Abstract
Coxiella burnetii vascular infections continue to be very severe diseases and no guidelines exist about their prevention. In terms of treatment, the benefit of the surgical removal of infected tissues has been suggested by 1 retrospective study.
We present a case of a C burnetii abdominal aortic graft infection for which we observed a dramatic clinical and biological recovery after surgery. We thus performed a retrospective cohort study to evaluate the impact of surgery on survival and serological outcome for patients with Q fever vascular infections diagnosed in our center.
Between 1986 and February 2015, 100 patients were diagnosed with Q fever vascular infections. The incidence of these infections has significantly increased over the past 5 years, in comparison with the mean annual incidence over the preceding 22 years (8.83 cases per year versus 3.14 cases per year, P = 0.001). A two-and-a-half-year follow-up was available for 66 patients, of whom 18.2% died. We observed 6.5% of deaths in the group of patients who were operated upon at 2 and a half years, in comparison with 28.6% in the group which were not operated upon (P = 0.02). Surgery was the only factor that had a positive impact on survival at 2 and a half years using univariate analysis [hazard ratio: 0.17 [95% CI]: [0.039–0.79]; P = 0.024]. Surgery was also associated with a good serological outcome (74.1% vs 57.1% of patients, P = 0.03). In the group of patients with vascular graft infections (n = 47), surgery had a positive impact on serological outcome at 2 and a half years (85.7% vs 42.9%, P < 0.001) [hazard ratio: 0.40 [95% CI]: [0.17–098]; P = 0.046] and tended to be associated with lower although not statistically significant mortality (11.1% vs 27.6% of deaths, P = 0.19).
Surgical treatment confers a benefit in terms of survival following C burnetii vascular infections. However, given the high mortality of these infections and their rising incidence, we propose a strategy that consists of screening for vascular graft and aneurysms in the context of primary Q fever, to decide when to start prophylactic treatment, similar to the strategy recommended for the prophylaxis of Q fever endocarditis.
INTRODUCTION
Q fever is a zoonosis caused by Coxiella burnetii. The primo infection can take several clinical forms such as pneumonia, influenza-like illness, and hepatitis. When the infection persists, it can lead to Q fever endocarditis in patients with valvulopathy and also to vascular infections.1 The main known risk factors for vascular infections are aneurysms and the presence of vascular grafts.2 Diagnosis of vascular infections can be made using several criteria, including high levels of Phase I IgG titers to C burnetii (see Table, Supplemental Digital Content 1),1 and new imaging tools such as 18 FDG PET/CT have been proposed recently to help detect these infections.3–5 A comprehensive literature review, including recent studies from the outbreak in the Netherlands, yielded a total of 230 reported cases of C burnetii vascular infections,5–27 including 90 cases of vascular graft infections. The majority of the reports were published in the last 10 years, suggesting an increase in clinicians’ awareness of these infections in association with the development of 18 FDG PET/CT in this indication.
These infections have a very poor outcome because of a major risk of death resulting from aneurysm, graft tear or fistulization to adjacent organs.6,10,28 Overall mortality varies between 18% and 25%.6,17 However, very few studies are available regarding the influence of surgical removal of the infected vascular tissue on prognosis. Some reports describe surgical treatment of these infections with successful outcomes8,20,21 and 1 study suggests that surgery confers a survival benefit6 when combined with the recommended antibiotics, doxycycline, and hydroxychloroquine. Conversely, a recent retrospective study from the Netherlands suggests that surgical treatment of chronic Q fever is associated with all-cause and chronic Q fever-mortality.17 These results seem difficult to interpret, since in the classification used by the authors, endocarditis and vascular C burnetii infections are grouped under the global term of “chronic Q fever.” However, patients with vascular graft infections often suffer from multiple vascular comorbidities that increase the anesthesia and surgical risks of these interventions, making the surgical decision difficult. Moreover, no large prospective study has been found in the published literature dealing with the treatment and prognosis of C burnetii vascular infections, because of the number of cases per center being too small.
A successful prevention strategy exists for Q fever endocarditis. As these infections occur in patients with a pre-existing valvulopathy or prosthetic valve, we propose a strategy to systematically search for a valvulopathy (using transthoracic echocardiogram) in patients with primary Q fever,29 and to initiate prolonged prophylactic treatment with doxycycline and hydroxychloroquine in such patients. This approach has resulted in a dramatic decrease in the incidence of endocarditis over a 6-year period.30 In infections of vascular aneurysms or prosthesis, a screening strategy to decide when to perform prophylactic treatment would be useful.
We describe the case of a patient who had a C burnetii infection of his aortic graft. Local surgeons contraindicated the operation and he presented poor evolution following antibiotic treatment alone. When he was finally operated on abroad, we observed a dramatic serologic decrease after surgery, testifying to its positive evolution. We retrospectively analyzed the incidence and characteristics of patients with Q fever vascular infections over a 29-year period in the French National Referral Center for Q fever. We also retrospectively assessed the role of surgery in C burnetii aneurysms and vascular graft infections on the survival and serological outcomes for these patients.
Case Presentation
A 34-year-old Lebanese patient was transferred from the hospital in Beirut to the Timone Hospital in Marseilles, France, on January 23, 2008 for fever and polyarthralgia. His medical history included aortic prosthesis surgery in March 2001, due to a chronic traumatic aortic rupture. The CT scan revealed an eso-aneurysmal fistula with an associated aortic collection and a renal abscess. Q fever serology was performed in our laboratory and was found to be positive (IgG phase I: 6400 and IgG phase II: 12,800). Our patient had 2B criteria, that is definite diagnosis of Q fever vascular prosthesis infection according to the C burnetii vascular infection score (see Table, Supplemental Digital Content 1).1 As recommended in our center, treatment with doxycycline and hydroxychloroquine was initiated. We put this patient forward for surgical treatment. All surgeons refused to operate on him, considering the surgical risks to be too high. Twelve months later, he suffered from several deep abscesses (renal, splenic, and pulmonary) and bacteremia. Only 1 dilution decrease of IgG phase I was noted, suggesting a poor outcome. At 18 months, a new fistula on the prosthesis appeared with an endoluminal vegetation. On our advice (DR), the patient contacted a surgeon (HS) practicing in Liverpool, United Kingdom, who decided to perform surgery. He was finally operated on there for surgical debridement with graft replacement and fistulous tract repair. Antibiotherapy with doxycycline and hydroxychloroquine was continued. C burnetii was detected by qPCR on the graft biopsy. After the intervention, we observed a dramatic clinical improvement and a 5-fold decrease in Q fever serological titers 4 months later. (IgG phase I titer = 100 and IgG phase II titer = 200) (Figure 1). Doxycycline and hydroxychloroquine were continued for 16 months, that is 3 and a half years in total. At the time of publication, 4 years after the surgery, the patient is in good health, with no clinical or serological relapses.
FIGURE 1.
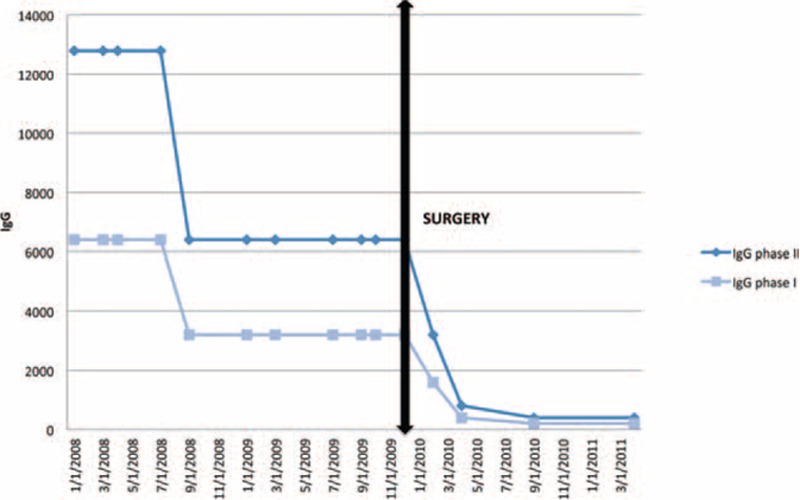
Serological and clinical outcome of the case patient before and after surgery.
Patients and Methods
Patients
We screened the French National Referral Center for Q fever database for patients with Q fever vascular infections between 1986 and February 2015. Over this period, we identified 100 patients who had the diagnosis criteria for vascular infections. Vascular infection was defined using published proposed criteria for diagnosis of Q fever vascular infection (see Table, Supplemental Digital Content 1).1 Patients were considered to have a vascular infection when C burnetii was isolated by culture or molecular detection in a vascular aneurysm or vascular graft biopsy. Major criteria were IgG titer to phase I C burnetii >6400 or molecular detection from the patient's blood combined with a CT scan or 18 FDG PET/CT showing vascular involvement (see Table, Supplemental Digital Content 1).
For each patient, we retrospectively collected their sociodemographic data (age and sex), clinical data (type and location of vascular prosthesis or aneurysm), and serological data. We also noted the presence of surgical treatment with removal of the infected tissue or device in addition to the recommended antibiotherapy, combining doxycycline, and hydroxychloroquine. Vital status at 1, 2 and a half, and 3 years of follow-up was sought by phone call when patients were not clinically followed in our center. Serological outcome at 1, 2 and a half, and 3 years was recorded in our center's database for serologic tests for Q fever.
Methods for Diagnosing C burnetii Vascular Infection
Serological diagnosis was performed in our laboratory using an indirect immunofluorescence assay to determine IgG titers to phases I and II of C burnetii, as previously described [6]. Molecular detection of C burnetii was performed on blood or surgical samples of vascular tissue or graft when available. DNA was extracted using the QiAmp tissue kit (Qiegen, Hilden, Germany) and detection of C burnetii was performed by qPCR targeting the IS1111 repetitive element.31 For culture, samples were inoculated HEL cells as previously described.32
Outcome Criteria
Good serological outcome was defined by a 2-fold decrease in the dilution titer of IgG antibody to C burnetii phase I, or a decrease of IgG I antibody below 800. Survival outcome was recorded by collecting dates of death for deceased patients, and the last time they were seen in consultation or by direct phone call to their medical doctor at 1, 2 and a half, and 3 years of follow-up. The outcome of interest was the elapsed survival time from diagnosis to either death or the end of the follow-up period. We chose to perform the final statistical analyses with a follow-up time of 2 and a half years because this was when data were available for the highest proportion of patients, providing the best statistical power.
Statistical Analysis
A bilateral Barnard exact test was used to test associations in 2 × 2 tables.33 This test was preferred to the classical exact Fisher test because it is more powerful for small samples.34 A P value <0.05 was considered to be significant. Comparison of means was performed using a Student t test. The impact of surgery on survival and serological outcome at 2 and a half years was identified initially by using the Kaplan–Meier estimate and log-rank test, and secondly using Cox proportional hazards regression. We computed univariate regressions and also considered age, sex, presence of a vascular prosthesis, and the prosthesis type as a possible confounder in a multivariate analysis. Statistical analysis was performed using SPSS Software 22 and SMP software for the Barnard test.
Ethics Statement
Patients’ medical data were retrospectively reviewed, and all collected data were anonymized in standardized forms according to procedures of the Commission Nationale de l’Informatique et des Libertés (the French commission for data protection). The study was approved by the local ethics committee (Comité de Protection des Personnes Sud Mediterranée 1) under registration number 1355. All patients gave informed consent.
RESULTS
From January 1, 1986 to February 2015, 100 patients were diagnosed with C burnetii vascular infection in our laboratory. The mean annual incidence of vascular infection in the last 5 years has increased when compared with the mean annual incidence of the preceding 22 years (8.83 cases per year vs 3.14 cases per year, P = 0.001) (Figure 2). In the same period, a total of 4691 cases of acute Q fever and 943 cases of endocarditis were diagnosed and we observed a 10-fold increase in the ratio of vascular infections to acute Q fever between 1986 and 2014 (Figure 2).
FIGURE 2.

Comparison of the ratio of endocarditis to acute Q fever cases versus the ratio of vascular infection to acute Q fever cases diagnosed from 1986 to 2014.
Clinical data and treatment at the time of diagnosis were available for 86 patients. At diagnosis, 39 patients (45.3%) had IgGI titers at 6400 or higher. The median value of IgGI titer was 3200 and the mean value was 11,926 due to extreme high values (3 patients had IgGI > 100,000). Of these 86 patients, 32 of 35 patients (91%) from whom a surgical sample was available had a positive qPCR for C burnetii on surgical vascular biopsy or perivascular collection. For 14 of these 32 patients (43%), the qPCR was positive after initiation of an adapted antibiotherapy and the average duration of treatment before positive qPCR on biopsy was 7.6 months (See Figure, Supplemental Digital Content 2). Of these patients, 28% (2 patients with vascular graft and 2 patients without vascular graft) had a positive qPCR 1 year after the start of treatment (Figure 2). Fourteen of 24 patients (58%) had a positive culture from surgical samples (see Figure, Supplemental Digital Content 2), and 9 of them (64%) had a positive culture once antibiotherapy had begun. The average duration of treatment before positive culture on biopsy was 2.3 months and 1 patient presented a positive culture after 1 year of treatment (see Figure, Supplemental Digital Content 2). All patients but 1 with positive culture had a positive qPCR. Only 8 (14%) out of 55 patients had a positive qPCR on blood samples.
Twenty-two patients (25%) benefited from an 18 FDG PET/CT showing signs of vascular involvement (see Figure, Supplemental Digital Content 3). For 15 patients, there was a hyperfixation of the vascular graft and for 7 patients a hyperfixation of the aneurysm wall. For 5 patients, a hyperfixation of a psoas abscess complicating the vascular infection was seen. Three patients had associated spondylodiscitis visualized on an 18 FDG PET/CT and 1 patient had an aorto-digestive fistula.
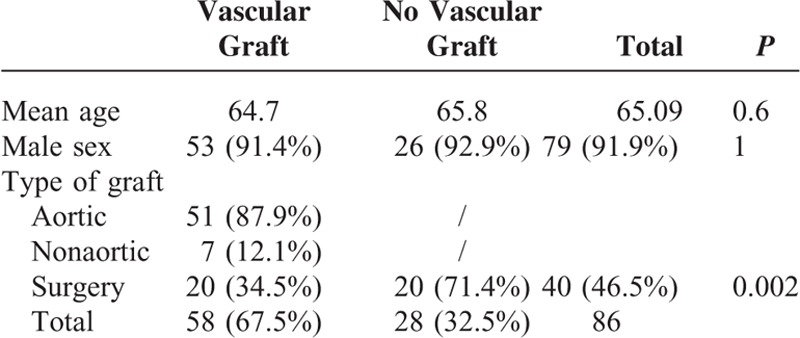
A total of 58 patients (67.5%) had a vascular graft infection and 28 (32.5%) had a vascular infection without graft. The mean age of patients was 65, and there was no difference in age between patients with or without a prosthesis (Table 1). 91.9% of patients (n = 79) were men. Forty-six percent (n = 40) of patients had a surgical treatment for their Q fever vascular infection. Patients with vascular graft infections were significantly less frequently operated on than patients with vascular aneurysm infections (34.4% vs 71.4%, P = 0.002). The majority of grafts involved the aorta (87.9%) (Table 1).
TABLE 1.
Characteristics of Patients Diagnosed With C burnetii Vascular Infections
Impact of Surgery on Survival Status
Survival status at 2 and a half years of follow-up was available for 66 patients. The overall mortality at 2 and a half years was 18.2% (n = 12 patients). Of the patients who were operated upon, 93.5% (n = 29) were alive at 2 and a half years vs 71.4% (n = 25) in the nonoperated group (P = 0.02). The mortality in the subgroup of patients without vascular graft who were not operated on was 33.3% (n = 2) in comparison with no patients in the operated group (P = 0.04) (Table 2). The same trend was observed in patients with a vascular graft between patients who had been operated upon and those who had not, although this was not statistically significant (11.1 % vs 27.6% respectively, P = 0.2) (Table 2). To seek whether surgical treatment had an impact on prognosis, a Kaplan–Meier estimator was performed (Figure 3). Surgery had a significant positive impact on survival at 2 and a half years of follow-up (P = 0.022) (Figure 3). Then the Kaplan–Meier estimator was stratified for the presence or absence of a vascular graft. In patients without a vascular graft, this impact was statistically significant (P = 0.029) and for patients with a vascular graft, the Kaplan–Meier survival analysis was not statistically significant (P = 0.19) (see Figure, Supplemental Digital Content 4).
TABLE 2.
Comparison of the Vital Status and the Serological Outcome Between Patients Who Were Operated Upon and Patients Who Were Not

FIGURE 3.
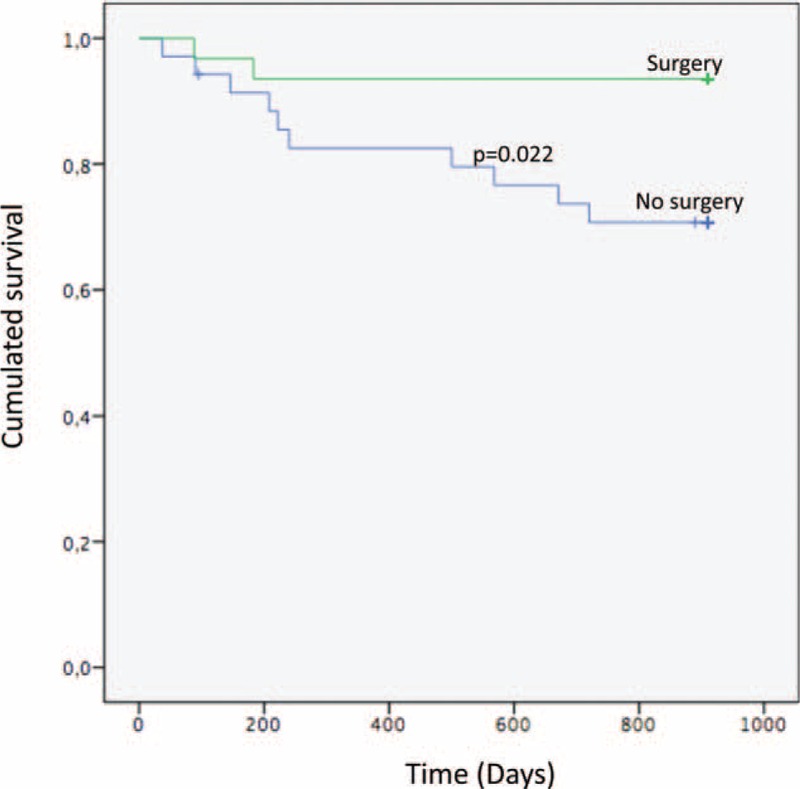
Kaplan–Meier survival curve analysis at 2 and a half years between patients who were operated upon and those who were not.
When univariate Cox proportional hazard regression analysis was performed on the entire cohort, age, sex, presence of vascular graft, and localization of vascular graft had no influence on survival at 2 and half years. Surgical treatment was the only factor significantly associated with survival at 2 and a half years [hazard ratio: 0.17 [95% CI]: [0.039–0.79]; P = 0.024].
Impact of Surgery on Serological Outcome
We analyzed the serological outcome at 2 and a half years and stratified the analysis on the presence or absence of a graft. These data were available for 55 patients. 74.1% (n = 20) of patients who were operated upon had a good serological outcome at 2 and a half years, vs 57.1% (n = 16) of patients who were not operated upon (P = 0.03). Regarding the group of patients with vascular graft infections, 85.7% (n = 12) of operated patients had a good serological outcome at 2 and a half years of follow-up, versus 42.9% (n = 9) of patients who were not operated upon (P <0.001) (Table 2). In this group, a univariate Cox proportional hazard regression analysis for each factor that could be associated with a good serological outcome at 2 and a half years was performed. Surgery was a predictor of good serological outcome at 2 and a half years of follow-up [hazard ratio: 0.40 [95% CI]: [0.17–098]; P = 0.046]. No other factor had a significant impact on serological outcome in this group. In the group of patients with no vascular graft, no significant difference was observed between patients who had been operated on and those who had not in terms of serological outcome at 2 and a half years (Table 2).
DISCUSSION
We report here a large detailed retrospective cohort of C burnetii vascular infections. Over the last 5 years, we have observed a significant increase in the annual incidence of vascular infections diagnosed in our center compared with the preceding 22 years (8.83 cases per year vs 3.14 cases per year, P = 0.001) (Figure 2). Botelho et al had already noted an increase in the number of diagnosed cases of C burnetii vascular infections between 1998 and 2006, due to a strategy to systematically screen for Q fever patients with aneurysms or vascular prosthesis and whose serum, blood, or vascular biopsies were sent to our laboratory. The recent elaboration of a diagnosis score1 and the new contribution of 18 FDG PET/CT may also have contributed to the better identification of cases3 (Figure 2). The mean age (65) and sex ratio (91.9% of men) in our study are fairly similar to those reported in previous studies.6,8
Our results confirm that C burnetii vascular infections are very severe with an overall mortality rate of 18.2% at 2 and a half years of follow-up. These results contrast with what we observed for Q fever endocarditis (Figure 2). Thanks to well-established management strategies, Q fever endocarditis is now a much less severe infection, with reported overall mortality rates of 7% at 3 years of follow-up.35 Over the same period, as the result of adopting a strategy to detect and systematically administer prophylaxis to patients with risk factors for endocarditis, we have observed a dramatic reduction in C burnetii endocarditis incidence.30 This is illustrated by the ratio of endocarditis to acute Q fever, which has decreased over time (Figure 2). Currently, no systematic strategy exists for detecting predisposing vascular aneurysm or vascular prosthesis in the context of primary Q fever, so no prophylaxis can be initiated to decrease the incidence of vascular infections. However, given their severity, it would seem urgent to elaborate a screening strategy for early diagnosis and prophylaxis. We therefore propose such a strategy (Figure 4). The presence of a vascular graft should be systematically looked for in the medical history of patients diagnosed with a Q fever primo infection. If a vascular graft is present and no other criteria for vascular infection are found (Figure 4), prophylaxis consisting of a 12-month treatment of hydroxychloroquine and doxycycline should be administered. Major risk factors for aortic aneurysms are: men over the age of 65 who smoke or have smoked, and a family history of aneurysms.36 We suggest that patients over the age of 65 who are diagnosed with a Q fever primo infection undergo a CT scan or abdominal ultrasound (if renal contraindication) to screen for the presence of an aortic abdominal aneurysm. If an aneurysm is detected, prophylaxis should also be given. Eighteen FDG PET/CT could be helpful in the presence of an aneurysm or vascular graft, to detect precocious signs of infection, particularly in the event of persistent fever or poor serologic evolution (Figure 4).
FIGURE 4.
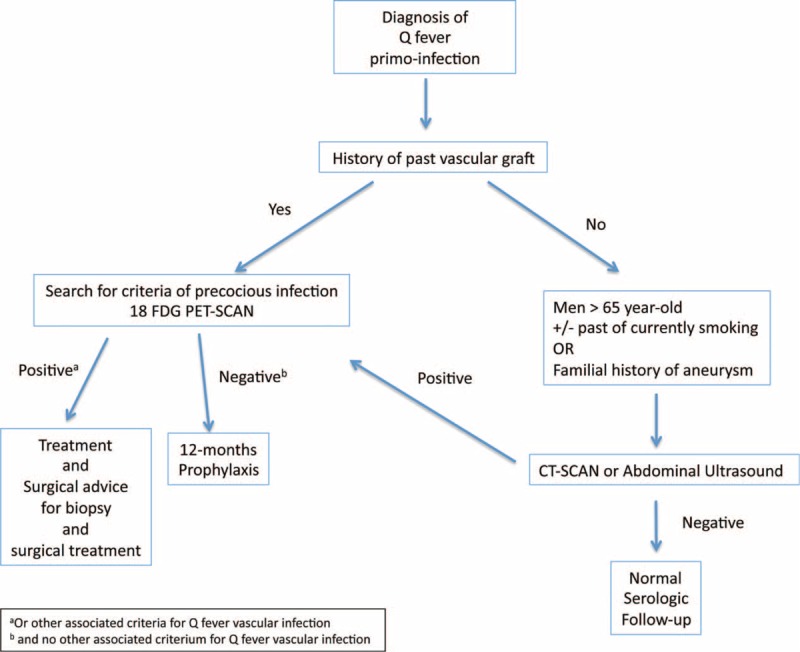
Screening strategy for vascular Q fever infection.
Because our study is retrospective, we obtained available data on survival at 2 and a half years for only 66 patients, which has reduced the statistical power of the exercise and which constitutes a limitation of this work. In particular, in patients with vascular grafts we observed a lower proportion of deceased patients in the group of patients who had been operated upon (11% vs 27%), but the difference was not statistically significant probably because the sample of patients was too small (n = 2 and 8 respectively).
Our study represents the largest cohort of patients with Q fever vascular infection (n = 22) who benefited from an 18 FDG PET/CT. For 9 of these patients (40%), this examination revealed complications of the infection such as psoas abscesses, spondylodiscitis, or digestive fistula, reflecting the usefulness of this tool in the initial evaluation of Q fever vascular infections. Globally, C burnetii vascular infections remain underdiagnosed because of a lack of awareness among clinicians leading to a considerable delay in treatment. In the Netherlands, a country that faced a significant Q fever outbreak between 2007 and 2010, recent work in 1 center over a 3-year period reports a seroprevalence of Q fever of 13.6 % among 149 patients with a vascular graft, of whom 25% (5 patients) had a serologic profile compatible with a vascular infection.7 Such studies in other European countries would be useful to better estimate the global prevalence of the disease to propose accurate treatment.
We found a positive impact of surgery on survival. These results back up those of a previous study, which showed the same link between surgical treatment and recovery.6 This surgical benefit is significant in the subgroup of patients without a vascular graft, where we observed no deaths in the group which had been operated upon (see Figure, Supplemental Digital Content 4).
For 43% of patients with a positive qPCR on vascular biopsy (n = 14), the biopsy was performed after initiating antibiotherapy (see Figure, Supplemental Digital Content 2) and for 3 patients (21%) the qPCR was positive after 2 years of treatment, with 1 patient also positive in culture after this delay. This result may illustrate the fact that in C burnetii vascular infections, the levels of bacteria are so high that even a long course of adapted antibiotherapy is insufficient to completely sterilize the focus of infection. This possibly explains why surgical removal of the infected tissues seems to be mandatory to completely eradicate the infection. This is another difference with respect to Q fever endocarditis, for which a recent study has demonstrated that an adapted medical treatment with doxycycline and hydroxychloroquine for 18 months in native valve endocarditis and 24 months for prosthetic valve endocarditis is sufficient to eradicate the infection.35
The aim of our study was also to check whether the positive impact of surgery on prognosis could be seen in the particularly high-risk population of patients with vascular graft infections (such as the case of our patient). In these patients, we found that surgical removal of the infected device was a predictor of good serologic outcome, which in turn is an indicator of recovery, exactly as we observed for our patient shortly after surgery (Figure 1). Although we saw a lower percentage of deaths in patients with vascular graft infections when they were operated upon, the comparison was not statistically significant. This is due to the fact that in the group which was operated upon, 2 patients died very shortly after the surgery (within 2 days), of immediate postoperative complications. Of the 2 patients, 1 succumbed on the day of his operation for graft removal from hemorrhagic shock and the other died from a myocardial infarction 2 days after surgery. Patient one, who died during the operation, was 80-year old and had a medical history comprising myocardial infarction, carotid endarterectomy, and an ischemic cerebral stroke 1 year previously. Patient two was 67-year old and died 2 days after surgery from a myocardial infarction. The coronarography revealed tritroncular lesions. His medical history was marked by a 22-year period of tobacco smoking and dyslipidemia. Currently, cardiac complications are responsible for 42% of noncardiac surgery perioperative mortality.37 More specifically, aortic and major vascular surgery are considered high-risk procedures with more than a 5% global risk of cardiovascular death in the 30 days following surgery without considering the patient's comorbidities.37 Within the population of patients with vascular graft infections, the assessment of cardiac risk depends on the patient's characteristics and on the type of surgery and emergency.37 Recent predictive models to assess global surgical risk have been built and 3 of them are recommended by the American and European College of Cardiology's updated guidelines.38,37 We applied 1 of these models, the universal ACS NSQIP surgical risk calculator39 (www.riskcalculator.facs.org), to the patient who died from myocardial infraction. This patient had a perioperative risk of cardiac arrest or myocardial infarction of 21%. Such new tools should be applied to patients with C burnetii vascular graft infections to better assist the therapeutic decision. Recently, Kloppenburg et al40 reported a case of C burnetii aortic bifurcated stent graft infection in a patient with high-risk comorbidities. He was treated with semiconservative surgery, with removal of the abscesses and aneurysm wall, but conservation of the endograft. Such a strategy may be a solution for patients presenting a high surgical risk.
Our study confirms that surgical treatment has a positive impact on survival in C burnetii vascular infections. In the subgroup of patients with vascular graft infections, this surgical treatment has no significant impact on survival, but a positive impact on serologic evolution. Because these infections remain very severe and the surgical risk continues to be very high, we propose, for the first time, a strategy involving systematic screening of vascular graft and aneurysms and prophylaxis for patients diagnosed with a Q fever primo infection. We hope that such a strategy will help to decrease the incidence and mortality of C burnetii vascular infections in the future.
Supplementary Material
Footnotes
Abbreviations: 18 FDG PET/CT = 18 F-Fluorodeoxyglucose positron emission tomography/computed tomography, C burnetii = Coxiella burnetii.
CE and MM should be considered as co-first authors.
Funding source: IHU Méditerrannée Infection Foundation. Funding sources had no role in the design and conduct of the study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Raoult D. Chronic Q fever: expert opinion versus literature analysis and consensus. J Infect 2012; 65:102–108. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis 2005; 5:219–226. [DOI] [PubMed] [Google Scholar]
- 3.Merhej V, Cammilleri S, Piquet P, et al. Relevance of the positron emission tomography in the diagnosis of vascular graft infection with Coxiella burnetii. Comp Immunol Microbiol Infect Dis 2012; 35:45–49. [DOI] [PubMed] [Google Scholar]
- 4.van Assen S, Houwerzijl EJ, van den Dungen JJ, et al. Vascular graft infection due to chronic Q fever diagnosed with fusion positron emission tomography/computed tomography. J Vasc Surg 2007; 46:372. [DOI] [PubMed] [Google Scholar]
- 5.Barten DG, Delsing CE, Keijmel SP, et al. Localizing chronic Q fever: a challenging query. BMC Infect Dis 2013; 13:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botelho-Nevers E, Fournier P-E, Richet H, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2007; 26:635–640. [DOI] [PubMed] [Google Scholar]
- 7.Hagenaars JCJP, Wever PC, van Petersen AS, et al. Estimated prevalence of chronic Q fever among Coxiella burnetii seropositive patients with an abdominal aortic/iliac aneurysm or aorto-iliac reconstruction after a large Dutch Q fever outbreak. J Infect 2014; 69:154–160. [DOI] [PubMed] [Google Scholar]
- 8.Fournier PE, Casalta JP, Piquet P, et al. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis Off Publ Infect Dis Soc Am 1998; 26:116–121. [DOI] [PubMed] [Google Scholar]
- 9.Sessa C, Vokrri L, Porcu P, et al. Abdominal aortic aneurysm and Coxiella burnetii infection: report of three cases and review of the literature. J Vasc Surg 2005; 42:153–158. [DOI] [PubMed] [Google Scholar]
- 10.Melenotte C, Million M, Hartung O, et al. Query rectal bleeding. Lancet 2012; 380:446. [DOI] [PubMed] [Google Scholar]
- 11.Senn L, Franciolli M, Raoult D, et al. Coxiella burnetii vascular graft infection. BMC Infect Dis 2005; 5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georghiou GP, Hirsch R, Vidne BA, et al. Coxiella burnetii infection of an aortic graft: surgical view and a word of caution. Interact Cardiovasc Thorac Surg 2004; 3:333–335. [DOI] [PubMed] [Google Scholar]
- 13.Prinsen J-HS, Boersma D, van Loenhout R, et al. Persistent endoleak after endovascular aneurysm repair for acute Q-fever-infected aortocaval fistula. Vascular 2015; 23:645–647. [DOI] [PubMed] [Google Scholar]
- 14.Hagenaars JCJP, Koning OHJ, van den Haak RFF, et al. Histological characteristics of the abdominal aortic wall in patients with vascular chronic Q fever. Int J Exp Pathol 2014; 95:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzaez-Del Vecchio M, Vena A, Valerio M, et al. Coxiella burnetii infection in hemodialysis and other vascular grafts. Medicine (Baltimore) 2014; 93:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagenaars JCJP, Wever PC, Shamelian SOA, et al. Vascular chronic Q fever: quality of life. Epidemiol Infect 2015; 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kampschreur LM, Delsing CE, Groenwold RHH, et al. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. J Clin Microbiol 2014; 52:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigterman TA, Bendermacher BLW, Welten RJTJ, et al. Primary aortoduodenal fistula and Q-fever. Vasc Med Lond Engl 2013; 18:347–349. [DOI] [PubMed] [Google Scholar]
- 19.Ikediobi UT, Streit J. Chronic Q fever causing aortitis. Am J Med 2013; 126:e9–e10. [DOI] [PubMed] [Google Scholar]
- 20.Aerts PDM, van Zitteren M, Van Kasteren MEE, et al. Report of two in situ reconstructions with a saphenous spiral vein graft of Coxiella burnetii-infected aneurysms of the abdominal aorta. J Vasc Surg 2013; 57:234–237. [DOI] [PubMed] [Google Scholar]
- 21.Wegdam-Blans MCA, ter Woorst JF, Klompenhouwer EG, et al. David procedure during a reoperation for ongoing chronic Q fever infection of an ascending aortic prosthesis. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 2012; 42:e19–e20. [DOI] [PubMed] [Google Scholar]
- 22.Bisharat N, Minuhin I. Prosthetic vascular graft infections between blood and concordance of graft culture pathogen. Am J Med Sci 2012; 344:431–435. [DOI] [PubMed] [Google Scholar]
- 23.Wegdam-Blans MCA, Vainas T, van Sambeek MR, et al. Vascular complications of Q-fever infections. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg 2011; 42:384–392. [DOI] [PubMed] [Google Scholar]
- 24.Bendermacher BLW, Peppelenbosch AG, Daemen JWHC, et al. Q fever (Coxiella burnetii) causing an infected thoracoabdominal aortic aneurysm. J Vasc Surg 2011; 53:1402–1404. [DOI] [PubMed] [Google Scholar]
- 25.Edouard S, Labussiere A-S, Guimard Y, et al. Q fever: a case with a vascular infection complication. BMJ Case Rep 2010; 2010: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepidi H, Fournier P-E, Karcher H, et al. Immunohistochemical detection of Coxiella burnetii in an aortic graft. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2009; 15 suppl 2:171–172. [DOI] [PubMed] [Google Scholar]
- 27.Mejia A, Toursarkissian B, Hagino RT, et al. Primary aortoduodenal fistula and Q fever: an underrecognized association? Ann Vasc Surg 2000; 14:271–273. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell ME, Manshani N, McCaughey C, et al. Coxiella burnetii infection of an aortic graft with multiple vertebral body erosion. J Vasc Surg 2007; 45:399–403. [DOI] [PubMed] [Google Scholar]
- 29.Million M, Walter G, Thuny F, et al. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis Off Publ Infect Dis Soc Am 2013; 57:836–844. [DOI] [PubMed] [Google Scholar]
- 30.Edouard S, Million M, Royer G, et al. Reduction in incidence of Q fever endocarditis: 27 years of experience of a national reference center. J Infect 2014; 68:141–148. [DOI] [PubMed] [Google Scholar]
- 31.Eldin C, Angelakis E, Renvoise A, et al. Coxiella burnetii DNA, but not living bacteria, is common in dairy products in France. Am J Trop Med Hyg 2013; 88:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouriet F, Fenollar F, Patrice J-Y, et al. Use of shell-vial cell culture assay for isolation of bacteria from clinical specimens: 13 years of experience. J Clin Microbiol 2005; 43:4993–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnard GA. A New Test for 2 × 2 Tables. Nature 1945; 156:783–784. [Google Scholar]
- 34.Lydersen S, Fagerland MW, Laake P. Recommended tests for association in 2 × 2 tables. Stat Med 2009; 28:1159–1175. [DOI] [PubMed] [Google Scholar]
- 35.Million M, Thuny F, Richet H, et al. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis 2010; 10:527–535. [DOI] [PubMed] [Google Scholar]
- 36.Svensjo S, Bjorck M, Wanhainen A. Update on screening for abdominal aortic aneurysm: a topical review. Eur J Vasc Endovasc Surg 2014; 48:659–667. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2014; 31:517–573. [DOI] [PubMed] [Google Scholar]
- 38.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 44:e77–e137. [DOI] [PubMed] [Google Scholar]
- 39.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kloppenburg GT, van de Pavoordt ED, de Vries J-PP. Endograft-preserving therapy of a patient with Coxiella burnetii-infected abdominal aortic aneurysm: a case report. J Med Case Rep 2011; 5:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


