Abstract
Acute kidney injury (AKI) and atrial fibrillation (AF) after cardiac surgery are common occurrences and increase patient morbidity and mortality. Inflammation plays a role in increased incidence of AF in patients with chronic kidney disease (CKD); reactive oxygen species and inflammatory markers which are increased in patients with CKD were found to affect the proper functioning of the intracellular ion channels, connexions (transmembrane proteins found in intercellular gap junctions), and electrical homogeneity of the extracellular matrix which are essential for electrical stability and proper conduction of electrical impulses in the atrium. However, it is not known if similar mechanisms are also involved in AKI. We tested the hypothesis that patients with AKI after cardiac surgery have a higher incidence of postoperative AF.
Data from 2885 patients, who had undergone cardiac surgery between August 2008 and July 2012 from the Singapore's 2 major heart centers, were obtained prospectively. Postoperative AKI was defined using the Acute Kidney Injury Network criteria. The primary outcome was postoperative AF, and subjects were considered to have postoperative AF if the AF lasted more than an hour, affected hemodynamics, or required medical treatment.
The incidence of AKI was 29.7% and the incidence of postoperative AF was 16.8%. A total of 27.7% of AKI patients developed AF. Patients with AKI had a 2-fold increased risk of developing AF (relative risk [RR], 1.716; 95% confidence interval [CI], 1.433–2.055; P < 0.001). The following factors were found to independently increase the risk of AF in patients with AKI: age (RR, 1.011; 95% CI, 1.000–1.022; P = 0.04), low preoperative hemoglobin (RR, 0.942; 95% CI, 0.888–1.000; P = 0.05), low preoperative estimated glomerular filtration rate (eGFR) (RR, 0.987; 95% CI, 0.980–0.994; P < 0.001), and lowest hematocrit during bypass (RR, 0.943; 95% CI, 0.910–0.978; P < 0.001).
Patients with AKI are more likely to develop postoperative AF. These patients were older and had lower preoperative hemoglobin, eGFR, and lower nadir hematocrit during bypass. Identification of high-risk AKI patients with early prevention and treatment of AF should reduce the long-term morbidity and mortality among Asian patients undergoing cardiac surgery.
INTRODUCTION
Acute kidney injury (AKI) and atrial fibrillation (AF) after cardiac surgery are common occurrences and increase patient morbidity and mortality.1–3 AF is the most common arrhythmia after cardiac surgery and can occur in patients with or without pre-existing AF.2 Cardiac comorbidities associated with AF include hypertension, coronary artery disease, valvular heart disease, congestive heart failure, and cardiomyopathy which are common in the cardiac surgical population. Evidence suggests that inflammation is involved in the pathogenesis of AF with several studies demonstrating elevated serum C-reactive protein levels in these patients.4 Cardiac surgery elicits an exaggerated inflammatory response with proinflammatory cytokine and complement activation. Although this can worsen pre-existing AF, it can also lead to postoperative AF in patients undergoing cardiac surgery.
Inflammation is also associated with renal dysfunction.5,6 Proposed mechanisms include decreased proinflammatory cytokine clearance, endotoxemia, oxidative stress, and reduced antioxidant levels. Reactive oxygen species and inflammatory markers which are increased in patients with chronic kidney disease (CKD) were found to affect the proper functioning of the intracellular ion channels, connexions (transmembrane proteins found in intercellular gap junctions), and electrical homogeneity of the extracellular matrix which are essential for electrical stability and proper conduct of electrical impulses in the atrium.7 CKD promotes inflammation and this in turn may lead to an increased incidence of AF in these patients.8
Although it has been shown that end-stage renal disease patients on hemodialysis and patients with CKD have an increased prevalence of AF,8 the incidence of postoperative AF in patients with AKI after cardiac surgery is unclear. The intense inflammatory reaction associated with cardiopulmonary bypass (CPB) may cause AKI in a patient with or without pre-existing renal dysfunction, and this in turn may increase the incidence of postoperative AF in these patients. This study thus aimed to test the hypothesis that patients with AKI after cardiac surgery have a higher incidence of postoperative AF.
METHODS
Study Design, Database, and Population Selection
With institutional review board approval, we prospectively recruited 2885 patients who underwent cardiac surgery at Singapore's 2 heart centers from August 2008 to July 2012. All patients provided written informed consent.
All perioperative data were collected prospectively and collated in a central database. Demographic variables were selected on the basis of previously reported risk factors for AF and AKI. These included age, height, weight, sex, history of diabetes, hypertension, and pre-existing AF. Procedural variables were recorded, including duration of CPB, aortic cross-clamp time, and need for inotropic or intraaortic balloon counterpulsation therapy after separation from CPB. Postoperative outcomes including AKI, AF, length of stay, and inpatient mortality were also recorded.
Exclusion criteria included the following: patients who were on preoperative dialysis and/or had other postoperative arrhythmias other than AF.
Perioperative Anesthesia and Surgical and Perfusion Management
Anesthesia was typically induced with common induction agents (etomidate or propofol) and maintained with a balanced anesthesia regime of low-dose fentanyl (10–20 μg/kg) and volatile agents (primarily sevoflurane). Conventional CPB circuits with roller pumps, membrane oxygenators, heat exchangers, venous reservoirs, cardiotomy suction, and arterial blood filters were used. The volume of prime used in the bypass circuits were between 1300 and 1400 mL. The perfusion targets were mild-to-moderate hypothermia (32–35 °C), hematocrit levels of >21%, an activated clotting time of >400 seconds, a glucose level of <10 mmol/L, a nonpulsatile flow rate of 2.2 to 2.4 L/min/m2, and mean arterial pressure of 50 to 70 mm Hg. Myocardial protection was achieved with cold blood cardioplegia. Aprotinin was not used in any of the patients.
Renal Function Assessment and Perioperative Renal data
The preoperative serum creatinine value used was obtained closest to surgery, typically at preadmission testing within 1 week of surgery. If a material change had occurred in the patient's condition, the serum creatinine measurement would be repeated the day before surgery, and that value would be taken as the preoperative serum creatinine. The peak serum creatinine was the highest creatinine value obtained within the 1st 48 hours postoperatively. Acute Kidney Injury Network (AKIN) criteria was used as the primary outcome, defined as an absolute increase in serum creatinine of ≥26.4 μmol/L, and/or a ≥50% increase in the serum creatinine to the peak postoperative from the preoperative serum creatinine. The estimated glomerular filtration rate (eGFR) was calculated via the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which has been previously validated in the local population.9
Assessment of Atrial Fibrillation and Arrhythmias
Postoperative AF was defined as AF in the postoperative period after cardiac surgery and before hospital discharge that lasted more than an hour, affected hemodynamics, or required medical treatment. Patients were on continuous telemetry for at least 72 hours while in the intensive care or high dependency units. Subsequently, patients had daily 12-lead electrocardiogram and as required, when symptomatic. Diagnosis of AF was made by clinicians managing patients in the postoperative period according to institutional protocol.
Statistical Analysis
Population demographics, medical history, preoperative risk assessment, intraoperative variables, and postoperative outcomes were analyzed descriptively. To identify if AKI was an independent risk factor for postoperative AF, univariate analysis followed by multivariate analysis was done. For comparison of data between the AF patients with AKI and no AKI, univariate analysis was performed using a 2-tailed unpaired t-test for numerical factors and Chi-square test for categorical factors. Clinically and statistically significant univariate factors were then added into the multivariate Poisson regression model to determine the independent risk factors which predispose AKI patients to develop AF, as compared to non-AKI patients who develop AF. The statistical significance of variables was taken as P < 0.05. All statistical analyses were performed using IBM SPSS, version 22.0 (IBM, Armonk, NY).
RESULTS
A total of 2885 patients who underwent cardiac surgery in the 2 heart centers were enrolled in our study during this period. Of these, 313 patients were excluded as 10 of them were on regular dialysis and 303 of the patients had other postoperative arrhythmias other than AF. Therefore, there were 2572 patients who met our inclusion and exclusion criteria. Of the 2572, 432 (16.8%) and 763 (29.7%) patients developed postoperative AF and AKI, respectively (Figure 1).
FIGURE 1.
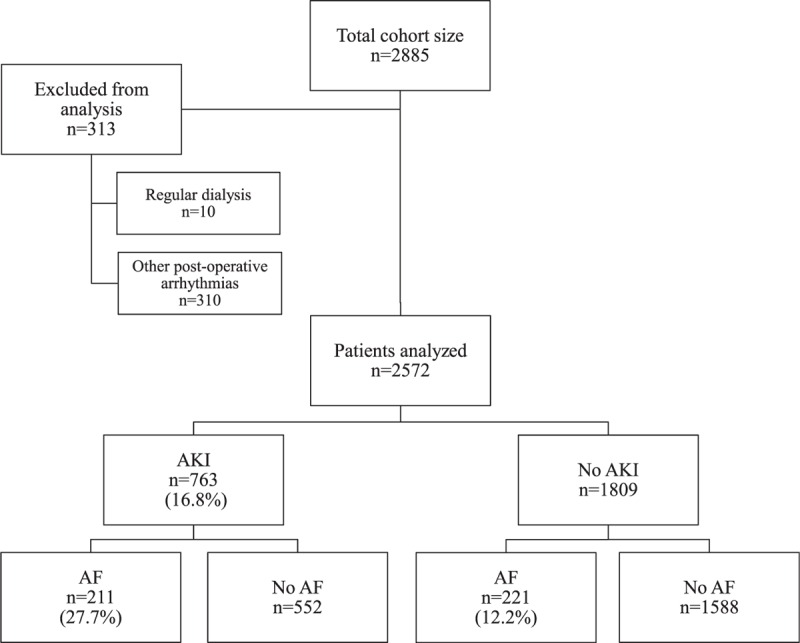
Flowchart from recruitment to outcomes. AF = atrial fibrillation; AKI = acute kidney injury.
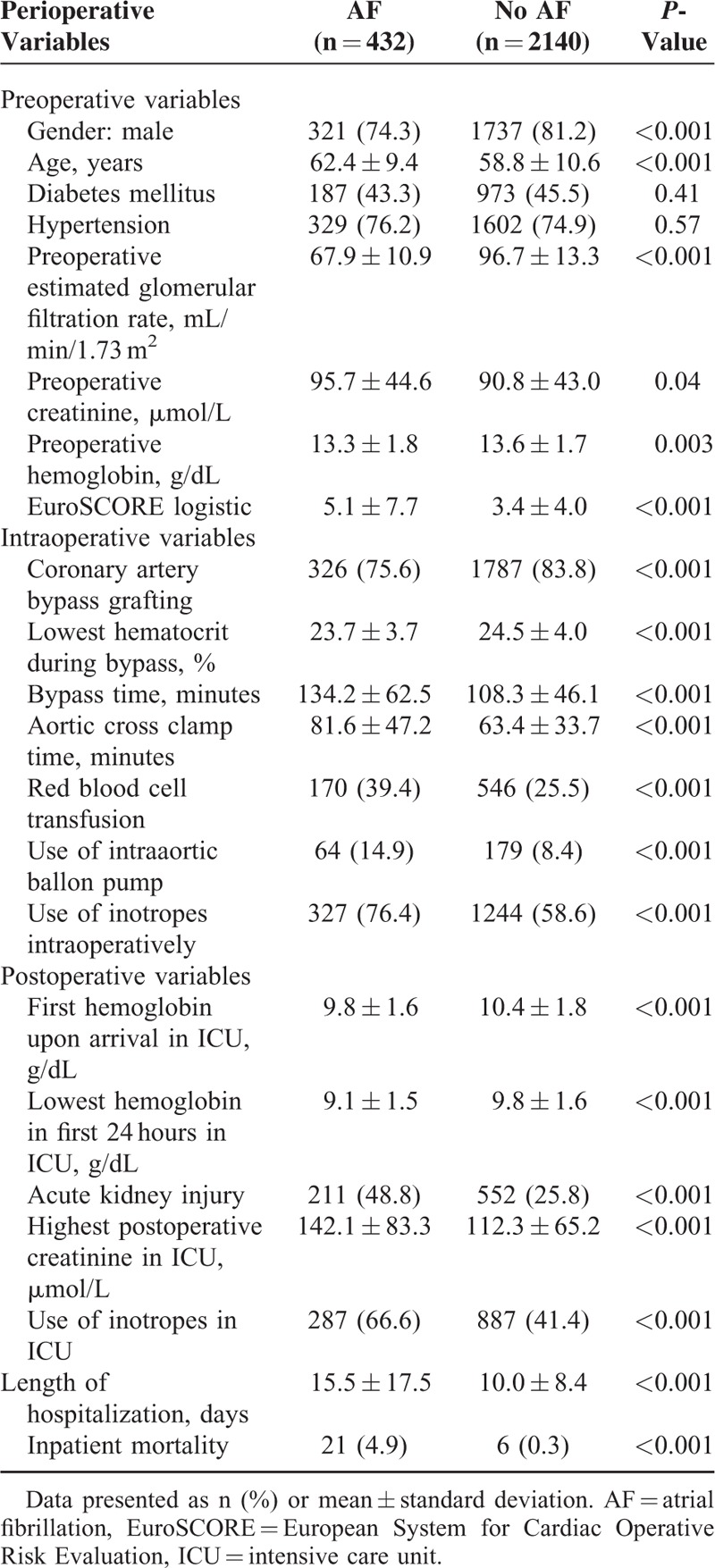
Patients who developed postoperative AF were more likely to be females, older, have a longer bypass time and had the use of inotropes intraoperatively (Table 1).
TABLE 1.
Univariate Analysis of Perioperative Variables and Atrial Fibrillation After Cardiac Surgery for the Whole Cohort
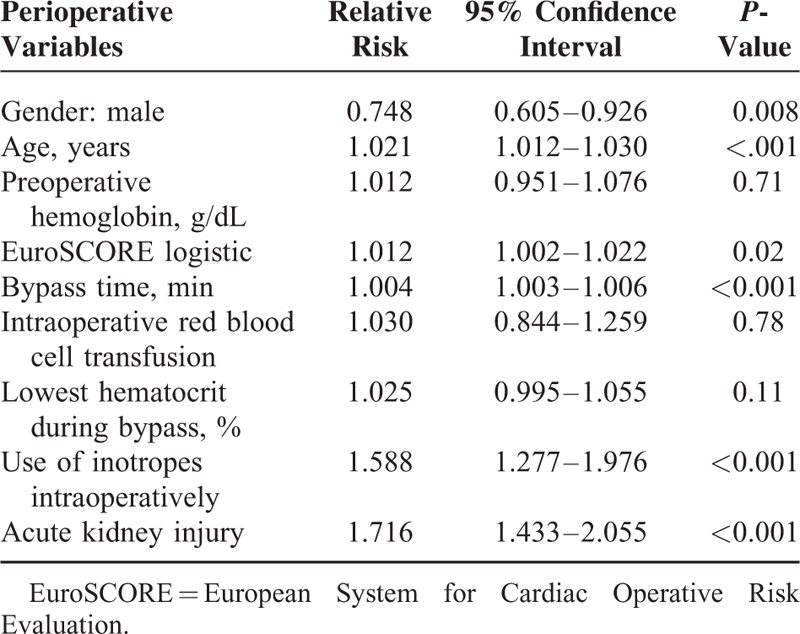
Postoperative AKI was independently associated with postoperative AF (Table 2).
TABLE 2.
Multivariate Analysis of Perioperative Variables and Atrial Fibrillation After Cardiac Surgery for the Whole Cohort
Of the 763 patients who developed AKI, 211 (27.7%) had postoperative AF, while only 221(12.2%) of the 1809 patients without AKI had postoperative AF. Patients with AKI were about twice as likely to develop AF (P < 0.001) (Figure 1).
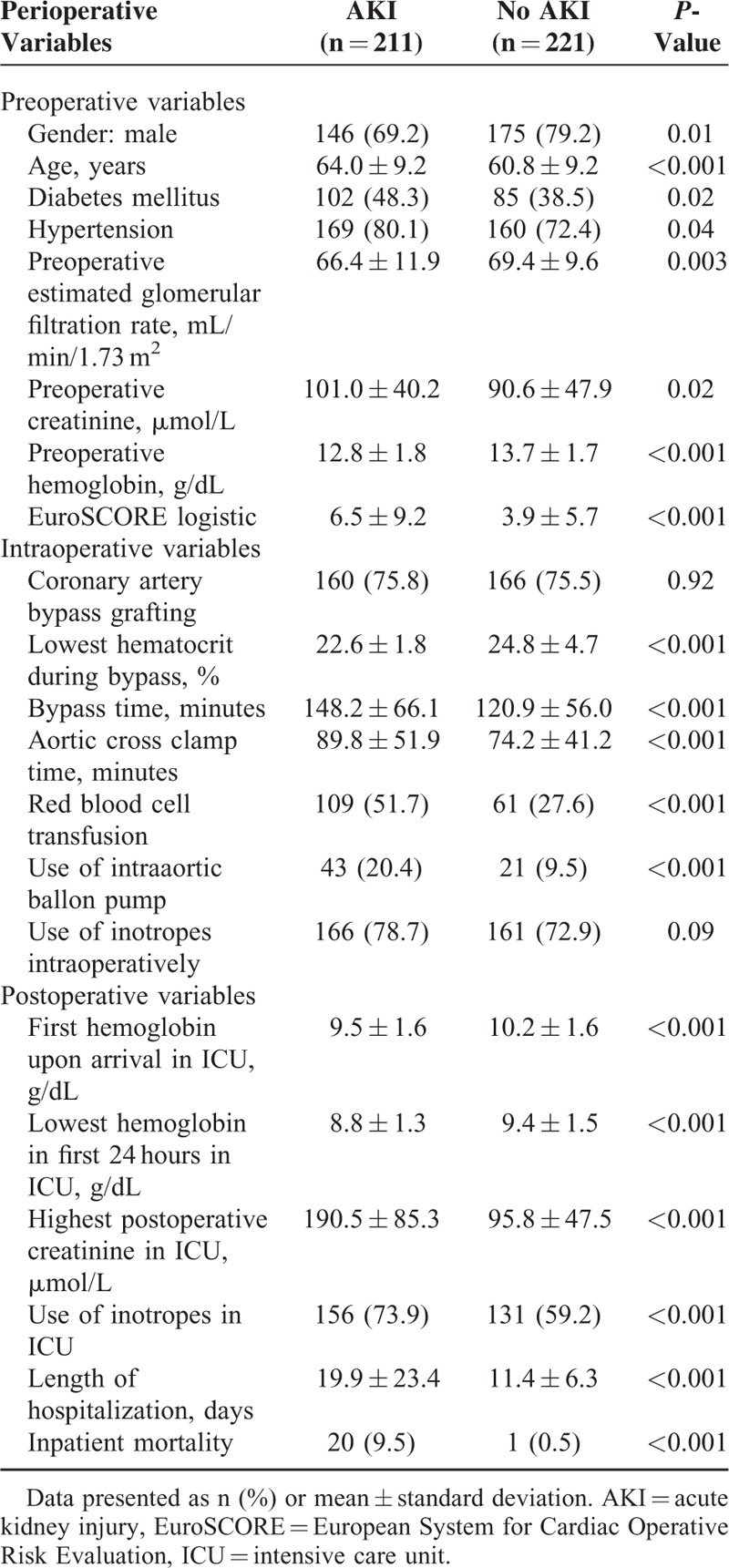
Patients with AKI who developed AF more likely to be females, older, diabetic, hypertensive, have a history of myocardial infarction and/or congestive heart failure, lower eGFR, and higher EuroSCORE. Intra-operatively, they were also more likely to have a lower nadir hematocrit on bypass, longer bypass time, required intraaortic balloon pump, and increased need for transfusion (Table 3).
TABLE 3.
Univariate Analysis of Perioperative Variables for Patients With Atrial Fibrillation After Cardiac Surgery
Multivariate analysis demonstrated that age, preoperative anemia, preoperative eGFR, and lowest hematocrit during bypass were independently associated with AKI and AF (Table 4).
TABLE 4.
Multivariate Analysis of Perioperative Variables and Acute Kidney Injury and Atrial Fibrillation After Cardiac Surgery
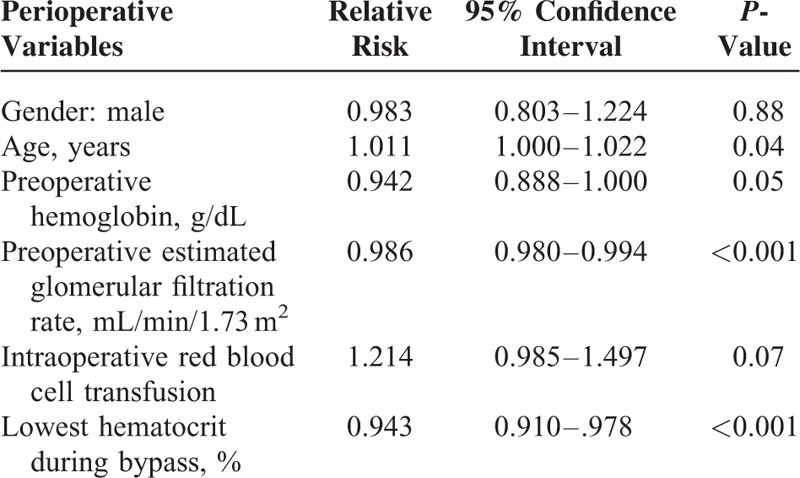
The length of hospitalization and mortality was significantly longer and higher, respectively, for patients who had AKI and AF, as compared to those who did not develop AKI but developed AF (Table 3).
DISCUSSION
We observe that postoperative AKI and AF were common occurrences after cardiac surgery in our cohort, with an incidence of 29.7% and 16.8%, respectively. Patients with AKI had a 2-fold increase risk of postoperative AF compared to patients without AKI. Furthermore, patients with AKI who developed postoperative AF not only have a longer length of stay but also have a 9-fold increase in mortality compared to patients who did not develop AKI.
Increasing age, anemia, and a low eGFR independently increase the risk of postoperative AF in patients with AKI in our cohort.
Age, Renal Impairment, and Postoperative AKI With AF
Our finding of age-related association between AKI and AF corroborates with previous studies and is consistent with a role for a common age-related pathophysiology.1 Age is a known risk factor for both AKI and AF.1 In our cohort, the mean age of patients who developed AKI and AF was 64.0 ± 9.2 years as compared to 60.8 ± 9.2 years for patients who did not develop AKI but developed AF (P < 0.001). With age, there is a physiologic decline of creatinine clearance of 0.75 mL/min/year.10 The aging kidney is associated with increased numbers of sclerotic glomeruli and interstitial fibrosis.11 Patients undergoing cardiac surgery may already have impaired renal function either as a result of the aging process or there could have superimposed CKD. As expected, the mean creatinine of patients who developed postoperative AKI was 101.0 μmol/L compared to the mean of 90.6 μmol/L in patients who did not develop AKI (P = 0.02).
Previous studies showed that patients with CKD and end-stage renal disease have an increased risk of AF, and this is partially explained by the fact that both conditions share a number of risk factors.8 It is also known that patients with CKD are at risk of developing AKI after cardiac surgery and the association with AF.
Cardiac surgery in itself is also associated with AF in patients with or without pre-existing AF. Cardiac comorbidities associated with AF include hypertension, coronary artery disease, valvular heart disease, congestive heart failure, and cardiomyopathy which are common in our cohort. Evidence suggests that inflammation is involved in the pathogenesis of AF with several studies demonstrating elevated serum C-reactive protein levels in patients with AF.4 The role of the inflammatory response in AF is also well documented in conditions such as myocarditis and pericarditis.12,13 Cardiac surgery elicits an exaggerated inflammatory response with proinflammatory cytokine and complement activation which can worsen pre-existing AF, or lead to new-onset AF.
Furthermore, inflammation is also associated with renal dysfunction.5,6 Proposed mechanisms include decreased proinflammatory cytokine clearance, endotoxemia, oxidative stress, and reduced antioxidant levels. Reactive oxygen species (ROS) and inflammatory markers which are increased in patients with CKD were found to affect the proper functioning of the intracellular ion channels, connexions (transmembrane proteins found in intercellular gap junctions), and electrical homogeneity of the extracellular matrix which are essential for electrical stability and proper conduct of electrical impulses in the atrium.7 The aging process is associated with dysregulation of the inflammatory process and increase in baseline cytokine levels.6 The peak incidence of AKI and AF occurring on the second and third postoperative day coincides with peak rise of inflammatory markers such as C-reactive protein suggests that inflammation is a common pathway in which AKI and AF are related. In particular, the older patient and those with CKD are vulnerable to AKI possibly due to the exaggerated inflammatory response to CPB and it is not inconceivable that the same exaggerated inflammatory process predisposes these patients to increased risk of postoperative AF.
Atheroembolism could be another mechanism to explain the risk for AF in AKI patients.1 Hemodynamic instability and emboli dislodgement resulting from AF could cause or accentuate acute damage to the kidneys. The stress response of surgery could precipitate paroxysmal AF in the susceptible patient. However, the temporal sequence of this etiology would require that AF precedes that of AKI but the close temporal relationship between the onset of AKI and AF, both peaking with 48 hours makes this a less plausible explanation.
Anemia and Postoperative AKI With AF
Patients with AKI who developed AF had a mean preoperative hemoglobin level of 12.8 g/dL as compared to 13.7 g/dL in those who did not develop AKI after surgery. Anemia contributes to AKI by reduction of renal oxygen delivery and impaired hemostasis.14 The lowest hematocrit is a reliable indicator of the degree of hemodilution. The kidneys are vulnerable to changes in renal blood flow because their metabolism is flow dependent.15 On the one hand, hemodilution increases kidney perfusion but also causes an increase in energy requirement of the kidneys due to increased tubular transport. This coupled with the decreased oxygen delivery of hemodilution and the physiologic hypoxic environment of the renal medulla can precipitate AKI especially in the oxygen sensitive renal medulla.16 Interestingly, it has also been shown that anemia is an independent predictor of mortality in patients with AF.17 Several mechanisms may explain this association. Anemia, resulting in tissue hypoxia, may impair diastolic function of the heart. In the absence of systolic dysfunction, diastolic dysfunction can present at an early stage of myocardial ischemia, and this corroborates with findings which show that the diastolic heart function is more susceptible to ischemia.18 In order for the body to adapt to an anemic state, the additional sympathetic and inotropic stress on the myocardium may lead to myocytes and vasculature remodeling.18 The hormonal and metabolic effects of anemia may also result in direct cardiotoxicity and myocardial ischemia.18
Although the univariate analysis showed that patients with AKI and AF have a higher blood transfusion rate compared to those without AKI and AF, this was not evident on multivariate analysis. This suggests that despite the inflammatory process associated with blood transfusion which may be detrimental, the defense of an adequate hemoglobin is far more important.
Outcomes With AKI and AF
Similar to other studies,19,20 patients who developed postoperative AKI and AF have poorer outcomes in our cohort. Patients with postoperative AKI and AF stayed about 8.5 days more on the average compared to those who did not develop AKI. This increases healthcare costs and utilization of healthcare resources.
Patients with postoperative AKI and AF also had a 21-fold increase in inpatient mortality as compared to their counterparts who developed postoperative AF only.
Strengths and Limitations
The strengths of this study included the prospective nature of the study with clearly defined data points and parameters for analysis. In addition, the study was protocol-driven, ensuring uniform practice among anesthetists and surgeons, thus affirming the reliability of the data collected.
Nevertheless, we acknowledge some limitations. The serum creatinine was used to assess renal function but is not reflective of renal damage. However, this reflects the clinical practice and the use of the AKIN which is based on serum creatinine is well established in AKI and other major adverse outcomes after cardiac surgery.21,22 The measurement of inflammatory markers would also enable the elucidation of the pathophysiology of the 2 conditions.
CONCLUSION
In conclusion, patients who developed AKI have a 2-fold increased risk of developing AF after cardiac surgery. They also have a 21-fold increase in inpatient mortality. Coupled with the identification and prevention of AKI, monitoring and prevention, or treatment of AF in a high-risk patient with AKI is key to significantly reduce morbidity and mortality.
Footnotes
Abbreviations: AF = atrial fibrillation, AKI = acute kidney injury, CKD = chronic kidney disease, CPB = cardiopulmonary bypass.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Albahrani MJ, Swaminathan M, Phillips-Bute B, et al. Postcardiac surgery complications: association of acute renal dysfunction and atrial fibrillation. Anesth Analg 2003; 96:637–643. [DOI] [PubMed] [Google Scholar]
- 2.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization – MultiCenter Study of Perioperative Ischemia Research Group. JAMA 1996; 276:300–306. [PubMed] [Google Scholar]
- 3.Auer J, Lamm G, Weber T, et al. Renal function is associated with risk of atrial fibrillation after cardiac surgery. Can J Cardiol 2007; 23:859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003; 107:87–92. [DOI] [PubMed] [Google Scholar]
- 6.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol 2004; 15:3184–3191. [DOI] [PubMed] [Google Scholar]
- 7.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med 2011; 17:556–563. [DOI] [PubMed] [Google Scholar]
- 8.Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 2010; 159:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teo BW, Xu H, Wang D, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis 2011; 58:56–63. [DOI] [PubMed] [Google Scholar]
- 10.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–285. [DOI] [PubMed] [Google Scholar]
- 11.Neugarten J, Gallo G, Silbiger S, et al. Glomerulosclerosis in aging humans is not influenced by gender. Am J Kidney Dis 1999; 34:884–888. [DOI] [PubMed] [Google Scholar]
- 12.Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 1976; 235:39–41. [PubMed] [Google Scholar]
- 13.Morgera T, Di Lenarda A, Dreas L, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J 1992; 124:455–467. [DOI] [PubMed] [Google Scholar]
- 14.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119:495–502. [DOI] [PubMed] [Google Scholar]
- 15.Karkouti K, Beattie WS, Wijeysundera DN, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg 2005; 129:391–400. [DOI] [PubMed] [Google Scholar]
- 16.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1:19–32. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Gage BF, Deych E, et al. Anemia: an independent predictor of death and hospitalizations among elderly patients with atrial fibrillation. Am Heart J 2009; 157:1057–1063. [DOI] [PubMed] [Google Scholar]
- 18.Simsek H, Gunes Y, Demir C, et al. The effects of iron deficiency anemia on p wave duration and dispersion. Clinics (Sao Paulo) 2010; 65:1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Chami MF, Kilgo P, Thourani V, et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol 2012; 55:1370–1376. [DOI] [PubMed] [Google Scholar]
- 20.Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, et al. Atrial fibrillation following cardiac surgery: risk analysis and long-term survival. J Cardiothorac Surg 2012; 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezeldin TH. Relation between serum creatinine and postoperative results of open-heart surgery. Saudi Med J 2013; 34:1020–1025. [PubMed] [Google Scholar]
- 22.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004; 15:1597–1605. [DOI] [PubMed] [Google Scholar]


