Abstract
We investigated the factors that might influence the development of diabetic foot ulcers (DFUs) in type 2 diabetes patients without diabetic polyneuropathy (DPN).
From January 2000 to December 2005, a total of 595 patients who had type 2 diabetes without DPN between the ages of 25 and 75 years, and had no prior history of DFUs were consecutively enrolled in the study. A cardiovascular autonomic function test was performed to diagnose cardiovascular autonomic neuropathy (CAN) using heart rate variability parameters.
The median follow-up time was 13.3 years. Among the 449 (75.4%) patients who completed the follow-up evaluation, 22 (4.9%) patients developed new ulcers, and 6 (1.3%) patients underwent the procedure for lower extremity amputations. The patients in the DFUs group had a longer duration of diabetes, higher baseline HbA1c levels, higher rates of nephropathy, and CAN. A Cox hazard regression analysis results revealed that the development of DFUs was significantly associated with the presence of CAN (normal vs definite CAN; HR, 4.45; 95% confidence interval, 1.29–15.33) after adjusting for possible confounding factors.
The development of DFUs was independently associated with CAN in patients with type 2 diabetes without DPN. We suggested the importance of CAN as a predictor of DFUs even in the patients without DPN, and the need to pay attention to patients with definite CAN and type 2 diabetes.
INTRODUCTION
Dramatic increases in the number of patients with type 2 diabetes and their accompanying diabetic-related vascular complications have made diabetic foot problems an increasingly important clinical concern. Diabetic foot ulcers (DFUs) are frequent and disastrous complication of diabetes, often leading to lower extremity amputation (LEA).1 The lifetime risk for developing foot ulcers among patients with diabetes is as high as 25%,2 and individuals with diabetes have a 15- to 40-fold higher risk of LEA compared with the general population.3 The incidence of diabetes-related LEA among patients with type 2 diabetes has increased compared with that of type 1- and nondiabetic-related LEA.4,5 Therefore, in addition to managing glycemic levels and controlling for cardiovascular risk, diabetic foot problems should not be neglected in management of patients with type 2 diabetes.
A number of risk factors contribute to the development of DFUs, including previous amputation, previous foot ulcer history, peripheral neuropathy or foot deformity, peripheral artery disease, impaired vision, smoking, and renal impairment.6,7 A large meta-analysis also reported DFUs to be associated with an increased risk of cardiovascular deaths and all-cause mortality.8 Therefore, identifying risk factors and patients at risk for DFUs are key for preventing these serious diabetic complications.
Cardiovascular autonomic neuropathy (CAN) is one of the chronic complications in type 2 diabetes. CAN is significantly related with cardiovascular disease (CVD) and CVD-related mortality due to silent myocardial ischemia, or life-threatening cardiac arrhythmia.9 CAN can be easily measured on the basis of outpatient using Ewing method, measuring heart rate variability (HRV) during a Valsalva maneuver, deep breathing, and upright posture.10 The association between peripheral autonomic neuropathy and DFUs has been well verified.11,12 However, little is known about the association between CAN and DFUs in patients with type 2 diabetes, especially among those without diabetic polyneuropathy (DPN).
This study investigated the association between CAN and the development of DFUs in patients with type 2 diabetes without DPN. To the best of our knowledge, this is the first long-term prospective study to show evidence of this relationship in an Asian population.
METHODS
From January 2000 to December 2005, a total of 1014 patients age 25 to 75 years with type 2 diabetes enrolled in a DPN study were consecutively recruited to participate in the present study. A cardiovascular autonomic function test (AFT) was performed at the university-affiliated diabetes center of St Vincent's Hospital in South Korea. Seventy-four patients were excluded from the study for arrhythmia or severe illness such as heart failure, liver cirrhosis, alcoholism, severe infection, or malignancy. Patients with type 1 diabetes, chronic kidney disease (CKD) stage 3 and higher, end-stage renal disease, former or current DFUs, or previous amputation were excluded. Three hundred forty-five patients diagnosed with DPN at baseline were also excluded. During the follow-up period from January 2000 to June 2015, 132 patients who dropped out and 14 patients who died before reaching the endpoint were excluded from the analyses. The Catholic Medical Center Ethics Committee approved this study. All participants provided their signed informed consent.
At the commencement of the study, patient height, body weight, and systolic and diastolic blood pressures were measured. Hypertension was defined as systolic blood pressures ≥140 mm Hg, diastolic blood pressures ≥90 mm Hg, or the use of antihypertensive medications. Fasting and postprandial plasma glucose levels were measured using an automated enzymatic method, and glycated hemoglobin (HbA1c) levels were measured using high-performance liquid chromatography with a reference range of 4.4% to 6.4% (25–46 mmol/mol) (Bio-Rad, Montreal, Quebec, Canada) every 6 months during the follow-up period. Blood lipid concentrations of total cholesterol, triglycerides, and high-density lipoprotein cholesterol were measured enzymatically using an automatic analyzer (model 736-40, Hitachi, Tokyo, Japan). Estimated glomerular filtration rates (eGFRs) were used to determine CKD classification using the 4-component Modification of Diet in Renal Disease equation.13 We defined smoking as current or past smokers within 3 years before enrollment in the study.14 Alcohol consumption was defined as drinking any type of alcoholic beverage at least once a week for a period of 6 months or longer.
Ophthalmologists reviewed diabetic retinopathy findings as assessed from retinal photographs taken at baseline. Urinary albumin excretion rates were measured from a 24-h urine collection using immunoturbidimetry (Eiken, Tokyo, Japan). Diabetic nephropathy was defined as a urine albumin excretion (UAE) rates >30 mg/d.15
DPN was assessed using the 5.07 Semmes–Weinstein monofilament test on 10 sites on both feet, comprehensive foot examination, and nerve conduction studies. DPN was defined as the presence of 2 or more abnormalities according to typical symptoms, signs, quantitative sensory tests, or nerve conduction studies.16
In the present study, CVD was defined as a diagnosed history of coronary artery disease (CAD) or cerebrovascular disease (CVA), while CAD was defined as a history of diagnosed angina pectoris, myocardial infarction, or coronary revascularization (coronary bypass surgery or coronary angioplasty). Stroke history included previous transient ischemic attack or cerebral infarction. Specialists, including cardiologists, neurologists, or neurosurgeons, confirmed the clinical diagnosis of CVD based on verified medical records.17 The cardiovascular AFT was performed by 1 examiner using the Ewing method, which included a test for HRV, such as the expiration/inspiration (E/I) ratio, responses to the Valsalva maneuver, and postural change from lying to standing position, as previously described.10,17 E/I ratios below the age-related reference value, Valsalva ratios <1.20, and posture ratios <1.03 were considered abnormal. Each these 3 ratios were scored as normal (0) or abnormal (1), for a total maximum score of 3. The CAN staging scores of 0 were defined as normal autonomic function, while scores of 1 and 2 or more were defined as early CAN and definite CAN, respectively.18,19
Assessment of DFUs and LEAs
During regular outpatient check-ups and diabetes care every 3 to 6 months, physicians evaluated participants for DFUs or amputations. The main outcome in this study was newly developed DFUs, including associated abscesses, gangrene, cellulitis, osteomyelitis, necrotizing fasciitis, and infected ulcers. Using the Wagner–Meggitt classification system, any patient with Wagner Grade 1 or greater foot ulcer lesions involving the foot or ankle was considered to have DFUs.20
Statistical Analyses
Baseline data were presented as the mean ± standard deviation values or median with interquartile range. The statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA). A P value of <0.05 was considered to be statistically significant. Continuous variables were compared with independent Student t tests, while categorical variables were compared using a χ2 tests. We used Cox proportional hazards regression models to assess the associations with the outcome and CAN. Models included sex, age, duration of diabetes, the presence of hypertension, smoking, alcohol consumption, mean HbA1c, diabetes treatment, the use of angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers, the use of statin or aspirin, and the presence of diabetic retinopathy and nephropathy. We evaluated the potential interaction between CAN stage and other prognostic factors, such as the mean HbA1c level, diabetes duration, and diabetic nephropathy during the study. The results were reported as hazard ratios (HRs) with 95% confidence intervals (CIs).
RESULTS
Baseline Characteristics of the Study Population According to DFUs
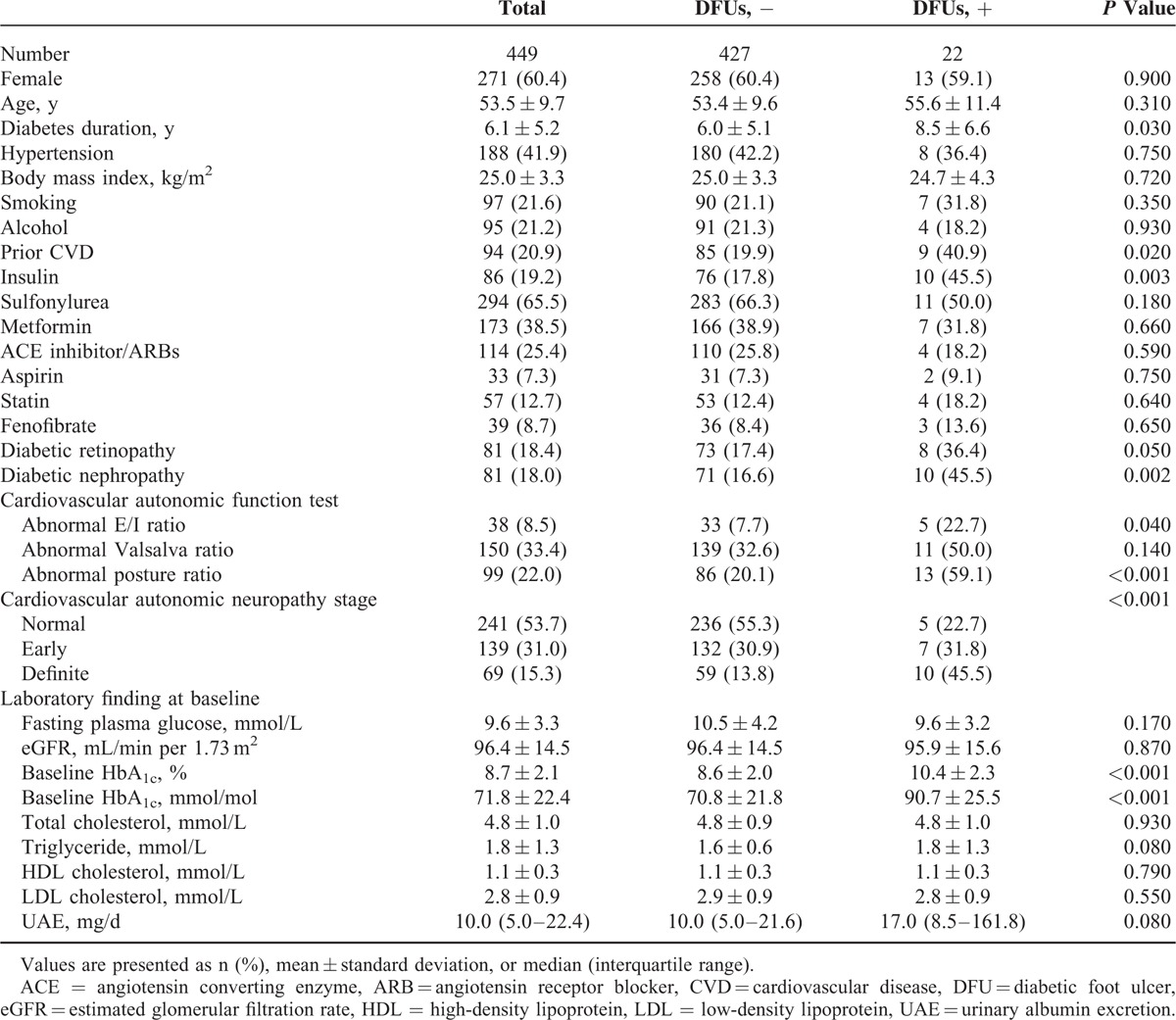
The median follow-up time was 13.3 years. Of 595 patients enrolled in this study, 449 (75.4%) completed the follow-up evaluation. At baseline, the total study population who completed the follow-up consisted of 178 (39.6%) men and 271 (60.4%) women, with a mean age and diabetes duration of 53.5 ± 9.7 and 6.1 ± 5.2 years, respectively. At the commencement of the study, 86 (19.2%) patients were receiving insulin therapy and 294 (65.5%) were receiving sulfonylurea treatment. Compared with participants who did complete the follow-up evaluation, 146 patients who did not complete the follow-up evaluation did not differ with respect to sex ratio (P = 0.67), age (53.5 ± 9.7 years vs 53.7 ± 10.3 years; P = 0.82), diabetes duration (6.1 ± 5.2 years vs 6.2 ± 5.9 years; P = 0.82), and baseline HbA1c (8.7 ± 2.1% [71.8 ± 22.4 mmol/mol] vs 9.1 ± 2.5% [75.9 ± 27.4 mmol/mol]; P = 0.10).
During the follow-up period, 22 (4.9%) patients developed DFUs, corresponding to an incidence of 3.7 per 1000 patient-years. The median time from enrollment to the first occurrence DFU was 4.4 years. Overall, patients with DFUs had a longer duration of diabetes and more often received insulin treatment. Diabetic microvascular complications at baseline, such as retinopathy and nephropathy, were observed more frequently in the group with DFUs (Table 1). Six (1.3% of total, 27.3% of patients with DFUs) LEAs were performed during the follow-up period. Among patients who required amputations, only 1 (16.6% of the patients with DFUs) required major amputation. The group with DFUs had a higher mean HbA1c level (9.2 ± 1.3% [77.2 ± 14.2 mmol/mol] vs 8.1 ± 1.3% [65.3 ± 13.4 mmol/mol]; P < 0.001) and mortality rate (9.1% vs 1.6%, P = 0.015) during the study.
TABLE 1.
Descriptive Characteristics at Baseline Examination
Clinical Characteristics of the Study Population According to CAN Stage
In this study, 139 (31.0%) and 69 (15.3%) patients had early and definite CAN at baseline; 38 (8.5%), 150 (33.4%), and 99 (22.0%) patients showed abnormal HRV responses to deep breathing, the Valsalva maneuver, and postural change. Among patients with DFUs, an abnormal response to it was observed in the majority (59.1%) of patients. Patients with DFUs had significantly more abnormalities than patients without DFUs, as shown in all of the subsections of the AFT.
Patients with higher CAN scores were older, were more often female, had diabetes for a longer period, had more diabetic retinopathy, were more likely to have hypertension, and were more often treated with insulin. Patients with higher scores also tended to exhibit higher fasting glucose and baseline HbA1c levels (Supplementary Table 1). Compared with subjects without DFUs, the incidence of DFUs increased with increasing CAN scores (5 [22.7%] patients with normal autonomic function vs 7 [31.8%] patients with early CAN vs 10 [45.5%] patients with definite CAN; P for trends <0.001). In addition, patients with higher CAN stages had a higher rate of LEAs during the follow-up (no patients with normal autonomic function vs 2 [1.4%] patients with early CAN vs 4 [5.8%] patients with definite CAN; P for trends <0.001).
Association Between CAN and DFUs
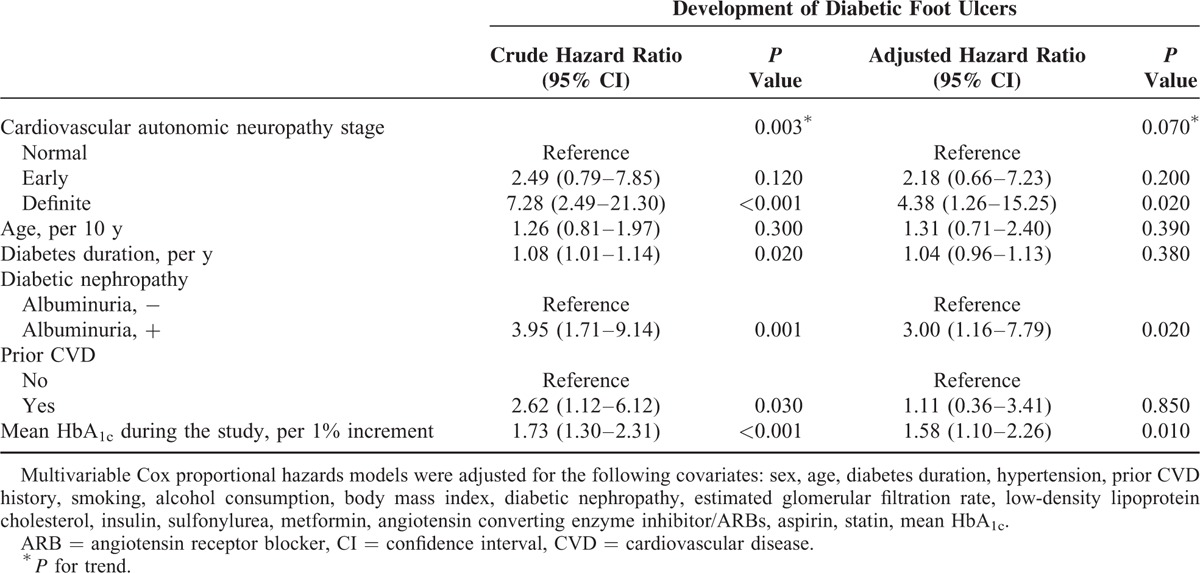
Univariate Cox regression analysis showed that diabetes duration, mean HbA1c level, diabetic retinopathy, diabetic nephropathy, and CAN were potential risk factors for DFUs. In multivariable Cox proportional hazard regression analysis, patients with definite CAN showed a 4.5-fold increased risk of DFUs compared with those with normal autonomic function during the observation period after adjusting for sex, age, diabetes duration, hypertension, smoking, alcohol consumption, body mass index, mean HbA1c, eGFR, low-density lipoprotein cholesterol, insulin treatment, ACE inhibitor or angiotensin receptor blocker use, and diabetic nephropathy (Table 2). Glycemic control was an important factor for future development of DFUs. The risk of DFUs increased about 60% for each 1% point increase in mean HbA1c level (HR, 1.58; 95% CI, 1.10–2.25; P = 0.01). In the patient group with both definite CAN and poor glycemic control (mean HbA1c > 9%), the HR for DFUs increased to 9.82 (P for interaction = 0.18). In addition, in the patient group with both definite CAN and albuminuria, the HR for DFUs increased to 9.10 compared with that of patients without either factor (P for interaction = 0.85).
TABLE 2.
Hazard Ratios for Diabetic Foot Ulcers Identified by Principal Component Analysis
DISCUSSION
This long-term, prospective, observational cohort study revealed a significant relationship between diabetic cardiovascular autonomic dysfunction and the development of DFUs in patients with type 2 diabetes during a 13-year follow-up period. We found that definite CAN increased the risk of DFUs even in patients with type 2 diabetes without DPN.
The Centers for Disease Control and Prevention's National Hospital Discharge Survey reported an annual rate of 8 hospital discharges with a diagnosis of DFU as 5.7 to 8.5 per 1000 diabetic population,21 compared with an incidence of foot ulcers among patients without DPN of 3.7 per 1000 patient-years in our Asian hospital-based cohort study. DFUs and associated limb loss are critical problems for patients with type 2 diabetes. Ethnic differences in social, economic, and geographical factors have been reported in patients with DFUs and LEAs.22 Several studies have reported a lower risk of LEAs in Asian populations compared with those of African-American and Caucasian populations. In a study in Singapore, 3.2% of diabetic patients admitted to the largest tertiary hospital underwent LEAs.23 Other studies have reported a low rate of LEAs among diabetic patients of East Asian ethnicity.24,25
Several risk factors for DFUs have been reported by epidemiologic studies. Previous history of ulcer or amputation, peripheral edema, plantar callus formation, ischemia, diabetic microvascular complications, uncontrolled diabetes, older age, kidney disease, and longer duration of diabetes have been suggested to be important risk factors for DFUs in patients with type 2 diabetes.23,26–28 DFU is an outcome of complicated risk factors including peripheral neuropathy, peripheral vascular disease, foot deformities, arterial insufficiency, trauma, and impaired resistance to infection.20 Among peripheral neuropathy manifestations, motor neuropathy accounts for the inability to coordinate movement, which often results in foot muscle atrophy or foot deformity.20,29 Loss of protective sensation with recurrent injury is associated with sensory neuropathy.30 These peripheral nerve dysfunctions predispose patients to diabetic foot problems. A previous 4-year prospective study and another other longitudinal double-blind multicenter study showed the predictive value of DPN in the development of DFUs.31,32
However, the contribution of CAN to diabetic foot problems in patients with type 2 diabetes has not been clearly reported. CAN is a well-known independent risk factor for cardiovascular events in patients with type 1 and 2 diabetes. CAN may increase mortality by more than 3-fold, independent of traditional cardiovascular risk factors.19 The Seattle Diabetic Foot Study33,34 is a prospective research on the association between DFUs and CAN. The researchers measured the HRV during deep breathing at 6 breaths/min in 749 patients with type 2 diabetes. They followed up the patients over 3.7 years and detected an incidence of 3.0 per 100 person-years. Aso et al35 reported that diabetic patients with neuropathic foot ulceration exhibited greater impairment in spectral indices of autonomic activity measured using power spectral analysis of heart rate variation in a cross-sectional study.
Previous studies have demonstrated that peripheral autonomic neuropathy may contribute to the development of DFUs due to sympathetic denervation of the skin, with reduced nutritive capillary blood flow, impaired wound healing, loss of sweating, dryness, and skin fissures associated with the development of foot ulceration in diabetic subjects.36,37 Abnormalities in sympathetic vasoconstriction were detectable in the majority of diabetic patients with early-phase autonomic neuropathy.38
CAN is a simple, noninvasive marker of an imbalance or disturbance between sympathetic and parasympathetic (vagal) activity in the lower extremities.35 The Seattle Diabetic Foot Study used cardiovascular reflexes as a measure of peripheral autonomic neuropathy.33 In a previous cross-sectional study in patients with type 2 diabetes, systolic ankle-brachial blood pressure ratio and peripheral artery stiffness were associated with autonomic neuropathy, as evaluated by conventional cardiovascular AFTs and HRV indices.39,40 In our study, CAN was a significant independent prognostic factor for DFUs in patients without DPN. CAN might be associated with endothelial dysfunction by enhancing angiotensin II production and inflammatory response41 which may affect development of DFUs. In addition, CAN is also reportedly related to progression of carotid atherosclerosis,42 CAD,43 and lower extremity arterial calcification.44 The association between CAN and atherosclerosis could be explained by a maladaptive phenomenon in vascular function and structural integrity. However, this relationship has not been fully clarified.
Similar to other types of diabetic complications, glycemic control is important for preventing DFUs.45,46 According to a meta-analysis, the overall relative risk for LEA was 1.26 (95% CI, 1.16–1.36) for each percentage point increase in HbA1c.46 One of the important findings in our study is that adequate glycemic control is essential for prevention of DFUs and LEAs. The overall HR for DFUs was 1.58 (1.10–2.25) for each percentage point increase in mean HbA1c among patients with type 2 diabetes in this study. Diabetic nephropathy is a well-known factor of DFUs.27,31 In our study, diabetic nephropathy was a significant risk factor for DFUs, and patients with diabetic nephropathy and definite CAN had an approximately 9-fold higher risk of developing DFUs compared with patients without either factor.
The major strength of this study was the long-term observation to ascertain the relationship between CAN and DFUs in a large cohort of patients with type 2 diabetes. Several limitations of this study need to be addressed. First, we could not check the blood pressure response to standing or the response to sustained handgrip which initially reflect sympathetic autonomic dysfunction. However, each of the 3 tests we performed and analyzed in this study had high reliability and good reproducibility.10 Second, we did not include the patients who did not receive complete follow-up evaluation in the analysis, and this may affect the interpretation of the results, including selection bias. Finally, this study adjusted for CAD and CVA; however, peripheral or other atherosclerosis was not excluded or adjusted for in this study. Although vascular disease and ischemia contribute to DFUs, 60% to 70% of DFUs are neuropathic in origin.47
In summary, the results of this study underscore the importance of CAN as a predictor of DFUs and emphasize the need for clinicians to be aware of patients with definite CAN and type 2 diabetes. The results of this study confirm that definite CAN predicts development of DFUs, even in patients without DPN, which is a major risk factor for DFUs. Screening for foot injuries, regular follow-up of foot status, and patient education are essential for patients with definite CAN in order to prevent DFUs and related LEAs. Subjects with type 2 diabetes and definite CAN, especially individuals with uncontrolled glycemic status, may require more aggressive foot surveillance and additional efforts for foot protection.
Supplementary Material
Acknowledgment
The authors thank S Hwang, HW Jeon (St Vincent's Hospital, The Catholic University of Korea) for their assistance analyzing data.
Footnotes
Abbreviations: AFT = autonomic function test, CAD = coronary artery disease, CAN = cardiovascular autonomic neuropathy, CKD = chronic kidney disease, CVA = cerebrovascular disease, CVD = cardiovascular disease, DFU = diabetic foot ulcer, DPN = diabetic polyneuropathy, eGFR = estimated glomerular filtration rate, HRV = heart rate variability, LEA = lower extremity amputation, PAD = peripheral artery disease, UAE = urine albumin excretion.
J-SY analyzed data, wrote the manuscript, and interpreted data. S-AC reviewed and edited the manuscript and contributed to discussions. T-SL reviewed and edited the manuscript. E-YL reviewed the manuscript and contributed to discussions. K-HS reviewed the manuscript and contributed to discussions. Y-BA researched data and reviewed the manuscript. K-DY researched data and reviewed the manuscript. J-SK researched data and reviewed the manuscript. Y-MP contributed to statistical analysis, data interpretation, and discussions. S-HK designed the study, collected and researched data, interpreted data, and wrote the manuscript. S-HK is the guarantor of this work and, as such, had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.van Battum P, Schaper N, Prompers L, et al. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet Med 2011; 28:199–205. [DOI] [PubMed] [Google Scholar]
- 2.Boyko EJ, Ahroni JH, Smith DG, et al. Increased mortality associated with diabetic foot ulcer. Diabet Med 1996; 13:967–972. [DOI] [PubMed] [Google Scholar]
- 3.Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006; 45 (Suppl):S1–S66. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-de-Andres A, Martinez-Huedo MA, Carrasco-Garrido P, et al. Trends in lower-extremity amputations in people with and without diabetes in Spain, 2001–2008. Diabetes Care 2011; 34:1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vamos EP, Bottle A, Majeed A, et al. Trends in lower extremity amputations in people with and without diabetes in England, 1996–2005. Diabetes Res Clin Pract 2010; 87:275–282. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008; 31:1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruun C, Siersma V, Guassora AD, et al. Amputations and foot ulcers in patients newly diagnosed with type 2 diabetes mellitus and observed for 19 years. The role of age, gender and co-morbidity. Diabet Med 2013; 30:964–972. [DOI] [PubMed] [Google Scholar]
- 8.Brownrigg JR, Davey J, Holt PJ, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia 2012; 55:2906–2912. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Lee KA, Jin HY, et al. Relationship between the Korean Version Survey of the Autonomic Symptoms Score and cardiac autonomic neuropathy parameters in patients with diabetic peripheral neuropathy. Diabetes Metab J 2014; 38:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinik AI, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care 2003; 26:1553–1579. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore JE, Allen JA, Hayes JR. Autonomic function in neuropathic diabetic patients with foot ulceration. Diabetes Care 1993; 16:61–67. [DOI] [PubMed] [Google Scholar]
- 12.Edmonds ME, Nicolaides KH, Watkins PJ. Autonomic neuropathy and diabetic foot ulceration. Diabet Med 1986; 3:56–59. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 14.Ghanassia E, Villon L, Thuan Dit Dieudonne JF, et al. Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5-year follow-up study. Diabetes Care 2008; 31:1288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun JS, Ko SH, Ko SH, et al. Presence of macroalbuminuria predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care 2013; 36:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996; 39:1377–1384. [DOI] [PubMed] [Google Scholar]
- 17.Yun JS, Ko SH, Ko SH, et al. Cardiovascular disease predicts severe hypoglycemia in patients with type 2 diabetes. Diabetes Metab J 2015; 39:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol 2012; 8:405–416. [DOI] [PubMed] [Google Scholar]
- 20.Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr 2015; 9:192–199. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). National Hospital Discharge Survey. www.cdc.gov/diabetes/statistics/hospitalization_national.htm Accessed September 17, 2015. [Google Scholar]
- 22.Ahmad N, Thomas GN, Chan C, et al. Ethnic differences in lower limb revascularisation and amputation rates. Implications for the aetiopathology of atherosclerosis? Atherosclerosis 2014; 233:503–507. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Ostbye T, Tan SB, et al. Risk factors for lower extremity amputation among patients with diabetes in Singapore. J Diabetes Complications 2011; 25:382–386. [DOI] [PubMed] [Google Scholar]
- 24.Chi ZS, Lee ET, Lu M, et al. Vascular disease prevalence in diabetic patients in China: standardised comparison with the 14 centres in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001; 44 suppl 2:S82–S86. [DOI] [PubMed] [Google Scholar]
- 25.Lee ET, Keen H, Bennett PH, et al. Follow-up of the WHO multinational study of vascular disease in diabetes: general description and morbidity. Diabetologia 2001; 44 suppl 2:S3–S13. [DOI] [PubMed] [Google Scholar]
- 26.Aleem MA. Factors that precipitate development of diabetic foot ulcers in rural India. Lancet 2003; 362:1858. [DOI] [PubMed] [Google Scholar]
- 27.Mayfield JA, Reiber GE, Sanders LJ, et al. Preventive foot care in people with diabetes. Diabetes Care 1998; 21:2161–2177. [DOI] [PubMed] [Google Scholar]
- 28.Aziz Z, Lin WK, Nather A, et al. Predictive factors for lower extremity amputations in diabetic foot infections. Diabet Foot Ankle 2011; 2: doi: 10.3402/dfa.v2i0.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad J. The diabetic foot. Diabetes Metab Syndr 2015; doi: 10.1016/j.dsx.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Gardner SE, Frantz RA. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol Res Nurs 2008; 10:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994; 17:557–560. [DOI] [PubMed] [Google Scholar]
- 32.Abbott CA, Vileikyte L, Williamson S, et al. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998; 21:1071–1075. [DOI] [PubMed] [Google Scholar]
- 33.Boyko EJ, Ahroni JH, Stensel V, et al. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999; 22:1036–1042. [DOI] [PubMed] [Google Scholar]
- 34.Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care 2006; 29:1202–1207. [DOI] [PubMed] [Google Scholar]
- 35.Aso Y, Fujiwara Y, Inukai T, et al. Power spectral analysis of heart rate variation in diabetic patients with neuropathic foot ulceration. Diabetes Care 1998; 21:1173–1177. [DOI] [PubMed] [Google Scholar]
- 36.Edmonds ME. The diabetic foot: pathophysiology and treatment. Clin Endocrinol Metab 1986; 15:889–916. [DOI] [PubMed] [Google Scholar]
- 37.Kozniewska E, Jung L, Skolasinska K, et al. Changes in blood flow and permeability of vessels to protein preceding the development of cutaneous ulcers in the hind limb of the rabbit. Microvasc Res 1980; 19:189–196. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Nishizawa Y, Emoto M, et al. Sympathetic function test of vasoconstrictor changes in foot arteries in diabetic patients. Diabetes Care 1998; 21:1495–1501. [DOI] [PubMed] [Google Scholar]
- 39.Canani LH, Copstein E, Pecis M, et al. Cardiovascular autonomic neuropathy in type 2 diabetes mellitus patients with peripheral artery disease. Diabetol Metab Syndr 2013; 5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care 2013; 36:2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe S, Amiya E, Watanabe M, et al. Simultaneous heart rate variability monitoring enhances the predictive value of flow-mediated dilation in ischemic heart disease. Circ J 2013; 77:1018–1025. [DOI] [PubMed] [Google Scholar]
- 42.Gottsater A, Ahlgren AR, Taimour S, et al. Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res 2006; 16:228–234. [DOI] [PubMed] [Google Scholar]
- 43.Liao D, Carnethon M, Evans GW, et al. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes 2002; 51:3524–3531. [DOI] [PubMed] [Google Scholar]
- 44.Costacou T, Huskey ND, Edmundowicz D, et al. Lower-extremity arterial calcification as a correlate of coronary artery calcification. Metab Clin Exp 2006; 55:1689–1696. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Kim DJ, Jang HC, et al. Epidemiology of micro- and macrovascular complications of type 2 diabetes in Korea. Diabetes Metab J 2011; 35:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler AI, Erqou S, Lima TA, et al. Association between glycated haemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus—review and meta-analysis. Diabetologia 2010; 53:840–849. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez ER, Oley MA. The management of lower-extremity diabetic ulcers. Manag Care Interface 2000; 13:80–87. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


