Abstract
Accurate assessment of the lymphatic system has been limited due to the lack of optimal diagnostic methods. Recently, we adopted noncontrast magnetic resonance (MR) lymphangiography to evaluate the central lymphatic channel. We aimed to investigate the feasibility and the clinical usefulness of noninvasive MR lymphangiography for determining lymphatic disease.
Ten patients (age range 42–72 years) with suspected chylothorax (n = 7) or lymphangioma (n = 3) who underwent MR lymphangiography were included in this prospective study. The thoracic duct was evaluated using coronal and axial images of heavily T2-weighted sequences, and reconstructed maximum intensity projection. Two radiologists documented visualization of the thoracic duct from the level of the diaphragm to the thoracic duct outlet, and also an area of dispersion around the chyloma or direct continuity between the thoracic duct and mediastinal cystic mass.
The entire thoracic duct was successfully delineated in all patients. Lymphangiographic findings played a critical role in identifying leakage sites in cases of postoperative chylothorax, and contributed to differential diagnosis and confirmation of continuity with the thoracic duct in cases of lymphangioma, and also in diagnosing Gorham disease, which is a rare disorder. In patients who underwent surgery, intraoperative findings were matched with lymphangiographic imaging findings.
Nonenhanced MR lymphangiography is a safe and effective method for imaging the central lymphatic system, and can contribute to differential diagnosis and appropriate preoperative evaluation of pathologic lymphatic problems.
INTRODUCTION
Major indications for lymphatic imaging include chylous leak, lymphangioma, and lymphatic obstruction. The most common etiology of chylous leak is iatrogenic, particularly due to thoracic or abdominal surgery.1 Accurate depiction of lymphatic channels plays an important role in accurate diagnosis and treatment planning in chylous leakage. Although there are no definite imaging features that permit specific diagnosis, imaging of lymphangioma helps determine disease extent and aids surgical planning. When lymphangioma is present along the course of the thoracic duct, it usually originates from the thoracic duct.2 To prevent postoperative chylothorax in such a case, the surgeon should note its relationship with the thoracic duct when removing the mass.
Although there have been advances in lymphangiographic imaging, accurate assessment of the lymphatic system remains limited due to the lack of optimal diagnostic methods. Direct lymphangiography requires cannulation of the peripheral lymphatic channel and infusion of oil-based contrast agent. Problems may occur with catheterization of small lymphatic channels, and significant respiratory complications may occur due to oily pulmonary embolism or pneumonitis.3 Lymphoscintigraphy can depict peripheral lymphatic channels with a relatively simple intradermal injection. However, it does not have sufficient spatial resolution to outline morphologic details.4,5 Magnetic resonance (MR) lymphangiography has also been used to evaluate lymph node and lymphatic channel disease.6–8 Its advantages include high spatial resolution, production of 3-dimensional (3D) images, and no exposure to ionizing radiation.8 However, there has been little discussion about visualization of the entire central thoracic duct and accurate localization of pathologic problems yet.
Therefore, we investigated the feasibility and the clinical usefulness of noncontrast MR lymphangiography not only for determining a differential diagnosis but also for identification of leakage point and anatomical variation of thoracic duct for treatment planning in patients with lymphatic problem.
METHODS
This prospective study was approved by the institutional review board and written informed consent was obtained (no. SMC 2015-NON2015–020).
Patients
Patients suspicious for lymphatic problems underwent MR lymphangiography between March 2014 and December 2014. The inclusion criteria were as follows:
Patients with clinical suspicion of chylothorax with increased chest tube output or a constant large amount of pleural effusion documented on chest computed tomography (CT) or chest radiography. With the gross appearance of milky pleural fluid from a chest tube and if the triglyceride level by biochemical analysis of the pleural fluid was higher than 110 mg/dL, a diagnosis of chylothorax was made. Also a daily chest tube output of ≥400 mL was indicative of chylothorax.
Patients with newly diagnosed mediastinal cystic mass located along the course of the thoracic duct and with suspicious connection to the lymphatic channel.
Patients with contraindications for MR (ie, ferromagnetic material, pacemaker, claustrophobia) were excluded.
MR Lymphangiography
All examinations were performed on a 3T MR system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). The appearance and morphologic characteristics of the thoracic duct were evaluated using Controlled Aliasing in Parallel Imaging Results in Higher Acceleration (CAIPIRINHA) accelerated 3D heavily T2 space coronal and axial images in all cases. To highlight the thoracic duct, maximum intensity projection (MIP) was obtained by processing the image data. Imaging parameters for the heavily T2-weighted sequence were: TR/TE, 2500/655 milliseconds to 695 milliseconds; section thickness 1.0 mm; FOV 360 to 400 mm; matrix size 320 × 320 to 384 × 384 pixels; voxel size 0.5 × 0.5 × 1.0 mm; and acquisition time 284 to 322 seconds. Other routine MR sequences appropriate for the clinical indication, including pre/postcontrast fat-saturated T1-weighted VIBE sequences and black-blood fat-saturated turbo spin echo T2-weighted sequences, were acquired during the same session. Additional parameters are detailed in Table 1.
TABLE 1.
MR Lymphangiographic Scanning Parameters
Two thoracic radiologists with experience in lymphatic imaging investigated the MR lymphangiography images, and final decisions were reached by consensus. Check points during the MR imaging review were as follows:
The thoracic duct from the level of the diaphragm to the thoracic duct outlet was evaluated on heavily T2-weighted images. The degree of visualization was evaluated as follows: poor, the anatomic part was not visible or was partially visible; good, the anatomic part was mostly visible; and excellent, the entire anatomic part was clearly visible.
Presence or absence of an area of dispersion around the chyloma or direct continuity between the thoracic duct and mediastinal cystic mass was assessed.
Additionally, the presence of an enlarged diameter and a tortuous configuration of the thoracic duct around the chyloma or mass were recorded for each case.
In all chylothorax cases, patients were initially treated by conservative dietary manipulation, such as medium chain triglyceride (MCT) diet or total parenteral nutrition (TPN). Patients who were not controlled with conservative treatment for persistent chylous leakage underwent operation. Surgical management was adopted when the chlye drainage rate was more than 1 L/day for a period of more than 5 days. At the time of operation, whether the site of chyle leaks was correlated with MR lymphangiographic findings was recorded. After surgery, chest tube output was monitored and follow-up chest radiography was performed in all patients.
RESULTS
All 10 patients (age range 42–72 years; 5 female, 5 male) underwent MR lymphangiography. Among them, 7 patients had the findings of chylothorax, and 3 patients were suspicious for lymphangioma. The entire thoracic duct was successfully delineated in all 10 patients in heavily T2-weighted MR lymphangiographic images. In 1 patient, the thoracic duct was faintly visualized, possibly due to reduced chyle production, secondary to total parenteral nutrition. The variant of thoracic duct configuration was identified in 3 of the 10 patients. The right-sided thoracic duct was observed in 2 patients and duplicated thoracic duct was seen in the remaining 1 patient. The clinical and imaging findings of 10 patients are summarized in Table 2.
TABLE 2.
Patient Characteristics and MR Lymphangiographic Findings
Postoperative Chyle Leak
Among 6 patients who developed postoperative chylothorax, chyle leakage occurred after esophagectomy for esophageal cancer (n = 1), after lobectomy for lung cancer (n = 4), and after mass excision for lipoma (n = 1). In 4 of 6 (67%) patients with postoperative chylothorax, the chyle leakage site was identified on MR lymphangiography (see video, Supplemental Digital Content 1, which demonstrates the chyle leakage site; annotated with arrows and course of thoracic duct; annotated with circle). Lymphatic leakage was not identified in the remaining 2 patients. Despite conservative management such as drainage tube insertion, medium-chain triglyceride diet, or total parenteral nutrition, 2 patients with persistent high-output chyle leakage underwent surgical exploration with thoracic duct ligation. The leakage sites founded to be matched with MR lymphangiographic imaging findings (Figure 1). In the remaining 4 patients, the daily amount of fluid drainage decreased after lymphangiography. For this reason, conservative treatment was continued in these patients.
FIGURE 1.
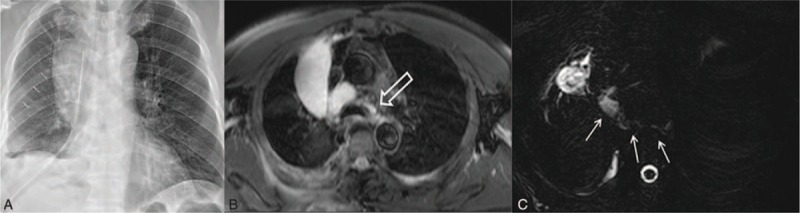
Postoperative chylothorax in a 68-year-old male who underwent right lower lobectomy due to lung cancer (T2aN0M0, stage IB). A, Posteroanterior chest radiography obtained 10 days after surgery shows large right pleural effusion despite chest tube drainage. B, Axial T2-weighted MR imaging obtained the same day demonstrates a large right pleural effusion continuous into the mediastinum between the aorta and azygos vein (white open arrow). C, Axial heavily T2-weighted MR imaging demonstrates a leak site (white arrows) in thoracic duct near the azygos arch. MR = magnetic resonance.
Lymphangioma
Three patients with mediastinal masses underwent chest CT and MR lymphangiography. Imaging showed a fluid-filled cystic mass with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Figure 2). These imaging findings suggested mediastinal cystic lymphangioma. In 2 of 3 patients who were suspected to have lymphangioma, a definite connection with the thoracic duct was noted in heavily T2-weighted MR lymphangiographic images. One of these patients underwent posterolateral thoracotomy. The thoracic duct was carefully ligated on the basis of MR lymphangiographic findings, and then the mass was resected without complication.
FIGURE 2.
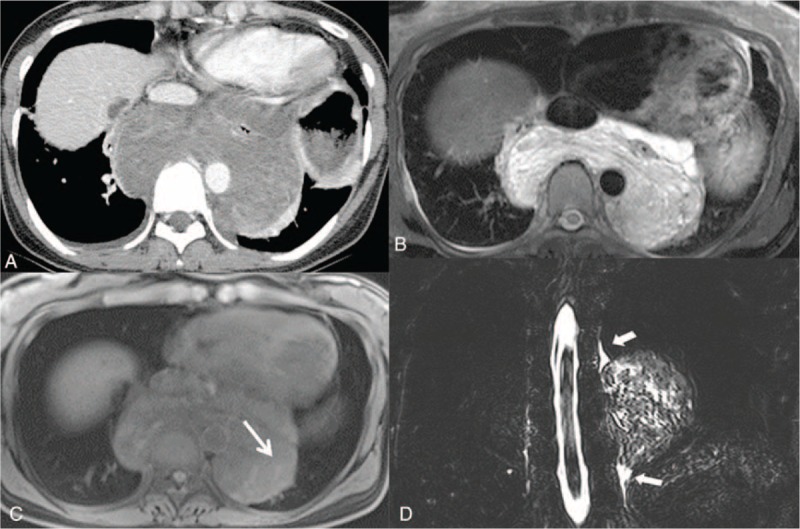
Cavernous lymphangioma in a 41-year-old female. A, Enhanced axial CT scan demonstrates a well-defined homogenous low attenuating mass encasing the esophagus in the posterior mediastinum. B, T2-weighted MR imaging shows high signal intensity with a multilocular septum within the mass. C, T1-weighted MR imaging shows an isosignal intensity with faint high signal intensity (white arrow), which led to suspicion of hemorrhage. D, Coronal heavily T2-weighted maximum intensity projection (MIP) image reveals continuation between the thoracic duct (arrow head) and mass. CT computed = tomography, MR = magnetic resonance.
Gorham Disease
One patient with recurrent idiopathic chylothorax underwent chest CT and MR lymphangiography. Imaging showed a large bilateral pleural effusion with suspicious leakage around the proximal thoracic duct and an abnormal cystic lesion in the anterior mediastinum (Figure 3, Supplemental Digital Content 3; which demonstrates chyle leakage site; annotated with open arrows and course of thoracic duct; annotated with arrows). There were also multiple osteolytic lesions in the thoracic spine with high signal intensity within that lesion on T2-weighted images, representing dilated lymphatic channels (Figure 3). On the basis of radiologic and clinical features, Gorham disease (osteolysis associated with intraosseous vascular anomalies) was diagnosed. Video-assisted thoracoscopic surgery (VATS) was performed and the thoracic duct was ligated at the significant leakage point seen on MR lymphangiographic images (see video, Supplemental Digital Content 2, which demonstrates the chyle leakage site; annotated with arrows and course of thoracic duct; annotated with circle).
FIGURE 3.
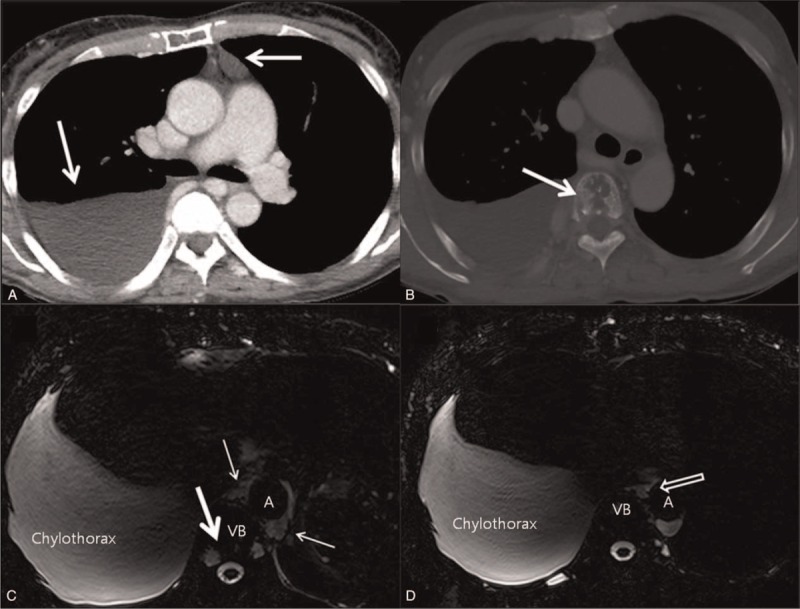
Gorham disease in a 63-year-old female with idiopathic chylothorax. A, Enhanced axial CT with mediastinal window setting shows large right pleural effusion and abnormal fluid density lesion (white arrow) in the anterior mediastinum. B, Axial CT with bone window setting reveals a mottled osteolytic lesion (white arrow) in vertebral body. C, D, Axial heavily T2-weighted MR imaging obtained the same day demonstrates abrupt discontinuation of the thoracic duct (open arrow) with dispersion of chyle into the mediastinum (thin arrows), suggesting leakage around the cistern chyli. The mottled osteolytic lesion mentioned above showed high T2 signal intensity (thick arrow), suggesting a dilated lymphatic channel within the vertebral body. CT computed = tomography, MR = magnetic resonance.
DISCUSSION
Most studies in the field of clinical lymphatic imaging have focused on peripheral lymph edema or lymph nodes.7–10 Only a few studies have investigated the central lymphatic channel.11,12 However, in fact, lymphatic circulation disorders that require prompt management or surgical correction may be caused by central lymphatic vessel problems. Therefore, critical cases provide an important opportunity to advance the understanding of lymphatic disorders.
The current study presents a clinical assessment of lymphatic disease with noninvasive MR lymphangiography that provided accurate visualization of the central lymphatic channel. As a new method of visualizing the lymphatic channel, noncontrast MR lymphangiography using heavily T2-weighted sequences is a type of hydrography that specifically displays static fluids in fluid-contacting structures with concurrent complete signal loss in solid tissue or flowing blood. It uses a long T2 relaxation time to demonstrate the thoracic lymphatic channel without requiring injection of contrast media.13 Our MR lymphangiography with T2WI was not the very first application for the evaluation for chylothorax, given that this has been reported before in several literatures.8,9,14 However, we found that our suggested MR sequences could visualize the entire thoracic duct clearly applying heavily T2-weighted sequence, particularly regardless of the patients’ various conditions, which could be points of difference from previous studies.
The reason of our superior imaging quality enabling diagnostic and therapeutic decision would be based on the application of a recent novel MR data acquisition technique—CAIPIRINHA—for heavily T2-weighted axial and coronal. CAIPIRINHA is a modern data acquisition technique basically using radial phase encoding, which improves the sensitivity variations in the receiver coil array in 2-phase encoding dimensions and provides higher acceleration factors with modified data acquisition patterns. Also, the sampling pattern of CAIPIRINHA helps allocate K-space points more consistently and could improve algorithms for reconstruction.15–17 Consequently, heavily T2-weighted MR lymphangiography using CAIPIRINHA could achieve higher degree of visualization for the central lymphatic system with good to excellent without breath holding. Even though several studies were reported about clinical application of CAIPIRINHA for T1-weighted 3D images,15,18,19 to our knowledge, CAIPIRINAH-accelerated heavily T2-weighted image for MR lymphangiography has not been investigated. We, moreover, applied MIP images obtained by processing raw data for the visual assessment of the lymphatic system, which enabled complete anatomic representation.
Aggressive surgical techniques to improve the curability of cancer patients may have contributed to the increased incidence of postoperative lymphatic leakage. Data from several previous studies have identified that postoperative chylothorax often occurs after esophagectomy with mediastinal lymphadenectomy in patients with esophageal cancer (0.42%), pneumonectomy (0.37%), lobectomy (0.26%–2.3%), or cervical lymph node dissection in patients with head and neck cancer or breast cancer.20–23 For patients with persistent high-output chylothorax, who failed conservative dietary treatment, reoperation to ligate the thoracic duct is necessary.24,25 In the presented patient series, MR lymphangiographic images enabled definition of the severity and extent of chylothorax. Leakage sites were successfully identified, which could aid in determining the surgical approach, allowing surgeons to look in a specific area. In all 3 patients with surgical treatment, leakage points were clearly noted on MR lymphangiographic image and chylous leakage was successfully trapped at the corresponding checkpoints during the VATS.
Magnetic resonance lymphangiography provides preoperative planning information regarding the entire course of the thoracic duct and anatomical variations. Additional information such as a leak at more than 1 site can also support the decision for the level of thoracic duct ligation. In addition, if the site of extravasation of chyle is once visualized, judgment for severity of leakage or prediction of resolution can be through the assessment of the amount of leakage. The typical thoracic duct course illustrated in Figure 4 is present in only 40% to 60% of patients. There are numerous variations in intrathoracic course, number of ducts, location of tributaries, and point of termination.1,26,27 These variations may contribute to a higher incidence of postoperative chylothorax. In this study, typical configurations of thoracic duct were detected in 70% (7/10) of cases. The right-side ducts were shown in 2 patients and duplication of thoracic duct was revealed in 1 patient. Before the surgery, the awareness of right thoracic duct variation would enable the successful thoracic duct ligation without further complications. According to previous reports,27–29 the cisterna chyli and entry of the thoracic duct in the subclavian region can be imaged with radiographic lymphangiographic images using an iodine contrast agent. This study has raised concerns about optimizing lymphangiography. As with whole body angiography, whole body lymphangiographic images could contribute to noninvasive diagnosis and treatment of lymphatic disorders.
FIGURE 4.
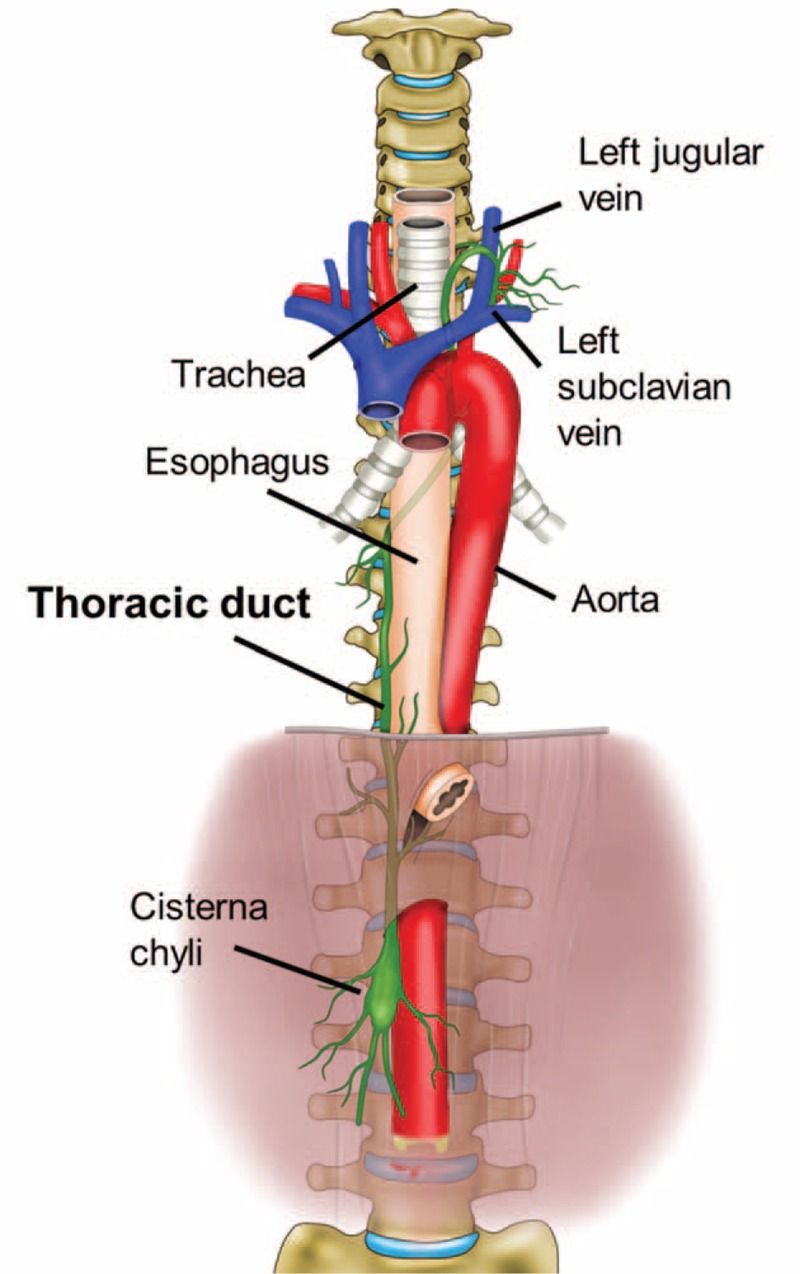
Schematic illustration of the anatomical course of the thoracic duct. The thoracic duct is a continuation of the cisterna chyli from its abdominal segment into the thorax. Typically, the lymphatic pathway originates in the cisterna chyli and enters into the thoracic cavity thorough the aortic hiatus. In the thorax, the thoracic duct ascends along the right anterior surface of the vertebral column between the aorta and the azygos vein posterior to the esophagus. At the T5 to T6 vertebral level, it crosses left of the midline and extends posterior to the aortic arch. It has a close relationship with the trachea, ascends approximately 2 to 3 cm above the clavicle, and then crosses anterior to the subclavian and thyrocervical trunk, making an arch inferiorly. Finally, it terminates at the junction of the left subclavian vein and the internal jugular veins.
Corresponding to previous MRI studies, lymphangioma was hypointense on T1-weighted sequences and high or isointense on T2-weighted images.30,31 MR provides the whole extent of the disease and reveals the relationship to adjacent structures. In the current series with patients suspicious for lymphangioma, MR lymphangiography provides a differential diagnostic clue by identifying continuity with the thoracic duct. MR lymphangiography also helps with preoperative planning to avoid the risk of postoperative chyle leak.
One patient presented in this study revealed a chylous pleural effusion and ascites, and also multiple osteolytic lesions characteristic of Gorham disease.32–34 In this case, MR lymphangiography exactly delineated the thoracic duct leak site and also captured the extent of the pathology with high resolution. This case demonstrates the important role of MR lymphangiography in diagnosis and treatment planning.
Several limitations must be considered in the present study. First, there were a small number of patients and each pathologic problem was heterogeneous. Second, MR lymphangiography was not compared with intraoperative findings in some patients. However, patients whose leakage site was not visible or who had mild chylous leak on MR lymphangiography were expected to relieve by conservative management, and conservative treatment was continued without further surgical treatments, based on MR findings. Consequently those patients had all improved with only dietary or tube drainage treatment. Therefore, these nonoperative cases also support the idea that MR lymphangiographic findings provide sufficient information for treatment decision-making. This is a prospective preliminary report involving selected patients who underwent MR lymphangiography in our institution over a short period. Further research in a larger population is required to refine the imaging findings and clinical utility of MR lymphangiography in lymphatic pathology.
In conclusion, nonenhanced MR lymphangiography is a safe and effective method for imaging the central lymphatic system. It can contribute to the differential diagnosis and appropriate preoperative evaluation of pathologic lymphatic problems.
Supplementary Material
Supplementary Material
Acknowledgments
We are thankful to Sung-kab Kim and Jaeho Shin who devoted their time and knowledge in technical support to provide graphic illustration and video supplements for this study.
Footnotes
Abbreviations: CAIPIRINHA = Controlled Aliasing in Parallel Imaging Results in Higher Acceleration, FOV = field of view, MCT = medium chain triglyceride, MIP = maximal intensity projection, MR = magnetic resonance, TE = echo time, TPN = total parenteral nutrition, TR = repetition time, VATS = video-assisted thoracic surgery.
EYK and HSH contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol 2011; 28:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raman SP, Pipavath SN, Raghu G, et al. Imaging of thoracic lymphatic diseases. AJR Am J Roentgenol 2009; 193:1504–1513. [DOI] [PubMed] [Google Scholar]
- 3.Silvestri RC, Huseby JS, Rughani I, et al. Respiratory distress syndrome from lymphangiography contrast medium. Am Rev Respir Dis 1980; 122:543–549. [DOI] [PubMed] [Google Scholar]
- 4.Clement O, Luciani A. Imaging the lymphatic system: possibilities and clinical applications. Eur Radiol 2004; 14:1498–1507. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto T, Yamagami T, Kato T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol 2009; 82:286–290. [DOI] [PubMed] [Google Scholar]
- 6.Notohamiprodjo M, Baumeister RG, Jakobs TF, et al. MR-lymphangiography at 3.0 T: a feasibility study. Eur Radiol 2009; 19:2771–2778. [DOI] [PubMed] [Google Scholar]
- 7.Lu Q, Delproposto Z, Hu A, et al. MR lymphography of lymphatic vessels in lower extremity with gynecologic oncology-related lymphedema. PLoS One 2012; 7:e50319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu N, Zhang Y. Magnetic resonance lymphangiography for the study of lymphatic system in lymphedema. J Reconstr Microsurg 2016; 32:66–71. [DOI] [PubMed] [Google Scholar]
- 9.Arrive L, Derhy S, El Mouhadi S, et al. Noncontrast magnetic resonance lymphography. J Reconstr Microsurg 2016; 32:80–86. [DOI] [PubMed] [Google Scholar]
- 10.Notohamiprodjo M, Weiss M, Baumeister RG, et al. MR lymphangiography at 3.0 T: correlation with lymphoscintigraphy. Radiology 2012; 264:78–87. [DOI] [PubMed] [Google Scholar]
- 11.Dori Y, Zviman MM, Itkin M. Dynamic contrast-enhanced MR lymphangiography: feasibility study in swine. Radiology 2014; 273:410–416. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy R, Hernandez A, Kavuk S, et al. Imaging the central conducting lymphatics: initial experience with dynamic MR lymphangiography. Radiology 2015; 274:871–878. [DOI] [PubMed] [Google Scholar]
- 13.Jara H, Barish MA, Yucel EK, et al. MR hydrography: theory and practice of static fluid imaging. AJR Am J Roentgenol 1998; 170:873–882. [DOI] [PubMed] [Google Scholar]
- 14.Yu DX, Ma XX, Wang Q, et al. Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol 2013; 23:702–711. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Winkler B, Pabst T, et al. Fast MR imaging of the paediatric abdomen with CAIPIRINHA-accelerated T1w 3D FLASH and with high-resolution T2w HASTE: a study on image quality. Gastroenterol Res Pract 2015; 2015:693654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewes P, Frellesen C, Al-Butmeh F, et al. Comparative evaluation of non-contrast CAIPIRINHA-VIBE 3T-MRI and multidetector CT for detection of pulmonary nodules: in vivo evaluation of diagnostic accuracy and image quality. Eur J Radiol 2016; 85:193–198. [DOI] [PubMed] [Google Scholar]
- 17.Breuer FA, Blaimer M, Heidemann RM, et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med 2005; 53:684–691. [DOI] [PubMed] [Google Scholar]
- 18.Budjan J, Ong M, Riffel P, et al. CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold examination (VIBE) for dynamic liver imaging: comparison of gadoterate meglumine, gadobutrol and gadoxetic acid. Eur J Radiol 2014; 83:2007–2012. [DOI] [PubMed] [Google Scholar]
- 19.Park YS, Lee CH, Kim IS, et al. Usefulness of controlled aliasing in parallel imaging results in higher acceleration in gadoxetic acid-enhanced liver magnetic resonance imaging to clarify the hepatic arterial phase. Invest Radiol 2014; 49:183–188. [DOI] [PubMed] [Google Scholar]
- 20.Cerfolio RJ, Allen MS, Deschamps C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996; 112:1361–1365.[discussion 1365-1366]. [DOI] [PubMed] [Google Scholar]
- 21.Yang DJ, Ren GS, Wang XY. Bilateral chylothorax following left supraclavicular lymph node dissection for breast cancer: one case report and literature review. Chin J Cancer 2014; 33:317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012; 93:897–903.[discussion 903-894]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 2007; 32:362–369. [DOI] [PubMed] [Google Scholar]
- 24.Cho HJ, Kim DK, Lee GD, et al. Chylothorax complicating pulmonary resection for lung cancer: effective management and pleurodesis. Ann Thorac Surg 2014; 97:408–413. [DOI] [PubMed] [Google Scholar]
- 25.Bryant AS, Minnich DJ, Wei B, et al. The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection. Ann Thorac Surg 2014; 98:232–235. [DOI] [PubMed] [Google Scholar]
- 26.Phang K, Bowman M, Phillips A, et al. Review of thoracic duct anatomical variations and clinical implications. Clin Anat 2014; 27:637–644. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberger A, Abrams HL. Radiology of the thoracic duct. Am J Roentgenol Radium Ther Nucl Med 1971; 111:807–820. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi H, Kuboyama S, Abe H, et al. Clinical feasibility of noncontrast-enhanced magnetic resonance lymphography of the thoracic duct. Chest 2003; 124:2136–2142. [DOI] [PubMed] [Google Scholar]
- 29.Pomerantz M, Herdt JR, Rockoff SD, et al. Evaluation of the functional anatomy of the thoracic duct by lymphangiography. J Thorac Cardiovasc Surg 1963; 46:568–575. [PubMed] [Google Scholar]
- 30.Sermon A, Gruwez JA, Lateur L, et al. The importance of magnetic resonance imaging in the diagnosis and treatment of diffuse lymphangioma. Acta Chir Belg 1999; 99:230–235. [PubMed] [Google Scholar]
- 31.Lohrmann C, Foeldi E, Langer M. Assessment of the lymphatic system in patients with diffuse lymphangiomatosis by magnetic resonance imaging. Eur J Radiol 2011; 80:576–581. [DOI] [PubMed] [Google Scholar]
- 32.Collins J. Case 92: Gorham syndrome. Radiology 2006; 238:1066–1069. [DOI] [PubMed] [Google Scholar]
- 33.Fujiu K, Kanno R, Suzuki H, et al. Chylothorax associated with massive osteolysis (Gorham's syndrome). Ann Thorac Surg 2002; 73:1956–1957. [DOI] [PubMed] [Google Scholar]
- 34.Yoo SY, Goo JM, Im JG. Mediastinal lymphangioma and chylothorax: thoracic involvement of Gorham's disease. Korean J Radiol 2002; 3:130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


