in this study. The LD pattern was analyzed using the parameters r2 and D’. Two main linkage blocks were observed across the locus (Fig. 1). Block 1 comprised of the 5 closely linked SNPs rs11573856, rs1485286, rs3102725, rs1905786, and rs1032128. Next, we analyzed the association between inferred haplotypes and alcohol-induced ONFH risk among the individuals and found no risk haplotypes between the 6 SNPs in block 1. Block 2 comprised of the 7 closely linked SNPs rs3134056, rs3134058, rs11573856, rs11573849, rs3102731, rs11573828, and rs1564861. A risk was confirmed between the haplotype “AAGTGTA” in the 7 SNPs in block 2 and alcohol-induced ONFH, even when adjusted by age (OR 1.54, 95% CI 1.08–2.19, P = 0.01; and adjusted OR 1.54, 95% CI 1.08–2.19, P = 0.01). A protective effect was confirmed between the haplotype “GAAGGCC” in the 7 SNPs in block 2 and alcohol-induced ONFH, even when adjusted by age (OR 0.72, 95% CI 0.53–0.98, P = 0.004; and adjusted OR 0.71, 95% CI 0.52–0.97, P = 0.03; Table 4). Furthermore, the formations of the primers were shown in Table 5.
Figure 1.
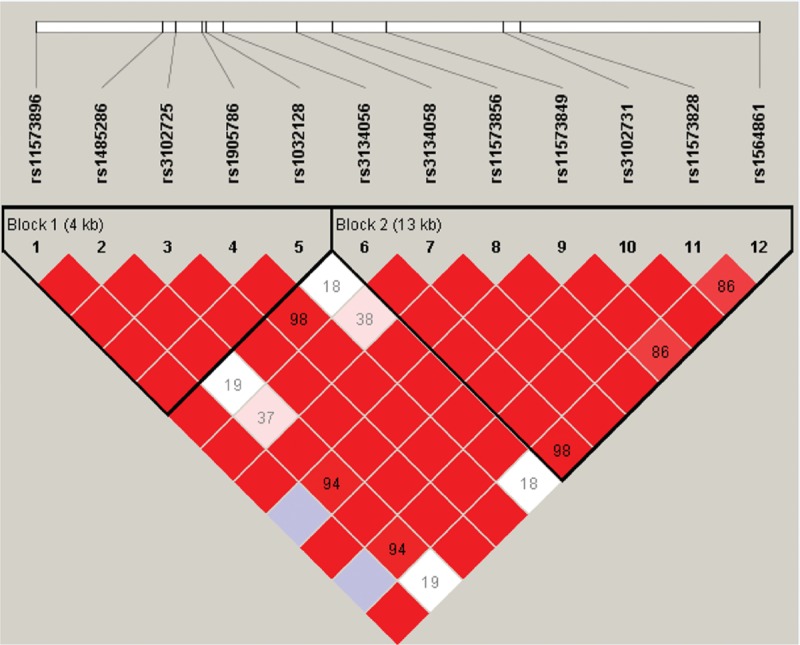
Linkage disequilibrium (LD) of polymorphic sites in the OPG on chromosome 8. The LD pattern was analyzed using the parameters r2 and D’.
Table 4.
Multiple gene haplotype frequencies and the association with the risk of alcohol-induced ONFH.
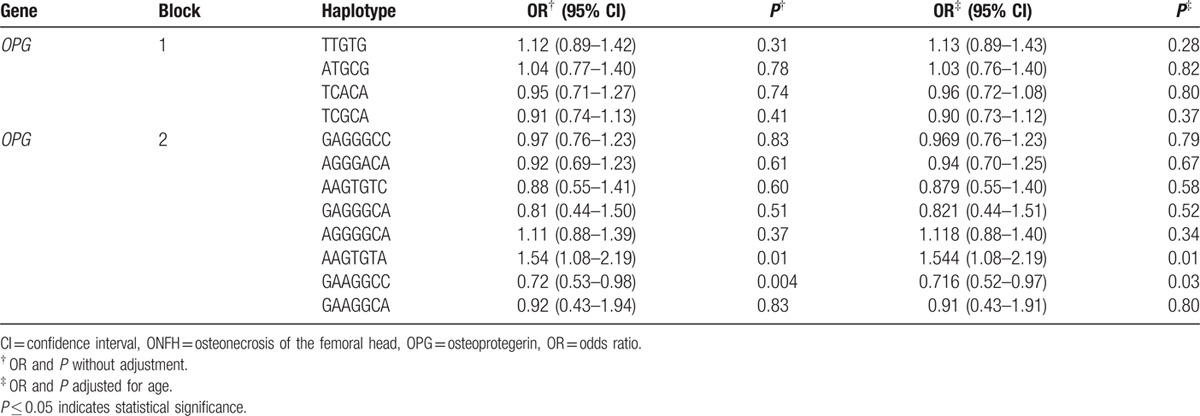
Table 5.
The formation of primers.

4. Discussion
Chronic alcohol consumption can induce the occurrence of alcohol-induced ONFH, which is one of the most important risk factors. Additionally, several genetic polymorphisms that are associated with susceptibility to alcohol-induced ONFH have been identified.[22] Although each polymorphism may only contribute to a small relative risk of alcohol-induced ONFH, the combination of several polymorphisms and other risk factors may be more impactful. To our knowledge, this study is the first to identify the association between alcohol-induced ONFH and OPG and RANKL polymorphisms. In this study, we detected 24 SNPs in OPG and RANKL and concluded that polymorphisms in OPG (rs1032128 and rs11573828) and in RANKL (rs2200287) might increase the risk of alcohol-induced ONFH. However, certain SNPs (rs11573856, rs3134056, and rs1564861) in OPG might confer protective effects.
The associations between OPG and RANKL polymorphisms and alcohol-induced ONFH found in the present study can be explained by the possible effect of ONFH on the OPG/RANKL ratio. First, both OPG and RANKL are members of the family of soluble TNF receptors. OPG is produced by osteoblasts and binds RANKL to prevent it from binding to RANK on osteoclasts and from subsequently causing cell activation,[5] and is secreted by mesenchymal stem cells (MSCs).[23] Second, recent experimental and epidemiological data strongly supported the theory that the ratio of OPG: RANKL, which is secreted by impaired endothelial cells, underlies a possible link between the osseous and vascular systems.[24] Alcohol has an inhibitory effect on the differentiation of MSCs into osteoblasts, and alcohol consumption might decrease the differentiation of MSCs into OPG. Hence, alcohol may indirectly decrease OPG expression, reduce the negative regulation of osteoclast differentiation, and block the pathophysiological induction of bone resorption. Moreover, ethanol consumption might influence the osseous blood supply to the femoral head by inducing vascular endothelial cell necrosis and cause the blood in the femoral head to be hypercoagulable. At the same time, OPG expression might have decreased because vascular endothelial cell necrosis decreased. In other words, reduced OPG expression might have reduced its ability to inhibit the progress of osteolysis, inducing bone formation and destroying the OPG/RANKL ratio, which gradually resulted in alcohol-induced ONFH.
A previous study supported the theory that OPG polymorphisms, including the rs1032128 SNP, were associated with volumetric BMD and bone geometry, which suggested that rs1032128 might influence serum levels of the N-terminal propeptide of procollagen I (PINP).[25] Furthermore, an OPG polymorphism (SNP rs1485286) has proven to be a strong candidate for the regulation of susceptibility to Paget disease of bone because the mutation increases bone turnover, and this association turned out to be stronger in females when males and females were subsequently analyzed separately.[26,27] However, some SNPs were not investigated, including rs11573828.[27] Furthermore, a recent study reported that the rs11573856 SNP in OPG has nothing to do with BMD and osteoporotic fracture and it did not destroy the OPG/RANKL ratio.[28] We also concluded that the rs11573856 SNP was not associated with increased risk of alcohol-induced ONFH; however, it might be a protective factor. Some previous studies found that rs2200287 in RANKL was associated with BMD mainly in the femoral neck in postmenopausal women, but was not found in males.[29,30] In our study, we found that rs2200287 was also associated with alcohol-induced ONFH in males.
The combination of our results and other findings indicated that alcohol consumption together with OPG and RANKL polymorphisms increased the susceptibility to ONFH.
Despite the power of the current study, some limitations were inherent in the case-control study. First, because our sample size (335 patients and 335 normal individuals) was not large relative to the alcohol-induced ONFH association studies published to date, we did not stratify the population by the amount of alcohol consumption, and we did not confirm that this locus was significant in drinkers. The negative results of major SNPs in this study might convert into positive findings when we increase the alcohol-induced ONFH sample size, which could make the conclusions more powerful. Second, the heterogeneity of alcohol consumption and comorbidities were not evaluated in this study, but evaluation of heterogeneity in drinking behaviors contributed to progress in elucidating the pathogenesis of alcohol-induced ONFH in other studies.[31] Third, in our study, we enrolled cases and matched controls in the same hospital so that selection bias can be avoided. In this case, this bias was not meaningful because the difference in the distribution of demographic segmentation and genetic frequencies were dissolved into nothingness. Last, alcohol-induced ONFH can be classified into different clinical stages to allow subgroup analysis for further study; however, it might make collecting samples more difficult.
Bonferroni correction is one of the most important multiple tests. When we used Bonferroni correction to our data, we found that there were no statistically significant associations between OPG and RANKL SNPs and alcohol-induced ONFH. This may be due to our sample size insufficiency and strict filtering criteria of SNPs and the weak point of Bonferroni correction test. Bonferroni correction test depends upon the amount of tests performed so that the results may be easier to be negative as long as more and more tests are performed. In this case, significant positive data may be deemed nonsignificant effects, and the results may be false-positive.
5. Conclusions
In conclusion, we provided new evidence for the association between SNPs and haplotypes of OPG and RANKL and alcohol-induced ONFH. These findings indicated that genetic variants of OPG and RANKL play an intricate role in the development of alcohol-induced ONFH and that the interaction of OPG and RANKL loci might be more important than a single locus. Thus, this study might offer important insights into the etiology of alcohol-induced ONFH.
Acknowledgments
It is our great honor to express heartfelt thanks to all of the patients and individuals for their participation. We are also grateful to the clinicians and other hospital staff who contributed to the blood sample and data collection for this study.
Footnotes
YL and YW contributed equally to this work and should be considered co-first authors.
This study was supported by the National Natural Science Foundation of China (No. 81160228 and 81260284).
The authors report no conflicts of interest.
References
- 1.Kim TH, Hong JM, Park EK, et al. Peroxisome proliferator-activated receptor-gamma gene polymorphisms are not associated with osteonecrosis of the femoral head in the Korean population. Molec Cells 2007; 24:388–393. [PubMed] [Google Scholar]
- 2.Hong JM, Kim TH, Kim HJ, et al. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Molec Med 2010; 42:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurel DB, Jaffre C, Rochefort GY, et al. Low bone accrual is associated with osteocyte apoptosis in alcohol-induced osteopenia. Bone 2011; 49:543–552. [DOI] [PubMed] [Google Scholar]
- 4.Youm YS, Lee SY, Lee SH. Apoptosis in the osteonecrosis of the femoral head. Clin Orthoped Surg 2010; 2:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rushbrook J, Pennington N. The effects of alcohol in orthopaedic patients. Orthopaed Trauma 2013; 27:164–170. [Google Scholar]
- 6.Chang JD, Hur M, Lee SS, et al. Genetic background of nontraumatic osteonecrosis of the femoral head in the Korean population. Clin Orthopaed Relat Res 2008; 466:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillai L, Bitto A, Burnett BP, et al. Genistein aglycone demonstrates a protective and reversible effect on the development of steroid-induced secondary osteoporosis and increases bone breaking strength in vivo. J Clin Densitometry 2010; 13:111. [Google Scholar]
- 8.Samara S, Dailiana Z, Chassanidis C, et al. Expression profile of osteoprotegerin, RANK and RANKL genes in the femoral head of patients with avascular necrosis. Exp Mol Pathol 2014; 96:9–14. [DOI] [PubMed] [Google Scholar]
- 9.Jiang XY, Chen Y, Xu L, et al. Association of LPR5 polymorphism with bone mass density and cholesterol level in population of Chinese Han. Exp Clin Endocrinol Diab 2010; 118:388–391. [DOI] [PubMed] [Google Scholar]
- 10.Hsu YH, Niu T, Terwedow HA, et al. Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Human Genet 2006; 118:568–577. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Yishake M, Li J, et al. Genetic susceptibility to prosthetic joint infection following total joint arthroplasty: a systematic review. Gene 2015; 563:76–82. [DOI] [PubMed] [Google Scholar]
- 12.Hadjigeorgiou G, Dardiotis E, Dardioti M, et al. Genetic association studies in osteonecrosis of the femoral head: mini review of the literature. Skelet Radiol 2008; 37:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Shang M, Lin L, Cui H. Association of genetic polymorphisms of Rank, Rankl and OPG with bone mineral density in Chinese peri- and postmenopausal women. Clin Biochem 2013; 46:1493–1501. [DOI] [PubMed] [Google Scholar]
- 14.Omar HS, Shaker OG, Nassar YH, et al. The association between RANKL and Osteoprotegerin gene polymorphisms with breast cancer. Molec Cell Biochem 2015; 403:219–229. [DOI] [PubMed] [Google Scholar]
- 15.Bonfa AC, Seguro LP, Caparbo V, et al. RANKL and OPG gene polymorphisms: associations with vertebral fractures and bone mineral density in premenopausal systemic lupus erythematosus. Osteop Int 2015; 26:1563–1571. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima W, Yamamoto T, Takahashi S, et al. The effect of alcohol intake and the use of oral corticosteroids on the risk of idiopathic osteonecrosis of the femoral head: a case-control study in Japan. Bone Joint J 2013; 95-b:320–325. [DOI] [PubMed] [Google Scholar]
- 17.Asano T, Takahashi KA, Fujioka M, et al. Genetic analysis of steroid-induced osteonecrosis of the femoral head. J Orthopaed Sci 2003; 8:329–333. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Current protocols in human genetics, 2009: 2.12. 1–2.12. 16. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007; 39:347–351. [DOI] [PubMed] [Google Scholar]
- 20.Adamec C. [Example of the use of the nonparametric test. Test x2 for comparison of 2 independent examples]. Cesk Zdrav 1964; 12:613–619. [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. The odds ratio. BMJ 2000; 320:1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TH, Baek JI, Hong JM, et al. Significant association of SREBP-2 genetic polymorphisms with avascular necrosis in the Korean population. BMC Med Genet 2008; 9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saki N, Abroun S, Farshdousti Hagh M, et al. Neoplastic bone marrow niche: hematopoietic and mesenchymal stem cells. Cell J 2011; 13:131–136. [PMC free article] [PubMed] [Google Scholar]
- 24.Benslimane-Ahmim Z, Heymann D, Dizier B, et al. Osteoprotegerin, a new actor in vasculogenesis, stimulates endothelial colony-forming cells properties. J Thromb Haemost 2011; 9:834–843. [DOI] [PubMed] [Google Scholar]
- 25.Roshandel D, Holliday KL, Pye SR, et al. Influence of polymorphisms in the RANKL/RANK/OPG signaling pathway on volumetric bone mineral density and bone geometry at the forearm in men. Calcified Tissue Int 2011; 89:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung PY, Van Hul W. Paget's disease of bone: evidence for complex pathogenetic interactions. Semin Arthr Rheum 2012; 41:619–641. [DOI] [PubMed] [Google Scholar]
- 27.Beyens G, Daroszewska A, de Freitas F, et al. Identification of sex-specific associations between polymorphisms of the osteoprotegerin gene, TNFRSF11B, and Paget's disease of bone. J Bone Mineral Res 2007; 22:1062–1071. [DOI] [PubMed] [Google Scholar]
- 28.Jurado S, Nogues X, Agueda L, et al. Polymorphisms and haplotypes across the osteoprotegerin gene associated with bone mineral density and osteoporotic fractures. Osteop Int 2010; 21:287–296. [DOI] [PubMed] [Google Scholar]
- 29.Tu P, Duan P, Zhang RS, et al. Polymorphisms in genes in the RANKL/RANK/OPG pathway are associated with bone mineral density at different skeletal sites in post-menopausal women. Osteop Int 2015; 26:179–185. [DOI] [PubMed] [Google Scholar]
- 30.Cheng BH, Wang TH, Kang HY, et al. Association between single nucleotide polymorphisms of the estrogen receptor 1 and receptor activator of nuclear factor kappa B ligand genes and bone mineral density in postmenopausal Taiwanese. Taiwan J Obstet Gynecol 2013; 52:197–203. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki S, Nagoya S, Tateda K, et al. Experimental rat model for alcohol-induced osteonecrosis of the femoral head. Int J Exp Pathol 2013; 94:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]


