Abstract
The aim of the study was to examine whether depression impacts medication nonadherence (MNA) over time and determine if race has a differential impact on MNA in patients with type 2 diabetes and comorbid depression.
Generalized estimating equations were used with a longitudinal national cohort of 740,197 veterans with type 2 diabetes. MNA was the main outcome defined by <80% medication possession ratio for diabetes medications. The primary independent variable was comorbid depression. Analyses were adjusted for the longitudinal nature of the data and covariates including age, sex, marital status, and rural/urban residence.
In adjusted models, MNA was higher in non-Hispanic blacks (NHBs) (odds ratio [OR] 1.58 [95% confidence interval—CI: 1.57, 1.59]), Hispanics (OR 1.34 [95% CI: 1.32, 1.35]), and the other/missing racial/ethnic group (OR 1.37 [95% CI: 1.36, 1.38]) than in non-Hispanic whites (NHWs). In stratified analyses, the odds of MNA associated with depression were highest in NHWs (OR 1.14 [95% CI: 1.12, 1.15]) and were significantly associated in the other 3 minority racial/ethnic groups. MNA was lower in rural than urban NHWs (OR 0.91 [95% CI: 0.90, 0.92]), NHBs (OR 0.92 [95% CI: 0.91, 0.94]), and the other/unknown racial/ethnic group (OR 0.89 [95% CI: 0.88, 0.90]), but higher in rural Hispanic patients (OR 1.12 [95% CI: 1.09, 1.14]).
Depression was associated with increased odds of MNA in NHWs, as well as in minority groups, although associations were weaker in minority groups, perhaps as a result of the high baseline levels of MNA in minority groups. There were also differences by race/ethnicity in MNA in rural versus urban subjects.
Keywords: depression, diabetes, medication adherence, race/ethnicity
1. Introduction
Type 2 diabetes affects approximately 26 million people in the United States.[1] Extensive literature exists indicating that patients with type 2 diabetes with comorbid depression have greater morbidity, higher mortality, and poorer quality of life than those with type 2 diabetes but no depression.[2–5] Longitudinal studies also indicate that depression is associated with worsening glycemic control and significantly higher risk of complications over time even after adjusting for diabetes severity and self-care behaviors.[6–8] A recent meta-analysis of longitudinal and prospective studies showed that patients with type 2 diabetes and depressive symptoms have a significantly increased risk for all-cause and cardiovascular disease mortality, with women at the greatest risk.[9]
Decreased self-care and poor medication adherence have been hypothesized to explain the association between diabetes with comorbid depression and worse patient outcomes.[10,11] Indeed, these patients have been shown to have decreased medication adherence in several prior studies.[4,5,12] However, interventions successful in improving depressive symptoms do not always demonstrate similar impacts on self-care or glycemic control, indicating successful management of patients may require a greater understanding and treatment of mediating factors.[13] In addition, interventions to improve medication adherence do not always demonstrate improved glycemic control, suggesting additional factors should be assessed.[14] Few studies have investigated the link between medication adherence and comorbid depression over time, but a prospective study by Dirmaier et al in Germany found that baseline depression predicted problems with medication adherence.[12]
Our research team and others have demonstrated that racial and ethnic differences exist in medication adherence among diabetic patients, and recent work suggests that differing race/ethnicity may modify effects of depression on medication adherence.[15–17] For example, although persistent racial differences in glycemic control exist, they are not completely explained by differences in medication adherence.[18] An association was found between medication adherence and race in a Medicaid population newly starting oral antidiabetic medication.[19] In a cohort of veterans, racial/ethnic differences in the association of glycemic control varied by medication adherence.[15] In addition, a differential impact of medication nonadherence (MNA) was seen on mortality by race/ethnicity.[20] This interaction may be a significant factor in understanding ethnic disparities, but limited research has been carried out on the topic and little is known about the association between ethnicity, medication adherence, and underlying factors influencing the association.[17] Furthermore, no work has been done on the differential impact of race on medication adherence in a population with both type 2 diabetes and depression.
The purpose of this study was to examine whether depression impacts medication adherence over time and determine if race has a differential impact on medication adherence in patients with type 2 diabetes and comorbid depression. This study addresses methodological weaknesses of previous studies by using a large sample with uniformity in measurement of ethnicity, assessment for depression, and adherence to investigate the question.[17]
2. Research design and methods
2.1. Study population
A national cohort of veterans with type 2 diabetes was created by linking multiple patient and administrative files from the Veterans Health Administration (VHA) National Patient Care and Pharmacy Benefits Management (PBM) databases. Veterans were included in the cohort if they had type 2 diabetes defined by 2 or more International Classification of Diseases, Ninth Revision (ICD-9) codes for diabetes (250, 357.2, 362.0, and 366.41) in the previous 24 months (2000 and 2001) and during 2002 from inpatient stays and/or outpatient visits on separate days (excluding codes from laboratory tests and other nonclinician visits) using a previously validated algorithm.[21] The National Patient Care Database was the source data for the VHA Medical SAS data sets, which were used to analyze veteran clinical data such as diagnosis and procedure codes for outpatient visits and inpatient admissions. The PBM database includes utilization information for every prescription filled in the Veterans Administration (VA). The Outpatient Pharmacy Package includes prescriptions dispensed at the site's pharmacy, either as a new fill or a refill, within that month. In addition, all prescriptions filled by a Consolidated Mail Outpatient Pharmacy are included. The PBM database is at the level of individual prescriptions, thus a veteran can have multiple records on a given day. The data sets were linked using patient scrambled Social Security Numbers.
Veterans were followed from time of entry into the study until death, loss to follow-up, or through December 2006. From a total of 892,223 veterans, there were 740,197 veterans on diabetes medications who qualified to the study cohort. These include 70% non-Hispanic white (NHW), 12.4% non-Hispanic black (NHB), and 6.1% Hispanic with type 2 diabetes. There were also 12.6% of veterans with either missing or unknown race/ethnicity information. The study was approved by the Medical University of South Carolina Institutional Review Board and local VA Research and Development Committee.
2.2. Outcome
The outcome variable was MNA based on mean medication possession ratio (MPR) over the study period for each veteran. MPR was calculated for individuals using insulin combined with oral hypoglycemic agents, insulin only, and oral hypoglycemic agents only. MPR was defined as the ratio of days for which a medication (insulin or oral hypoglycemic agents, i.e., VA classes HS501 or HS502, respectively) is supplied to the total days in a specified time interval. In this study, MPR was initially calculated in quarterly (90 days) intervals for both insulin and oral hypoglycemic agents for each patient. Subsequently, an annual average was calculated by taking the mean of the 4 quarterly MPR values in each year the patient was in the study from 2002 to 2006. Finally, mean MPR was calculated across all study years and utilized in the analysis. If the MPR exceeded 1, the MPR was set to 1. An MPR value of 1 implies perfect medication adherence, whereas an MPR value of 0 would imply that no medication had been taken. A mean MPR of 80%, which is an average of 90-day window MPRs over the entire study period, means that a patient has, on average, 80% of their medication supply over the entire study period. The greater the MPR percentage within each 90-day interval, the more medication veterans had in their possession and likely took as prescribed (i.e., greater adherence). MPR was dichotomized with a cutoff value at 80% where MNA was defined as an indicator of MPR values <0.8 which are considered to imply poor adherence.[22,23]
2.3. Primary covariates
The primary covariate was history of comorbid depression defined based on Elixhauser's AHRQ-Web ICD-9-CM coding algorithm as reported by Quan et al for comorbid depression (300.4, 301.12, 309.0, 309.1, and 311).[24] Moreover, the VA provides comprehensive evidence-based recommendations for improving screening and treatment of depression. The systematic use of the Patient Health Questionnaire-2 (PHQ-2) and PHQ-9 to screen for depression in the VA likely reduces undiagnosed depression within the cohort.[25] The second primary covariate of interest was race/ethnicity, with NHW serving as the reference group. Race/ethnicity was retrieved from the 2002 outpatient and inpatient Medical SAS data sets. When missing or unknown, the variable was supplemented using the inpatient race1-race6 fields from the 2003 Medical SAS data sets, the outpatient race1-race7 fields from the 2004 Medical SAS data sets, and the VA Vital Status Centers for Medicare and Medicaid Services field for race.
Other potential confounders were location of residence, age, sex, service connected disability, marital status, and comorbidities. Age was categorized into 4 groups (<50 years, 50–64 years, 64–74 years, ≥75 years). Location of residence was classified as urban or rural with highly rural being categorized as rural.[4] Marital status was classified as single/never married, married, divorced, separated, and widowed, and region was classified based on the 5 geographic regions of the country (combining Veterans Integrated Service Networks [VISNs]): Northeast (VISNs 1, 2, and 3), Mid-Atlantic (VISNs 4, 5, 6, 9, and 10), South (VISNs 7, 8, 16, and 17), Midwest (VISNs 11, 12, 15, 19, and 23), and West (VISNs 18, 20, 21, and 22).[5] Service connected disability was defined as dichotomous (<50% vs ≥50%) serving as proxy variable to measure copay status, as has been done in multiple prior studies.[26–28] We also included comorbidity variables such as anemia, cancer, cerebrovascular disease, congestive heart failure, cardiovascular disease, hypertension, hypothyroidism, liver disease, lung disease, fluid and electrolyte disorders, obesity, psychoses, substance abuse, and others (AIDS, rheumatoid arthritis, renal failure, peptic ulcer disease and bleeding, weight loss), and were defined based on ICD-9 codes at entry into the cohort. These comorbidities, including mental health comorbidities, were defined by Elixhauser's AHRQ-Web ICD-9-CM coding algorithm as reported by Quan et al.[24] Mental health comorbidities of interest included ICD-9 diagnosis of substance abuse (292.0, 292.82–292.89, 292.9, 304.x, 305.2–305.9, and 648.3), depression (300.4, 301.12, 309.0, 309.1, and 311), or psychosis (295.x–298.x, 299.1) as defined by Elixhauser's AHRQ-Web ICD-9-CM coding algorithm.[24] Comorbidity was modeled in 3 different ways, as count, individual disease indicators and 4 categories of the overall count (none, 1, 2, 3+). The results were very similar, so results based on categories of the count of comorbidities are reported.
2.4. Statistical analysis
Generalized linear model with a logit link and Binomial distribution was estimated via generalized estimating equations to study the association between MNA and depression adjusted for covariates and the longitudinal nature of the MNA data. For each covariate, odds ratios (ORs) and corresponding 95% confidence intervals (CIs) are reported. We tested for the possibility that race/ethnicity modifies the observed association between MNA and depression using interaction terms. Because the interaction terms were significant, we performed a race/ethnicity stratified analysis of the association between race and MNA. Additional analysis was made to examine the association between multimorbidity burden (count of comorbidities categorized into 0, 1, 2, and 3+) and MNA. To deal with missing race data, we used a full information maximum likelihood approach[29] assuming the missing race data mechanism is at random based on our previous sensitivity analysis work on missing race data. Finally, we used residual analysis to assess goodness-of-fit of each of the models. All data analyses were conducted using SAS 9.3. (Cary, NC).
3. Results
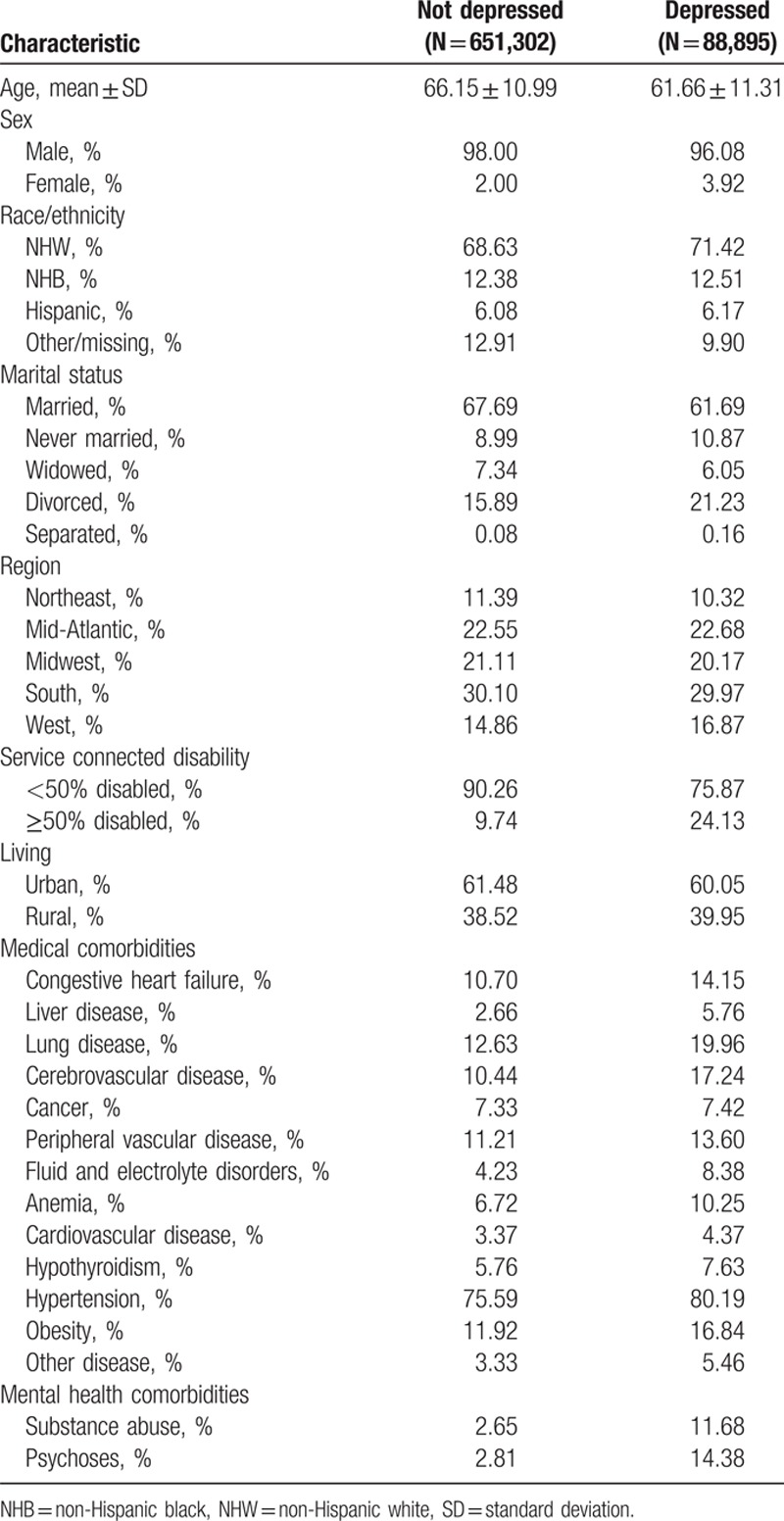
The study population consisted of 740,197 veterans with diabetes in 2002, including 88,895 (12.0%) who were diagnosed with depression. Depressed individuals with diabetes were younger (61.66 vs 66.15 years), more likely to be NHW, and less likely to be married than individuals with diabetes without depression (Table 1). Relative to those without depression, depressed individuals with diabetes were also more likely to have ≥50% service connected disability (24.13% vs 9.74%), were more likely to have each of the 13 medical comorbidities we examined, and were more likely to have diagnosed substance abuse (11.68% vs 2.65%) and psychoses (14.38% vs 2.81%).
Table 1.
Sample characteristics for veterans with type 2 diabetes taking medication during study period (N = 740,197).
The prevalence of MNA is shown in Figure 1 for patients with diabetes with and without depression stratified by racial/ethnic group. The prevalence of MNA was highest in 2002 across all strata of racial/ethnicity and in those with and without depression. In NHWs, the prevalence of MNA was higher in patients with depression than in those without depression (33.36% vs 30.64% using data from 2003), whereas in NHBs (43.71% vs 42.95% using data from 2003) and Hispanics (38.32% vs 39.79% using data from 2003) the prevalence of MNA was similarly high in both those with and without depression.
Figure 1.
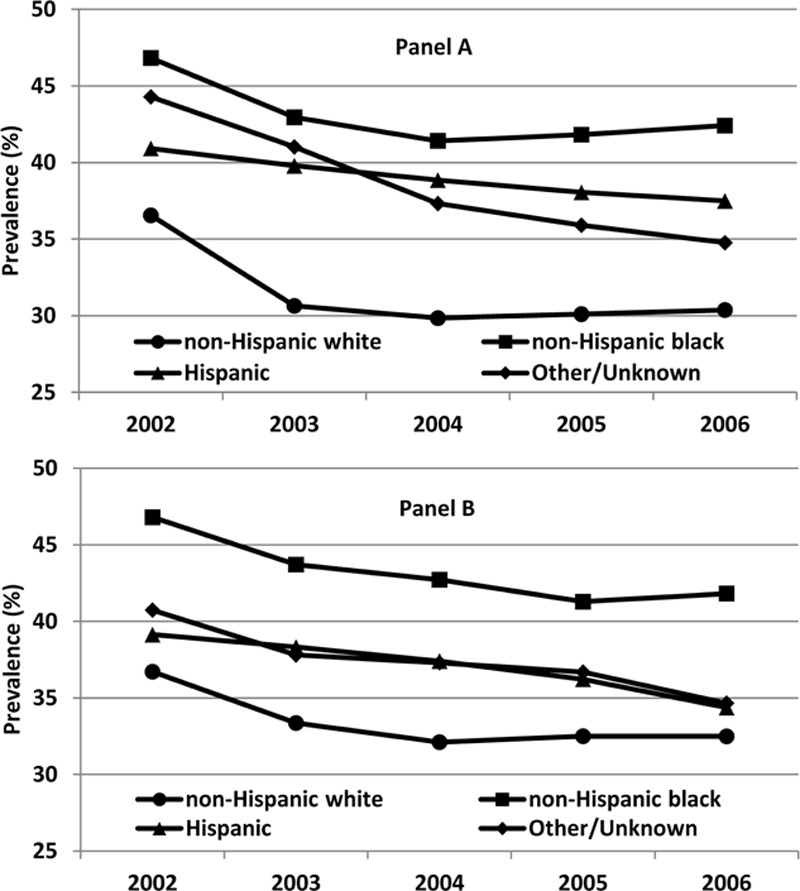
Medication nonadherence for diabetes medications in veterans without (panel A) and with (panel B) depression.
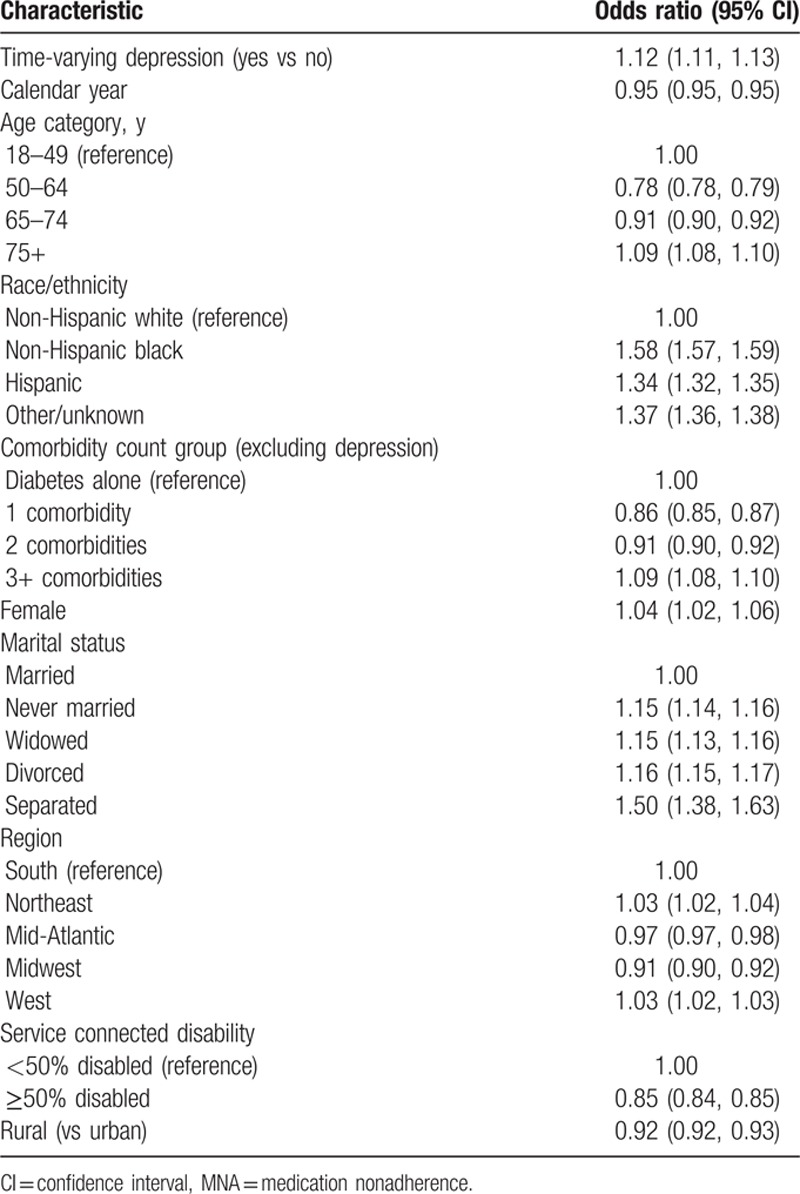
Table 2 shows ORs for being medication nonadherent for a model which included depression status, calendar year, age, race/ethnicity, number of comorbidities, marital status, geographic region, rural residence, and level of service connected disability. The OR for MNA comparing those with depression to those without depression was 1.12 (95% CI: 1.11, 1.13). MNA decreased over time (OR 0.95 [95% CI: 0.95, 0.96]) and was higher in NHBs (OR 1.58 [95% CI: 1.57, 1.59]), Hispanics (OR 1.34 [95% CI: 1.32, 1.35]), and the other/missing racial/ethnic group (OR 01.37 [95% CI: 1.36, 1.38]) than in NHWs. There were differences in MNA by marital status with each of the nonmarried categories having higher odds of MNA than the married group. The odds of MNA were 1.5 times for the separated group compared with the married group. Rates of MNA were also lower in patients with 50% or greater service connected disability (OR 0.85 [95% CI: 0.84, 0.85]) and in rural versus urban veterans (OR 0.92 [95% CI: 0.91, 0.93]).
Table 2.
Odds ratios and 95% CIs for patients with MNA on depression, adjusted for demographics.
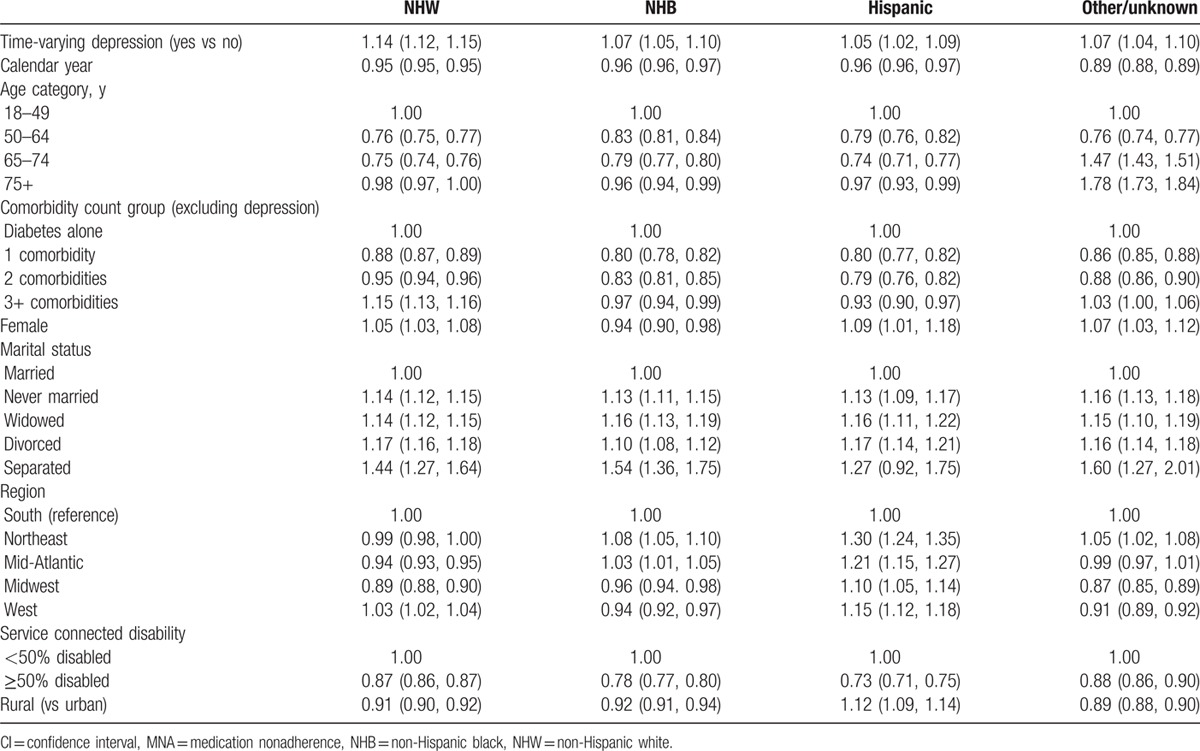
Subsequent analyses indicated that the relationship between depression and MNA was modified by race (P values for interaction terms between each race/ethnic group and depression status were all <0.0001, data not shown); hence, analyses were completed stratified by racial/ethnic group (Table 3). Covariates included in the model are identical to those included in the model shown in Table 2. In NHWs, the odds of MNA were higher in those with depression than in those without depression (1.14 [95% CI: 1.12, 1.15]). Results were in the same direction, but weaker in the other 3 minority racial/ethnic groups (i.e., NHB, Hispanic, and other/unknown) with ORs ranging from 1.05 (95% CI: 1.02, 1.09) in Hispanics to 1.07 in NHB as well as the other/unknown racial ethnic group. Across all race/ethnic groups, MNA decreased with calendar time and was lower in patients who had ≥50% service connected disability than in patients with <50% service connected disability. MNA was lower in rural than urban NHWs (OR 0.91 [95% CI: 0.90, 0.92]), NHBs (OR 0.92 [95% CI: 0.91, 0.94]), and patients with other/unknown racial/ethnic group (OR 0.89 [95% CI: 0.88, 0.90]). In contrast, MNA was higher in rural than urban Hispanic patients (OR 1.12 [95% CI: 1.09, 1.14]).
Table 3.
Odds ratios (95% CIs) for patients with MNA stratified by race.
4. Conclusions
Consistent with prior research, we found that MNA was higher in depressed patients with type 2 diabetes than those without comorbid depression. However, we observed a previously unreported and significant interaction between race and nonadherence among depressed subjects. Once stratified by race, our analysis shows that depression is a more significant factor among NHW patients as compared with minority patients. However, this must be weighed in light of high levels of MNA among racial ethnic minority groups in those without depression which ranged from 34% to 47% throughout the study (Fig. 1, panel A). Rurality also seems important, as there was significantly higher MNA in Hispanic patients with diabetes living in rural areas, though this was not observed among NHW or NHB patients.
First, our results support work showing medication adherence is one of several key self-care behaviors which are required for good diabetes control and optimal disease outcomes. Other behaviors include healthy diet, physical activity, and glucose self-monitoring. Indeed, subjects with depression are more likely to report self-care control problems, lower perceived self-care ability, and lower rates of self-care adherence as well as lower quality of life and higher levels of distress.[30,31] In a similar national cohort of type 2 diabetic patients, our research team previously reported longitudinal MPR, and were significantly lower among NHBs, Hispanics, and individuals with other/missing/unknown race/ethnicity (6.07%, 1.76%, and 2.83% lower, respectively) relative to NHWs. Lower MPR values represent higher rates of nonadherence. Individuals with minor and major depression are also less likely to engage in leisure-time physical activity, more likely to be current smokers, and less likely to engage in preventive care such as dilated eye examinations.[32]
Second, from a clinical perspective, our findings once again highlight the need for providers to be vigilant for MNA among racial and ethnic minority patients with or without depression. However, providers may also do well to consider depression among their NHW patients whose adherence has waned or to screen for adherence among patients who display depressive signs or symptoms. A number of treatment approaches have been applied to patients with comorbid depression and diabetes. These include pharmacotherapy with antidepressant medications, nonpharmacological approaches such as cognitive behavioral therapy, and health systems interventions such as collaborative care teams.[11] Each of these approaches has shown effectiveness at reducing depressive symptoms, but effects on glycemic control are less robust. For example, a majority of randomized trials of antidepressant medications in patients with comorbid diabetes and depression reported favorable trends in depression symptoms, but overall effects on hemoglobin A1c were low (−0.4%, 95% CI −0.6, −0.1; P = 0.002; 238 participants; 5 trials).[33] Several small trials have demonstrated modest effects of cognitive behavioral therapy on glycemic control among depressed diabetics.[34–36] In the Cognitive Behavioral Therapy for Adherence and Depression Trial, hemoglobin A1c values were significantly decreased among subjects receiving CBT compared with care as usual (−0.72%, P = 0.001).[32] However, a recent trial of mindfulness-based cognitive therapy failed to demonstrate any significant effects on glycemic control despite improvement in depressive symptoms, stress, and anxiety.[37]
Collaborative treatment approaches are another strategy for managing patients with comorbid type 2 diabetes and depression. Katon and colleagues recently reported results of a randomized trial of an intervention utilizing nurses to provide guideline-based, patient-centered depression and chronic disease management. They found that subjects in the intervention group had greater improvement in hemoglobin A1c (−0.58%), low-density lipoprotein cholesterol (−6.9 mg/dL [0.2 mmol/L]), systolic blood pressure (−5.1 mm Hg), and SCL-20 depression scores (−0.40 points) (P < 0.001 for the combined composite endpoint). Other trials using similar approaches are currently underway.[38] This is notable because nurse-directed diabetes case management with behavioral modification is also being used in other trials focused specifically on African Americans.[39,40]
This study should be evaluated in light of certain limitations. First, our sample was of veterans receiving care at the VA and >97% male, which might seem to limit generalizability to the general population and female patients specifically. However, given the large sample size, 3% female corresponds to 16,000 female subjects included in the analysis, which provides adequate statistical power to make meaningful inferences. Moreover, although veterans may not be representative of the general population, the standardized care received within the VA removes bias often found within administrative databases strengthening the internal validity of the study. Second, we analyzed diabetes medication adherence, but we were not able to analyze medication treatment or adherence for depression, disease severity for depression, or chronological development of comorbidities including depression. In addition, we were unable to measure nonpharmacological treatments for depression such as cognitive behavioral therapy. However, prior longitudinal studies and clinical trials have demonstrated lingering effects of diagnosed depression on glycemic control despite successful treatment of depressive symptoms which are consistent with our observations.[8,12] Third, we were unable to assess for additional self-care behaviors associated with poor diabetes control such as diabetes monitoring, diet, and physical activity. In light of our findings related to racial/ethnic differences in medication adherence in the presence of depression, future research should determine the degree to which these other factors differ. Fourth, we are limited in that we do not have information on socioeconomic status, but have controlled for service connectedness which is one indicator of access to the VA. Finally, we were limited by the use of administrative data from the VA, which restricted us to care received within the VA and limited our ability to identify onset or chronological relationship between development of depression and diabetes.
To our knowledge, the present study is the largest one to date to report the longitudinal association between depression and medication adherence among patients with type 2 diabetes. This study reinforces prior work highlighting racial and ethnic differences in medication adherence, and it adds to our understanding of the impact of depression on medication adherence among patients with type 2 diabetes. The finding of differential impact of depression on MNA by race may prove useful to both clinicians and policymakers developing treatment programs focused on racial and ethnic minority groups and rural patients.
Footnotes
Authorship: Study concept and design—LEE, MG, RNA, KJH, and CPL; acquisition of data—LEE; analysis and interpretation of data—MG, KJH, and EP; drafting of the manuscript—LEE, MG, RNA, KJH, CPL, and RJW; critical revision of the manuscript for important intellectual content—LEE, MG, RNA, KJH, CPL, RJW, and EP; final approval of manuscript—LEE, MG, RNA, KJH, CPL, RJW, and EP.
Funding: This study was supported by grant #IIR-06-219 funded by the VHA Health Services Research and Development (HSR&D) program. The funding agency did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The manuscript represents the views of the authors and not those of the VA or HSR&D. Drs LEE and MG are the guarantor's of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure: None of the authors disclosed any financial or other conflicts of interest.
References
- 1.Center for Disease Control Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. In: U.S. Department of Health and Human Services Centers for Disease Control Prevention, ed. Atlanta, GA; 2011. [Google Scholar]
- 2.Ali S, Stone M, Skinner TC, et al. The association between depression and health-related quality of life in people with type 2 diabetes: a systematic literature review. Diabetes Metab Res Rev 2010; 26:75–89. [DOI] [PubMed] [Google Scholar]
- 3.Andreoulakis E, Hyphantis T, Kandylis D, et al. Depression in diabetes mellitus: a comprehensive review. Hippokratia 2012; 16:205–214. [PMC free article] [PubMed] [Google Scholar]
- 4.Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract 2010; 87:302–312. [DOI] [PubMed] [Google Scholar]
- 5.Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology 2011; 36:1276–1286. [DOI] [PubMed] [Google Scholar]
- 6.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care 2010; 33:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathew CS, Dominic M, Isaac R, et al. Prevalence of depression in consecutive patients with type 2 diabetes mellitus of 5-year duration and its impact on glycemic control. Indian J Endocrinol Metab 2012; 16:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson LK, Egede LE, Mueller M, et al. Longitudinal effects of depression on glycemic control in veterans with type 2 diabetes. Gen Hosp Psychiatry 2008; 30:509–514. [DOI] [PubMed] [Google Scholar]
- 9.Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry 2013; 35:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract 2011; 65:314–322. [DOI] [PubMed] [Google Scholar]
- 11.Penckofer S, Doyle T, Byrn M, et al. State of the science: depression and type 2 diabetes. West J Nurs Res 2014; 36:1158–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirmaier J, Watzke B, Koch U, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom 2010; 79:172–178. [DOI] [PubMed] [Google Scholar]
- 13.Katon W, Lin EH, Von Korff M, et al. Integrating depression and chronic disease care among patients with diabetes and/or coronary heart disease: the design of the TEAMcare study. Contemp Clin Trials 2010; 31:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strom Williams J, Walker RJ, Smalls BL, et al. Effective interventions to improve medication adherence in type 2 diabetes: a systematic review. Diabetes Manag 2014; 4:29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt KJ, Gebregziabher M, Lynch CP, et al. Impact of diabetes control on mortality by race in a national cohort of veterans. Ann Epidemiol 2013; 23:74–79. [DOI] [PubMed] [Google Scholar]
- 16.Lopez JM, Bailey RA, Rupnow MF, et al. Characterization of type 2 diabetes mellitus burden by age and ethnic groups based on a nationwide survey. Clin Ther 2014; 36:494–506. [DOI] [PubMed] [Google Scholar]
- 17.Peeters B, Van Tongelen I, Boussery K, et al. Factors associated with medication adherence to oral hypoglycaemic agents in different ethnic groups suffering from type 2 diabetes: a systematic literature review and suggestions for further research. Diabet Med 2011; 28:262–275. [DOI] [PubMed] [Google Scholar]
- 18.Adams AS, Trinacty CM, Zhang F, et al. Medication adherence and racial differences in A1C control. Diabetes Care 2008; 31:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenolikar RA, Balkrishnan R, Camacho FT, et al. Race and medication adherence in Medicaid enrollees with type-2 diabetes. J Natl Med Assoc 2006; 98:1071–1077. [PMC free article] [PubMed] [Google Scholar]
- 20.Egede LE, Lynch CP, Gebregziabher M, et al. Differential impact of longitudinal medication non-adherence on mortality by race/ethnicity among veterans with diabetes. J Gen Intern Med 2012; 28:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004; 27 suppl 2:B10–21. [DOI] [PubMed] [Google Scholar]
- 22.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004; 27:1218–1224. [DOI] [PubMed] [Google Scholar]
- 23.Salas M, Hughes D, Zuluaga A, et al. Costs of medication nonadherence in patients with diabetes mellitus: a systematic review and critical analysis of the literature. Value Health 2009; 12:915–922. [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 25.VA/DoD Depression Practice Guidelines. Management of Major Depressive Disorder (2009). Available at: http://www.healthquality.va.gov/guidelines/MH/mdd/ Accessed January, 2016. [Google Scholar]
- 26.Axon RN, Gebregziabher M, Echols C, et al. Racial and ethnic differences in longitudinal blood pressure control in veterans with type 2 diabetes mellitus. J Gen Intern Med 2011; 26:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care 2011; 34:938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and ethnic differences in medication adherence among adults with type 2 diabetes (February). Ann Pharmacother 2011; 45:169–178. [DOI] [PubMed] [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: Wiley; 2002. [Google Scholar]
- 30.Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther 2008; 10:213–219. [DOI] [PubMed] [Google Scholar]
- 31.Egede LE, Grubaugh AL, Ellis C. The effect of major depression on preventive care and quality of life among adults with diabetes. Gen Hosp Psychiatry 2010; 32:563–569. [DOI] [PubMed] [Google Scholar]
- 32.Egede LE, Ellis C, Grubaugh AL. The effect of depression on self-care behaviors and quality of care in a national sample of adults with diabetes. Gen Hosp Psychiatry 2009; 31:422–427. [DOI] [PubMed] [Google Scholar]
- 33.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev 2012; 12:CD008381. [DOI] [PubMed] [Google Scholar]
- 34.Lustman PJ, Griffith LS, Freedland KE, et al. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1998; 129:613–621. [DOI] [PubMed] [Google Scholar]
- 35.Penckofer SM, Ferrans C, Mumby P, et al. A psychoeducational intervention (SWEEP) for depressed women with diabetes. Ann Behav Med 2012; 44:192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safren SA, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care 2014; 37:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Son J, Nyklicek I, Pop VJ, et al. The effects of a mindfulness-based intervention on emotional distress, quality of life, and HbA(1c) in outpatients with diabetes (DiaMind): a randomized controlled trial. Diabetes Care 2013; 36:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coventry PA, Lovell K, Dickens C, et al. Collaborative Interventions for Circulation and Depression (COINCIDE): study protocol for a cluster randomized controlled trial of collaborative care for depression in people with diabetes and/or coronary heart disease. Trials 2012; 13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egede LE, Strom JL, Durkalski VL, et al. Rationale and design: telephone-delivered behavioral skills interventions for Blacks with type 2 diabetes. Trials 2010; 11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egede LE, Strom JL, Fernandes J, et al. Effectiveness of technology-assisted case management in low income adults with type 2 diabetes (TACM-DM): study protocol for a randomized controlled trial. Trials 2011; 12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]


