Abstract
The aim of this study was to explore the incidence and outcomes of patients with oral cavity squamous cell carcinoma (OSCC) and fourth primary tumors (PTs) in a betel-chewing endemic area.
We retrospectively examined the records of 1836 OSCC patients who underwent radical tumor resection between 1996 and 2014. The outcome measures included the incidence and number of multiple PTs, the main risk factors, and their associations with overall survival (OS).
Of the 1836 patients, 1400 (76.3%) had a single PT, 344 (18.7%) a second PT, 67 (3.6%) a third PT, and 25 (1.4%) a fourth PT. Univariate analyses (log-rank test) identified the following factors as significantly associated with a fourth PT: simultaneous first and second PTs, betel quid chewing, buccal subsite, and pT3–4 status. After allowance for the potential confounding effect of other risk factors, all of these factors retained their independent prognostic significance in stepwise multivariate analyses, the only exception being betel chewing. The incidences of second, third, and fourth PTs at 5 and 10 years were 20.2%/34.6%, 4.0%/8.6%, and 1.0%/2.3%, respectively. The 5 and 10-year OS rates (calculated from the diagnosis of each PTs) for patients with a single, second, third, and fourth PTs were 68%/61%, 43%/37%, 45%/39%%, and 30%/30%, respectively (P < 0.0001). Among patients with a fourth PT, those who underwent radical surgery showed a significantly higher 3-year OS than those who did not (57% vs 13%; P = 0.0442).
Fourth PTs are rarely observed in OSCC patients in a betel quid-chewing endemic area. Long-term survival rates of patients treated with radical surgery seems acceptable, being 4-fold higher than their counterparts.
INTRODUCTION
Studies examining the occurrence of multiple primary tumors (PTs) in the same patient can contribute toward earlier diagnosis, better therapy, and improved follow-up care, ultimately resulting in survival benefits.1 In general, the main risk factors for multiple PTs include an increased surveillance of cancer survivors, inherited genetic predisposition to cancer, specific environmental factors and/or cancer-promoting aspects of lifestyle, and treatment of the initial primary cancer with radiotherapy (RT) and/or chemotherapy.2 In patients with head and neck neoplasms, most studies of multiple cancers have been limited to second PTs. In this scenario, data on the incidence and clinical outcomes of subjects with more than 2 PTs remain scarce, particularly on fourth PTs.3–7
Since field cancerization was initially described in the upper gastrointestinal tract in 1953, the occurrence of multifocal precancerous changes surrounding the PT have been reported in several organs (including the head and neck area).8–11 Early field cancerized areas represent genetically abnormal, but not yet histologically detectable, altered cells. Field cancerization in oral cavity squamous cell carcinoma (OSCC) is generally secondary to long-term exposure to certain carcinogens.11 In this regard, south Asia is well-recognized as being a unique environment in terms of practice of betel quid chewing.9 Areca nut (Areca catechu), the major constituent of a betel quid, is an addictive substance and a well-known carcinogenic to humans.12–14 OSCC has a high incidence in Taiwan and comprises approximately 4% to 5% of all malignancies occurring in the country.15 Notably, the prevalence of betel quid chewing in the Taiwanese population is as high as 16.9% (31% in men and 2.4% in women, respectively),16 and approximately 85% of OSCC patients are habitual betel quid chewers.17 Interestingly, betel quid chewing is also the major etiologic agent in the development of oral submucous fibrosis,18–20 a precancerous condition characterized by epithelial atrophy and fibrosis of the subepithelial connective tissue.21 Arecoline and arecaidine—the most abundant alkaloids of betel quid—play a pivotal role in the process of oral carcinogenesis, being able to deregulate mitotic spindle, promote genomic instability, and induce inflammatory events in keratinocytes.22–24
The high prevalence of betel quid chewing in Taiwan provides an opportunity to investigate the incidence of multiple PTs in OSCC patients, thus addressing the question as to whether multiple PTs can also be related to betel quid chewing. In this study, multiple PTs were defined as the occurrence of any malignancy arising in the head and neck region or in any other site of the body (Warrens and Gates criteria25) after an initial diagnosis of OSCC. We also examined the clinical outcomes of OSCC patients with multiple PTs according to the number of primary malignancies, with special emphasis on fourth PTs.
METHODS
Patients
The study was conducted from January 1996 to April 2014. Consecutive patients (n = 1836) with a diagnosis of first primary OSCC who were previously untreated were considered for inclusion. Radical surgery was planned in all participants, whereas neck dissection was performed when indicated. Preoperative work-up and staging were performed as described previously,9 including the use of panendoscopy (after 2002) and whole-body 2-deoxy-2[(18)F]fluoro-D-glucose–positron emission tomography (FDG-PET, after 2001). The seventh edition of the American Joint Committee on Cancer (AJCC) staging manual was used.26 Tumors that appeared anatomically separated from the first PT (ie, ≥2 cm of normal tissue identifiable between distinct lesions) were considered as second (or multiple) PTs. When ≥2 malignancies were simultaneously evident in the oral cavity, the index tumor was defined as the neoplasm with the most advanced stage. We carefully ruled out the presence of metastases and local relapses. Malignancies that developed at the same site were not classified as second (or multiple) PTs, independent from the time elapsed between the date of analysis and first definitive treatment. The surgical principles and the approach used for postoperative adjuvant therapy have been previously described in detail.9,27 Ethical approval was granted by the Institutional Review Board of the Chang Gung Memorial Hospital. Because of the retrospective nature of the study, the need for informed consent was waived.
Data Analysis
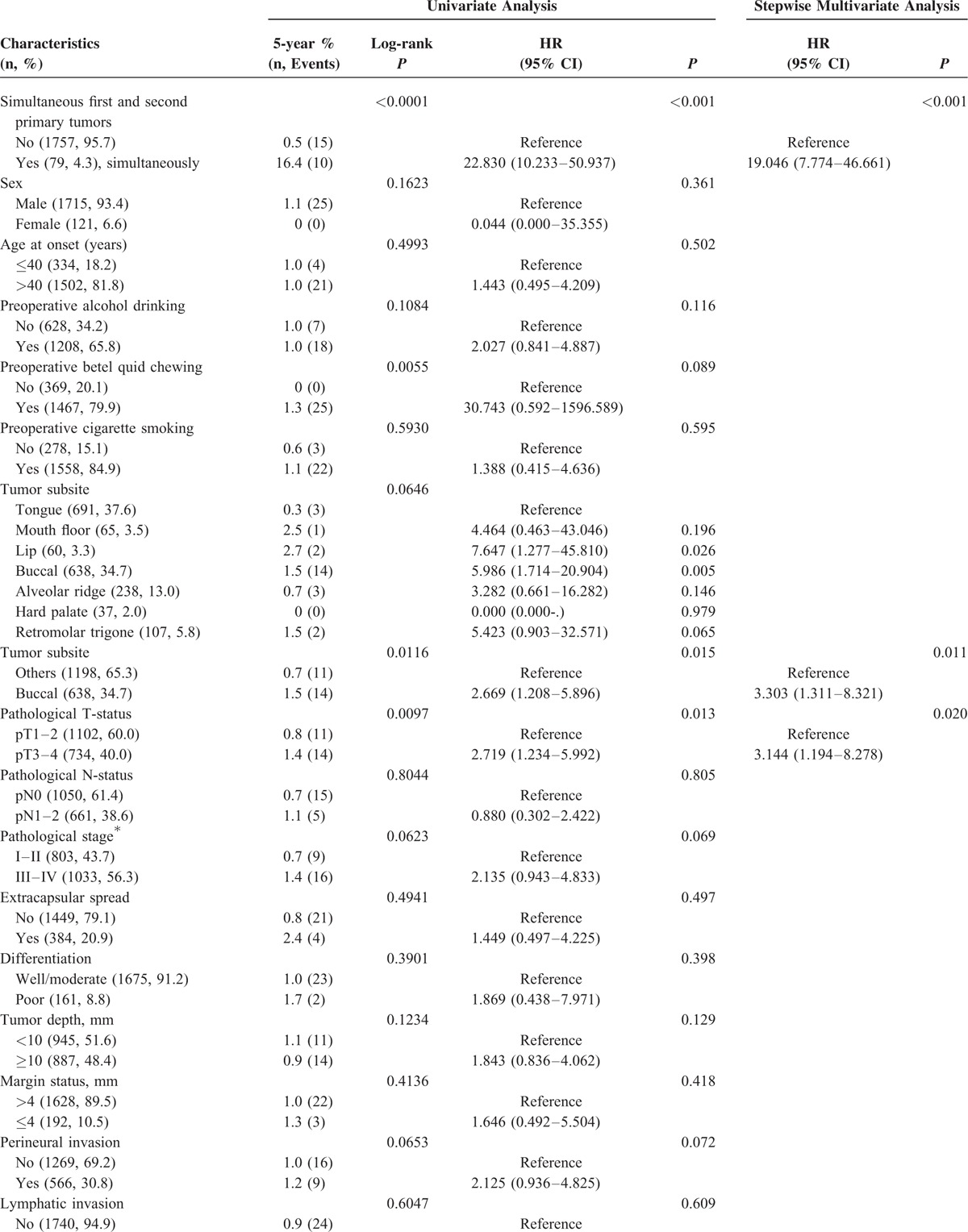
The closing date for follow-up was April 2015. At least 1 year of follow-up after primary definitive treatment was available for all patients who were not death-censored. The study endpoints consisted of the 5, 10, and 15-year overall survival (OS) rates. OS was defined as the time elapsed from surgery to the date of death or the censoring date. Kaplan–Meier curves were constructed that evaluated OS using the log-rank test for comparison. We developed univariate and multivariate Cox regression models to identify the significant predictors of a fourth primary malignancy (Table 1 ) and OS (Supplement Table) in the entire study cohort (n = 1836). All variables were examined as potential predictors and entered in both univariate analysis (UVA) and multivariate analysis (MVA). MVA was conducted using a stepwise forward selection procedure. The statistical computations were performed with the Statistical Package for Social Sciences, version 17.0 (SPSS Inc., Chicago, IL). The alpha error was set at 0.05 (2-tailed).
TABLE 1.
Univariate and Multivariate Analyses of Factors Predicting a Fourth Primary Malignancy in OSCC Patients
RESULTS
General Characteristics of OSCC Patients According to the Number of Second Primary Tumors
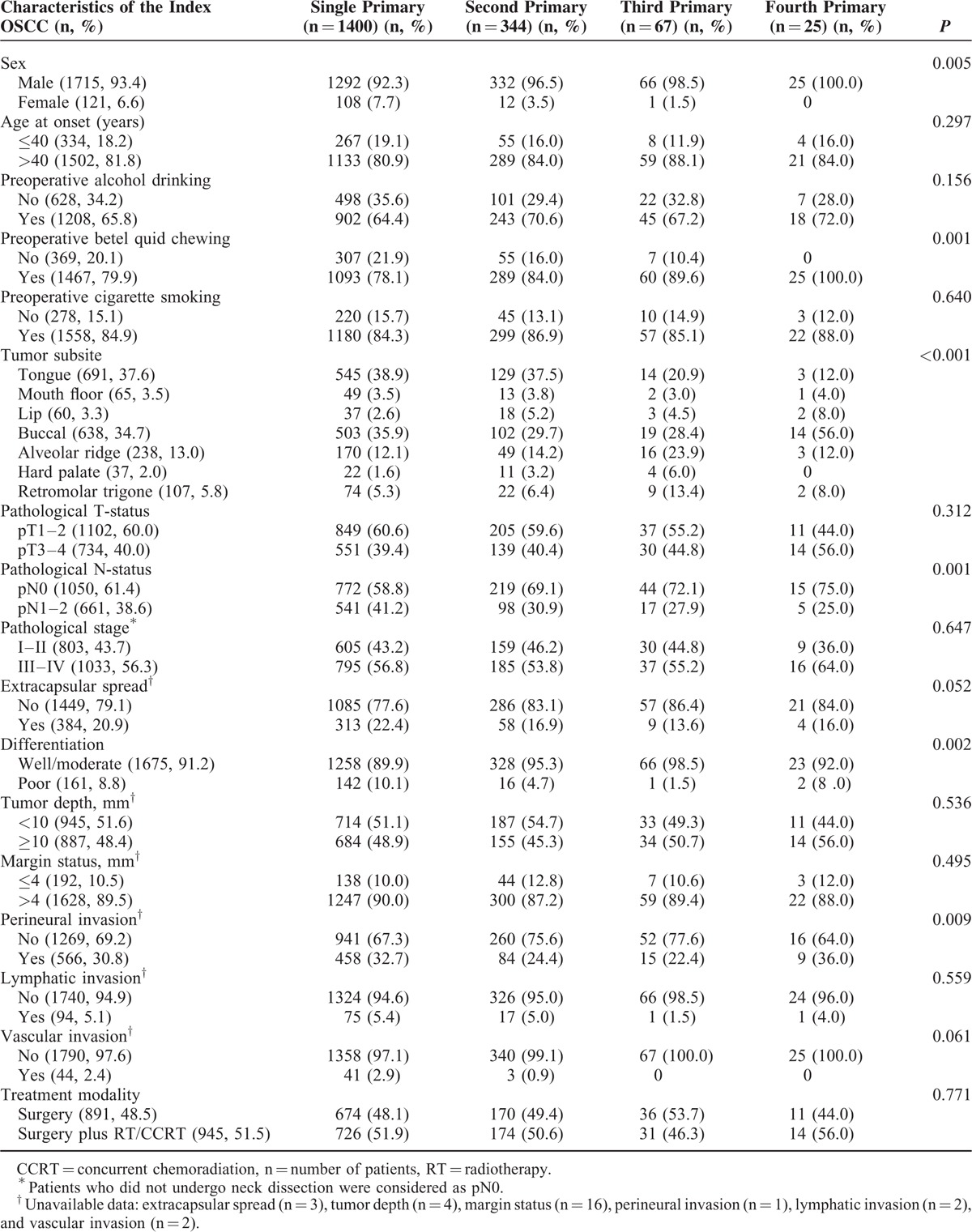
Of the 1836 patients, 1400 (76.3%) had a single PT, 344 (18.7%) a second PT, 67 (3.6%) a third PT, and 25 (1.4%) a fourth PT. The incidences of second, third, and fourth PTs for all patients (n = 1836) at 5 and 10 years were 20.2%/34.6%, 4.0%/8.6%, and 1.0%/2.3%, respectively (Figure 1A). The time intervals between the first and the second, the second and the third, and the third and the fourth PTs were 0 to 203 months (median: 36 months, mean: 47 months), 0 to 121 months (median: 26 months, mean: 28 months), and 0 to 82 months (median: 22 months, mean: 25 months), respectively. Table 2 summarizes the general characteristics of the study patients who presented with a single, second, third, and fourth PTs. Compared with other groups (single PT, second PT, or third PT), patients with a fourth PT were found to differ in terms of the following risk factors: sex (P = 0.005), preoperative betel quid chewing (P = 0.001), tumor subsite (P < 0.001), pathological N-status (P = 0.001), tumor differentiation (P = 0.002), and perineural invasion (P = 0.009).
FIGURE 1.
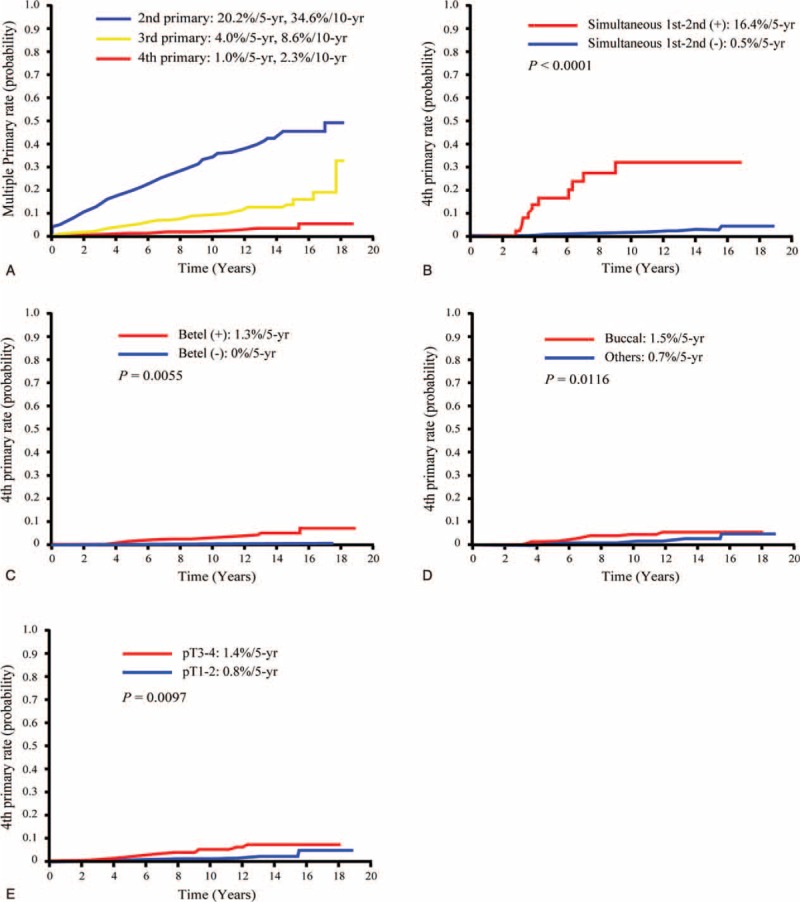
Kaplan–Meier plots of the incidence of second, third, and fourth primary tumors in OSCC patients (A), and 5-year incidence of fourth primary tumors in OSCC patients stratified according to the presence of simultaneous first and second primary tumors (B), betel quid chewing (C), buccal subsite (D), and pT status (E). OSCC = oral cavity squamous cell carcinoma.
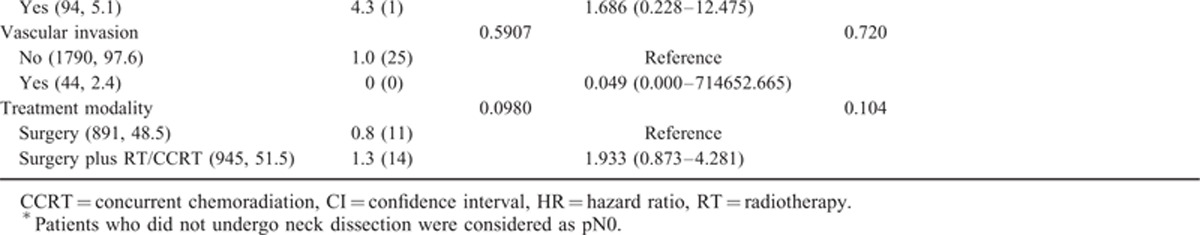
TABLE 1 (Continued).
Univariate and Multivariate Analyses of Factors Predicting a Fourth Primary Malignancy in OSCC Patients
Factors Associated With Fourth Primary Malignancies
The UVA (log-rank test) identified the following factors as significantly associated with the development of a fourth PT: simultaneous first and second PTs (P < 0.0001), betel quid chewing (P = 0.0055), buccal subsite (P = 0.0116), and pT3–4 status (P = 0.0097; Table 1 and Figure 1B–E). After allowance for the potential confounding effect of other risk factors, all of these variables retained their independent prognostic significance in stepwise MVA, the only exception being betel quid chewing (Table 1 ).
Factors Associated With Overall Survival
Univariate Cox regression analysis identified the following factors as significantly associated with OS: second PT (compared with a single PT; P = 0.024), age at onset >40 years (P = 0.008), preoperative alcohol drinking (P = 0.011), mouth floor subsite (as compared with tongue subsite; P = 0.019), buccal subsite (as compared with tongue subsite; P = 0.041), alveolar ridge subsite (as compared with tongue subsite; P = 0.001), hard palate subsite (as compared with tongue subsite; P < 0.001), retromolar subsite (as compared with tongue subsite; P = 0.044), pT3–4 tumor (P < 0.001), pN1–2 status (P < 0.001), pathological stage III–IV (P < 0.001), nodal extracapsular spread (P < 0.001), poor differentiation (P < 0.001), tumor depth ≥10 mm (P < 0.001), margin status ≤4 mm (P = 0.003), perineural invasion (P < 0.001), lymphatic invasion (P < 0.001), vascular invasion (P < 0.001), and surgery alone (P < 0.001). After allowance for potential confounders in MVA, the following factors were found to retain their independent prognostic significance: age at onset >40 years (P = 0.003), pT3–4 tumor (P < 0.001), pN1–2 status (P < 0.001), nodal extracapsular spread (P < 0.001), poor differentiation (P = 0.001), tumor depth ≥10 mm (P = 0.005), margin status ≤4 mm (P = 0.031), and lymphatic invasion (P = 0.003; Supplement Table).
Characteristics of OSCC Patients Who Developed Fourth Primary Malignancies
Table 3 depicts the characteristics of the 25 OSCC patients who developed fourth PTs. Notably, 3 of these 25 cases also developed a fifth PT. Among the OSCC patients who developed fourth PTs, we identified a total of 103 different primary cancers, as follows: 88 oral cavity tumors (location: tongue cancer, n = 22; alveolar ridge cancer, n = 21; buccal cancer, n = 20; lip cancer, n = 13; retromolar trigone cancer, n = 5; hard palate cancer, n = 4; and mouth floor cancer; n = 3); 11 oropharyngeal tumors (location: tongue base cancer, n = 4; soft palate cancer, n = 3; tonsil cancer, n = 3, and anterior pillar cancer, n = 1), 1 nasal cavity cancer, and 3 malignancies located outside the head and neck area (1 colorectal adenocarcinoma as a third PT, 1 inguinal sarcoma as a third PT, and 1 prostatic adenocarcinoma as a fourth PT). Fourteen of the 25 patients presented simultaneous PTs at different stages, as follows: 7 patients presented with simultaneous first and second PTs (patients #5, 9, 12, 13, 15, 18, and 25); 3 patients presented with simultaneous second and third PTs (patients #6, 14, and 17), 1 patient presented with simultaneous third and fourth PTs (patient #4), 1 patient presented with 3 simultaneous PTs (ie, simultaneous first, second, and third PTs; malignancies located at mouth floor, tongue, and lip; patient #20); and 2 patients presented with simultaneous primary/secondary and third/fourth PTs (patients #23 and 24). Therefore, a total of 10 patients had simultaneous first and second PTs (patients #5, 9, 12, 13, 15, 18, 25, 20, 23, and 24). Regarding the treatment modality offered to the 25 patients with fourth PTs, 12 (44%) were treated with surgery alone, 2 with surgery plus RT/concurrent chemoradiation (CCRT), 1 with surgery plus hormone therapy (for prostate cancer, patient #18), 6 with RT/CCRT, and 4 with best supportive care. Ten of the 25 patients underwent reirradiation (patients #2, 9, 10, 11, 13, 14, 16, 17, 18, and 24).
TABLE 2.
General Characteristics of OSCC Patients Who Presented With a Single, Second, Third, and Fourth Primary Malignancy
Survival of OSCC Patients Who Developed Fourth Primary Malignancies
The 5-year disease-free, disease-specific, and OS rates in the entire cohort were 73%, 81%, and 68%, respectively (Figure 2A). The 5, 10, 15-year OS rates (calculated from the date of surgery) for patients with single, second, third, and fourth PTs were 68%/61%/57%, 67%/47%/36%, 75%/57%/41%, and 87%/58%/32%, respectively (Figure 2B). At 5 years, patients with fourth PTs had acceptable OS rates. Patients who developed second, third, and fourth PTs showed a tendency toward lower OS rates after 10 years compared with those with a single primary malignancy. The 5 and 10-year OS rates (calculated from the diagnosis of single and multiple PTs) for patients with a single, second, third, and fourth PTs were 68%/61%, 43%/37%, 45%/39%, and 30%/30%, respectively (P < 0.0001; Figure 2C). Among patients with a fourth PT (n = 25), those who underwent radical surgical removal showed a significantly higher 3-year OS than those who did not (57% vs 13%, respectively; P = 0.0442; Figure 2D).
FIGURE 2.
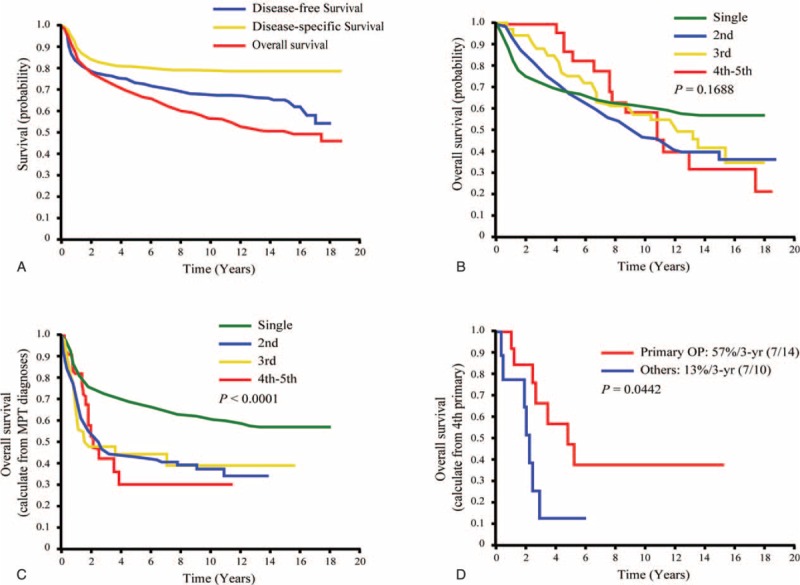
Kaplan–Meier plots of 5-year disease-free survival, disease-specific survival, and overall survival in all patients (A). Kaplan–Meier plots of 5-year overall survival in OSCC patients with a single, second, third, and fourth primary tumors calculated from date of treatment for the index OSCC (B), and from the data of diagnosis of each primary tumor (C). Kaplan–Meier plots of 5-year overall survival in OSCC patients with a fourth primary malignancy with and without treatment with radical surgery (D). OSCC = oral cavity squamous cell carcinoma.
DISCUSSION
Despite recent declines in the use of risky oral habits, the incidence of OSCC in Taiwan continues to increase.9,28 This paradoxical observation can be explained by the long-term (10–15 years) carcinogenic effects of betel quid chewing, either with or without the concomitant detrimental action of alcohol drinking and cigarette smoking.9 In the current study, we have shown that OSCC patients can continue to develop multiple subsequent PTs despite aggressive surgical and adjuvant treatment. All of our patients who developed a fourth PT had a history of betel quid chewing, although 99% of them quit chewing after their initial OSCC diagnosis. These findings are comparable with the results of Qaisi et al29 who showed that patients who quit smoking after treatment of a first primary malignancy continue to be at high risk of developing multiple PTs. Conversely, other studies demonstrated that avoidance of alcohol drinking and cigarette smoking can reduce the risk of developing a second PT in the head and neck region.30–33 Based on our observations, we believe that quitting betel quid chewing can be effective in the primary but not secondary prevention of oral cancers among subjects living in endemic areas.
The results of UVA (log-rank test) indicated that simultaneous first and second PTs, betel quid chewing, buccal subsite, and pT3–4 status were significantly associated with the development of a fourth PT in OSCC patients. After allowance for other potential confounding factors, all of the above-mentioned variables (the only exception being betel quid chewing) retained their independent prognostic significance in stepwise MVA. We have previously reported similar risk factors in studies focusing on the development of second PTs.9,27 Notably, the simultaneous presence of both a first and a second PT was the strongest predictor of a fourth PT (hazard ratio 19.046; Table 1 ). Consequently, such high-risk patients are ideal candidates for inclusion in future prospective multimodality clinical trials aimed at reducing the burden of multiple PTs.
In areas where betel quid chewing is not endemic, lower oral cavity subsites (ie, tongue, floor of mouth, and lower alveolar ridge) are the most common areas for the development of second PTs in patients with a history of heavy smoking.30 In the current study, the most common site of second PTs was the tongue (followed by the buccal mucosa and the alveolar ridge), whereas the buccal cavity was the main site of development for both third and fourth PTs (Table 2). Notably, 56% (n = 14) of all patients who developed a fourth PT had an index OSCC located in the buccal mucosa. In nonbetel-chewing areas, the lower alveolar ridge and the tongue are the most common sites from which multiple PTs originate.29 In contrast, the tongue (followed by the alveolar ridge, the buccal mucosa, and the lips) was the most common site of development of multiple PTs in the current study (Table 3). We believe that such differences may be at least in part explained by a specific effect of betel quid chewing.
Our current data are in line with previous studies conducted in patients with oral cavity cancer showing that most multiple PTs arise from the oral cavity, the oropharynx, and the nasal cavities.30–33 One intriguing finding of our study is the unexpectedly high 5-year OS rate in OSCC patients who developed a fourth PT, even though this observation cannot be easily explained. Our patients with fourth PTs tended to have a high pT status (pT3−4 tumors; Table 1 ) despite a low nodal status (pN0; Table 2). Of note, the outcomes of pT4N0 disease were in line with those previously observed in our study for patients with p-stage III disease.34 Here, the stage of the index OSCC was not a significant risk factor for the development of a fourth PT.30,35
In the current study, the incidence of second PTs was 3% per year, being in accordance with previously reported rates (1.5%−3.7%).30,31,36 However, we have shown for the first time that the development of a fourth PT in OSCC patients is a rare event (incidence of 2.3% at 10 years). Subsequent PTs have a detrimental prognostic impact,37, resulting in a 10% to 30% reduction of 5-year OS rates compared with patients who have a single malignancy.27,36,38,39 In the current study, we found that the presence of multiple PTs resulted in an approximately 25% reduction of 5-year OS compared with participants with a single neoplasm (Figure 2C). Leon et al38 have shown that survival decreased progressively with every new second PT after the initial index head and neck tumor (the majority of neoplasms [51.6%] arising from the larynx), with their 5-year survival rates after a first, second, third, and fourth malignancies being 67.6%, 56.1%, 45.0%, and 32.1%, respectively. In the present study, the observed 5-year OS rates of OSCC patients with a single, second, third, and fourth PTs were similar, being 68%, 43%, 45%, and 30%, respectively (Figure 2C), with the exception of lower OS rates after a second tumor and a loss of the progressively decreasing survival trend.38 The potential reasons that might explain the different clinical outcomes in our study as compared with that of Leon et al may reflect the different study populations. Specifically, the following points should be noted: betel quid chewing can play a role in tumor etiology in our current study, but not in the Leon et al's study; higher rates of second, third, and fourth PTs in our current study as compared with the Leon et al's study (18.7%, 3.6%, and 1.4% vs 8.1%, 1.4%, and 0.3%, respectively); higher prevalence of patients with oral cavity subsite in our current study as compared with the Leon et al's study (100% vs 11.6%, respectively); and higher rates of initial radical surgery with or without adjuvant therapy (100% vs 29.3%, respectively). Table 4 summarizes the available literature focusing on the outcomes of OSCC patients who developed third or fourth PTs.29,38,40,41
TABLE 3.
Clinicopathological Characteristics of OSCC Patients Who Developed a Fourth Primary Tumor
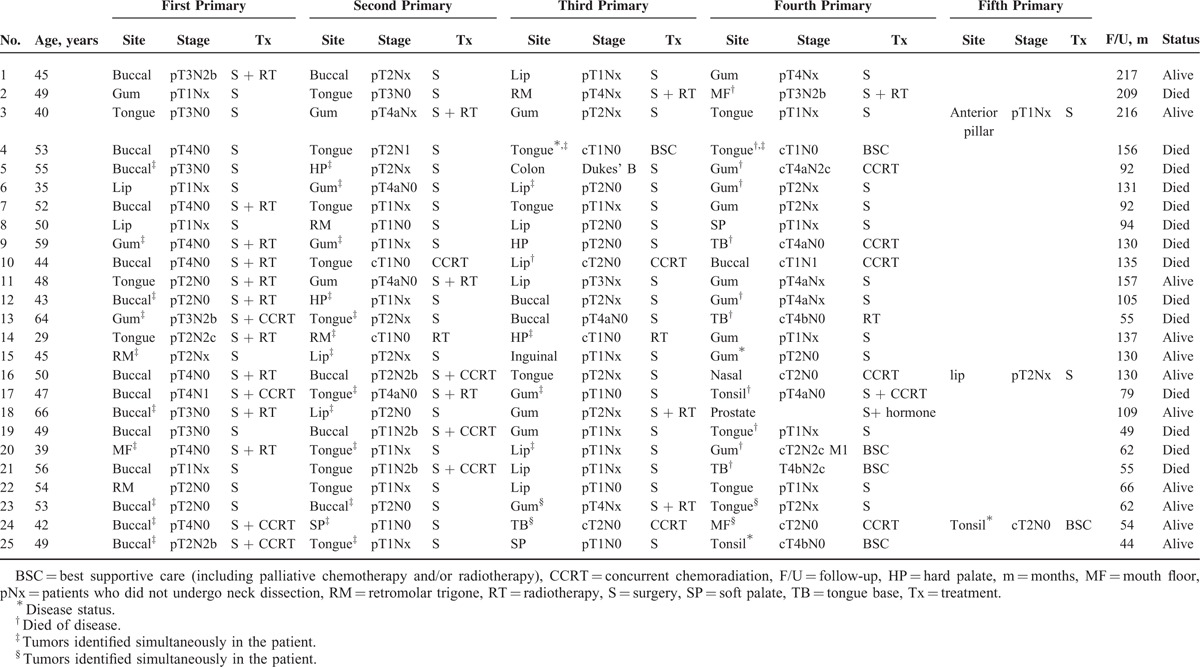
TABLE 4.
Comparison of Outcomes in OSCC Patients Presenting With Third and Fourth Primary Malignancies (Summary of Published Studies)
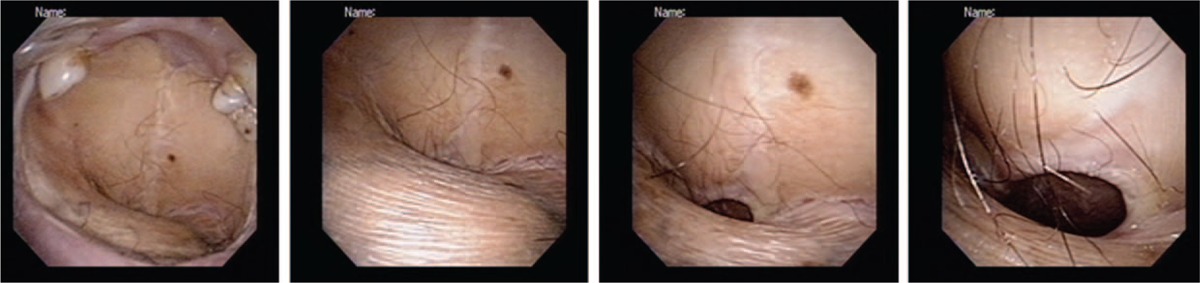
Surgery remains the mainstay of treatment for OSCC (both for the index malignancy and subsequent multiple PTs).42 Because multiple PTs are expected to portend a poor prognosis, a more aggressive surgical approach is warranted.43 In line with our previously described approach for second PTs,27 fourth and fifth PTs arising from the oral cavity/soft palate were treated as the first OSCC. In general, radical surgery aimed at obtaining adequate safety margins was the approach of choice for all operable patients (according to their performance status). Among the subgroup of patients who developed a fourth PT, subjects who underwent radical surgery showed a significantly higher 3-year OS than those who did not (57% vs 13%, respectively; P = 0.0442). Obviously, the surgical strategy needs to be carefully weighted in each patient against potential cosmetic and functional complications. For example, some patients in the current study underwent a total or subtotal replacement of the normal oral mucosa by multiple tissue flaps (used for reconstruction) after removal of each newly diagnosed PT. Figure 3 shows the current status of a patient (#14; Table 3) who underwent surgery from a fourth primary malignancy and 3 consecutive free flap reconstructions (after removal of tumors located in the central tongue, left retromolar trigone, hard palate, and right upper gum). In this specific case, the anterior part of the upper gum was the only portion of the native oral cavity that was actually preserved. Serial demolitions and reconstructions may significantly affect the patient's communication and swallowing abilities, ultimately requiring, in some cases, the insertion of a nasogastric tube or a permanent tracheostomy.
FIGURE 3.
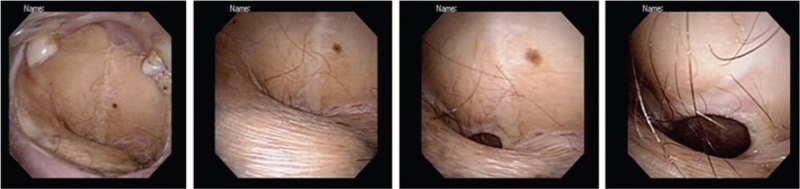
Postoperative images (from left to right: from the oral cavity to the oropharynx) of a representative OSCC patient who underwent 3 free flap reconstructions after removal of 4 primary malignancies (squamous cell carcinoma of the central tongue followed by squamous carcinomas of the retromolar trigone, hard palate, and upper gum). OSCC = oral cavity squamous cell carcinoma.
Three main limitations of our study need to be acknowledged. First, the single-center nature of our work and its retrospective design limit the generalizability of the findings. Nonetheless, this is the largest study to date focusing on OSCC patients who developed a fourth PT, a population for which available data remain scanty (Table 4). Second, all of the study patients were recruited in a betel quid-chewing endemic area. Consequently, the extent to which our data are applicable to other geographic locations remains unknown. Finally, this study was specifically focused on OSCC patients who developed multiple PTs in a betel-chewing endemic area. Consequently, we should keep in mind that the predictors of long-term OS rates identified in the current study may differ from those identified in OSCC patients in general. Consistent with the current study, we have previously shown a univariate association between alcohol drinking and survival.44 Betel quid chewing has been previously associated with poor 5-year disease-free and disease-specific survival rates.9 Similarly, an univariate association with OS was observed in this study. Future ad hoc studies should specifically compare the prognostic factors for OSCC in general with those OSCC patients who developed multiple PTs.
In summary, the results of our study indicate that fourth PTs are rarely observed in OSCC patients living in a betel quid-chewing endemic area. Long-term survival rates of patients treated with radical surgery seems acceptable, being 4-fold higher than their counterpart.
Supplementary Material
Acknowledgments
We appreciate the contribution and the valuable assistance of the Linkou Chang Gung Memorial Hospital Cancer Center databank and case managers.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CCRT = concurrent chemoradiation, MVA = multivariate analysis, OS = overall survival, OSCC = oral cavity squamous cell carcinoma, PTs = primary tumors, RT = radiotherapy, UVA = univariate analysis.
MA and C-TL contributed equally to this work
Disclosure: This study did not receive any specific funding. The authors report no conflicts of interest.
REFERENCES
- 1.Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007; 12:20–37. [DOI] [PubMed] [Google Scholar]
- 2.Coyte A, Morrison DS, McLoone P. Second primary cancer risk: the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer 2014; 14:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris LG, Sikora AG, Patel SG, et al. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol 2011; 29:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin K, Patel SG, Chu PY, et al. Second primary malignancy of the aerodigestive tract in patients treated for cancer of the oral cavity and larynx. Head Neck 2005; 27:1042–1048. [DOI] [PubMed] [Google Scholar]
- 5.León X, Quer M, Diez S, et al. Second neoplasm in patients with head and neck cancer. Head Neck 1999; 21:204–210. [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Roh JL, Baek S, et al. Second cancer incidence, risk factor, and specific mortality in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 2013; 149:579–586. [DOI] [PubMed] [Google Scholar]
- 7.Jones AS, Morar P, Phillips DE, et al. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer 1995; 75:1343–1353. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer 1953; 6:963–968. [DOI] [PubMed] [Google Scholar]
- 9.Liao CT, Wallace CG, Lee LY, et al. Clinical evidence of field cancerization in patients with oral cavity cancer in a betel quid chewing area. Oral Oncol 2014; 50:721–731. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal G, Jaiswal S, Kumar R, et al. Field cancerization: concept and clinical implications in head and neck squamous cell carcinoma. J Exp Ther Oncol 2013; 10:209–214. [PubMed] [Google Scholar]
- 11.Braakhuis BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res 2003; 63:1727–1730. [PubMed] [Google Scholar]
- 12.Winstock A. Areca nut-abuse liability, dependence and public health. Addict Biol 2002; 7:133–138. [DOI] [PubMed] [Google Scholar]
- 13.Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol 2001; 37:477–492. [DOI] [PubMed] [Google Scholar]
- 14.Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum 2004; 85:1–334. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C. An epidemiological study of oral squamous cell carcinoma in southern Taiwan. J Formosan Dent Assoc 1987; 10:268–274. [Google Scholar]
- 16.Guh JY, Chen HC, Tsai JF, et al. Betel-quid use is associated with heart disease in women. Am J Clin Nutr 2007; 85:1229–1235. [DOI] [PubMed] [Google Scholar]
- 17.Chang KM. Betel nut chewing and mouth cancer in Taiwan. 2. Observation of the oral mucosa in the betel nut chewer. Taiwan Yi Xue Hui Za Zhi 1966; 65:79–85. [PubMed] [Google Scholar]
- 18.Secretan B, Straif K, Baan R, et al. A review of human carcinogens: part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009; 10:1033–1034. [DOI] [PubMed] [Google Scholar]
- 19.Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol 2002; 7:115–125. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Reichart PA. A review of betel quid chewing, oral cancer and precancer in Mainland China. Oral Oncol 2007; 43:424–430. [DOI] [PubMed] [Google Scholar]
- 21.Cox SC, Walker DM. Oral submucous fibrosis. A review. Aust Dent J 1996; 41:294–299. [DOI] [PubMed] [Google Scholar]
- 22.Jeng JH, Wang YJ, Chiang BL, et al. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis 2003; 24:1301–1315. [DOI] [PubMed] [Google Scholar]
- 23.Wang YC, Tsai YS, Huang JL, et al. Arecoline arrests cells at prometaphase by deregulating mitotic spindle assembly and spindle assembly checkpoint: implication for carcinogenesis. Oral Oncol 2010; 46:255–262. [DOI] [PubMed] [Google Scholar]
- 24.Guo SE, Huang TJ, Huang JC, et al. Alcohol, betel-nut and cigarette consumption are negatively associated with health promoting behaviors in Taiwan: a cross-sectional study. BMC Public Health 2013; 13:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren SGO. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer 1936; 16:1358–1414. [Google Scholar]
- 26.Edge SB BD, Compton CC, Fritz AG, et al. AJCC Cancer Staging Manual. 7th edNew York: Springer-Verlag; 2010. [Google Scholar]
- 27.Liao CT, Kang CJ, Chang JT, et al. Survival of second and multiple primary tumors in patients with oral cavity squamous cell carcinoma in the betel quid chewing area. Oral Oncol 2007; 43:811–819. [DOI] [PubMed] [Google Scholar]
- 28.Cancer registry annual report, Taiwan, 2011. http://www.bhp.doh.gov.tw/ Accessed September 29, 2015. [Google Scholar]
- 29.Qaisi M, Vorrasi J, Lubek J, et al. Multiple primary squamous cell carcinomas of the oral cavity. J Oral Maxillofac Surg 2014; 72:1511–1516. [DOI] [PubMed] [Google Scholar]
- 30.Cianfriglia F, Di Gregorio DA, Manieri A. Multiple primary tumours in patients with oral squamous cell carcinoma. Oral Oncol 1999; 35:157–163. [DOI] [PubMed] [Google Scholar]
- 31.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer 1992; 70:14–19. [DOI] [PubMed] [Google Scholar]
- 32.de Vries N, Van der Waal I, Snow GB. Multiple primary tumours in oral cancer. Int J Oral Maxillofac Surg 1986; 15:85–87. [DOI] [PubMed] [Google Scholar]
- 33.Shikhani AH, Matanoski GM, Jones MM, et al. Multiple primary malignancies in head and neck cancer. Arch Otolaryngol Head Neck Surg 1986; 112:1172–1729. [DOI] [PubMed] [Google Scholar]
- 34.Liao CT, Chang JT, Wang HM, et al. Survival in squamous cell carcinoma of the oral cavity: differences between pT4N0 and other stage IVA categories. Cancer 2007; 110:564–571. [DOI] [PubMed] [Google Scholar]
- 35.Licciardello JT, Spitz MR, Hong WK. Multiple primary cancer in patients with cancer of the head and neck: second cancer of the head and neck, esophagus, and lung. Int J Radiat Oncol Biol Phys 1989; 17:467–476. [DOI] [PubMed] [Google Scholar]
- 36.Tepperman BS, Fitzpatrick PJ. Second respiratory and upper digestive tract cancers after oral cancer. Lancet 1981; 2:547–549. [DOI] [PubMed] [Google Scholar]
- 37.Robinson E, Neugut AI, Murray T, et al. A comparison of the clinical characteristics of first and second primary head and neck cancers. A population-based study. Cancer 1991; 68:189–192. [DOI] [PubMed] [Google Scholar]
- 38.Leon X, Martinez V, Lopez M, et al. Second, third, and fourth head and neck tumors. A progressive decrease in survival. Head Neck 2012; 34:1716–1719. [DOI] [PubMed] [Google Scholar]
- 39.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancer: patients’ characteristics and survival patterns. Eur J Cancer B Oral Oncol 1994; 30B:381–386. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki Y, Harada H, Ikuta M, et al. Clinical characteristics of multiple primary carcinomas of the oral cavity. Oral Oncol 2015; 51:182–189. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Takahashi H, Yao K, et al. Multiple primary malignancies in the head and neck: a clinical review of 121 patients. Acta Otolaryngol Suppl 2002; 547:88–92. [DOI] [PubMed] [Google Scholar]
- 42.Shah JP, Gil Z. Current concepts in management of oral cancer: surgery. Oral Oncol 2009; 45:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson E, Neugut AI. Clinical aspects of multiple primary neoplasms. Cancer Detect Prev 1989; 13:287–292. [PubMed] [Google Scholar]
- 44.Liao CT, Chang JT, Wang HM, et al. Analysis of risk factors predictive of local tumor control in oral cavity cancer. Ann Surg Oncol 2008; 15:915–922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


