Abstract
The prognostic value of serum lipid-profile in renal cell cancer (RCC) remains unknown. The purpose of the study is to explore the association between the serum lipid-profile and RCC patients.
The levels of preoperative serum lipid-profile (including cholesterol, triglycerides, high-density lipoprotein-cholesterol [HDL-C], low-density lipoprotein-cholesterol [LDL-C], apolipoprotein A-I [ApoA-I], and apolipoprotein B [ApoB]) were retrospectively performed in 786 patients with RCC. The cutoff values of the lipids were determined by the receiver-operating characteristic (ROC) curve analysis. Univariate and multivariate Cox regression analyses were performed to investigate the prognostic value of serum lipids in RCC.
Combined ROC analysis and univariate and multivariate Cox regression analyses, for overall survival (OS), revealed patients with low ApoA-I (<1.04) had significantly lower OS than the high ApoA-I (≥1.04) group (multivariate Cox regression analyses, hazard ratio [HR], 0.57; P = 0.003). Not only in the whole RCC cohort but also in the subgroups stratified according to the pT1-2 (P = 0.002), pN0 (P < 0.001), and pM0 (P = 0.001) status, respectively. Moreover, in the 755 patients with nonmetastasis, the low ApoA-I group was also associated with shortened disease-free survival (DFS) time compared to the high ApoA-I group (multivariate Cox regression analyses, HR, 0.65; P = 0.033). However, the other lipids were not independent prognostic factors for surgical RCC.
An elevated level of preoperative ApoA-I was demonstrated to be related with better survival in patients with surgical RCC. Measuring the preoperative ApoA-I might be a simple way for finding the poor prognostic patients who should enrolled in further clinical trials and management.
INTRODUCTION
Renal cell carcinoma (RCC) comprises approximately 3.8% of all new malignancies in adults.1About 90% of renal tumors are RCC, and almost 80% of these are clear cell tumors.2,3 Due to the increased use of imaging techniques, often ultrasound and computed tomography, the frequency of incidental detection of tumors has increased and patients with RCC typically present with a suspicious mass involving the kidney that has been visualized.4–6 Renal tumors are both unresponsive to chemotherapy and radiotherapy and surgical resection is the only known curative treatment for localized disease.7 After radical or partial nephrectomy, metastasis develops in about 2 years.8 Although the tumor–node–metastasis (TNM) stage and Fuhrman grade are currently the most extensively used prognostic tools, they are not entirely reliable.9 Other prognostic variables are pretreatment neutrophil-to-lymphocyte ratio,10 different histologic subtypes,11 and positive surgical parenchymal margin.12 Thanks to the insufficiency of these prognostic factors, new clinical and laboratory markers have started to be studied and established.
Serum lipid and lipoprotein abnormalities occur regularly in many experimental tumor systems.13,14 Cholesterol synthesis is raised in malignant cells compared with normal cells. Malignant cells need excess cholesterol and intermediates of the cholesterol biosynthesis pathway to maintain a high level of proliferation.15 Plasma high- and low-density lipoproteins are the probable major suppliers of cholesterol to cancer cells and tumors, potentially via receptor-mediated mechanisms.15 Some researchers reveal that overexpression of human apolipoprotein A-I (ApoA-I) in transgenic mice inhibits tumor growth and improves survival in a mouse model of ovarian cancer.16 The relationship between serum lipids and lipoproteins and tumors in humans is being explored. The increased cancer risk (such as breast, prostate, lung, and colon cancer) has been found to be associated with increasing dietary fat or cholesterol.17–19 Several studies have also reported that greater circulating total cholesterol concentration was related with cancer morbidity.20,21 Recently, ApoA-I had been revealed to be a potentially useful biomarker in metastatic nasopharyngeal carcinoma.22 However, no study was performed on the potential association of serum lipids and lipoproteins with surgical RCC. The purpose of this research was to analyze the prognostic value of serum lipids and lipoproteins in patients with surgical RCC.
PATIENTS AND METHODS
Patients
We retrospectively enrolled 912 patients diagnosed with RCC who were undergoing resection of primary tumor at Sun Yat-sen University Cancer Center (SYSUCC) between January 2000 and December 2012. The inclusion criteria of the study were as follows: no previous or coexisting tumor, have pretreatment blood sampling for lipids (including cholesterol, triglycerides, high-density lipoprotein-cholesterol [HDL-C], low-density lipoprotein-cholesterol [LDL-C], ApoA-I, and apolipoprotein B [ApoB]), and staged on the basis of the 2010 TNM staging system and the Fuhrman grading system. Patients were excluded: concomitant diseases, such as diabetes, hyperlipidemia, or metabolic syndrome, had an effect on serum lipid levels; using hormone replacement therapy or any drugs influencing in lipid metabolism. A total of patients who met abovementioned criteria were enrolled in this study. The study was approved by the Institutional Review Board of SYSUCC, and written informed consent was obtained for each patients.
Clinical Data Extraction
We collected baseline characteristic of participants from the case files, including age, body mass index (BMI), gender, stage, pathological types, Fuhrman-grading, and pTNM stage. Urine sample was tested preoperative urine protein. Blood samples were tested prior to initial surgical resection for levels of alkaline phosphatase (ALP), lactate dehydrogenase (LDH), serum creatinine (CRE), uric acid (UA), serum cholesterol, serum triglycerides, serum HDL-C, serum LDL-C, serum ApoA-I, and serum ApoB. Categories of each characteristic divided into as following: elevated ALP level was defined, if ALP value in serum was >135 U/L. Similarly, elevated LDH was defined when the LDH > 245 U/L. Elevated CRE was defined when the CRE > 130μmol/L. Elevated UA was defined when the UA > 420 μmol/L.
Patients Follow-up
Follow-up was carried out by telephone interview and complimentary medical records review. Important follow-up data included postoperative adjuvant therapy, living status, progression, and sites of tumor metastases. The last follow-up was completed in November 01, 2015, and after that, the whole data were analyzed. The primary endpoint was overall survival (OS), which was defined as the interval between surgery and last follow-up or death. The secondary endpoint was disease-free survival (DFS) which was calculated as the interval between surgery and last follow-up or recurrence or death.
Statistical Analysis
Continuous variables and categorical variables were presented as means and standard deviations, and frequencies and percentages, respectively. Percentage differences between groups were compared with the χ2 test or Fisher exact test. Comparison of continuous data was done by use of the Mann–Whitney test. Receiver-operating characteristic (ROC) curve analysis was used to determine the joint maximum sensitivity and specificity of a cutoff value to stratify patients at high risk of death for serum cholesterol, serum triglycerides, serum HDL-C, serum LDL-C, serum ApoA-I, and serum ApoB. The predictive value of the established model was assessed by using the area under the ROC curve (AUC) and the pairwise comparison of AUC values of significant biomarkers was carried out by using z statistic. OS and DFS after surgery were measured by using of Kaplan–Meier curves and the log-rank test. Univariate Cox regression analyses were done to compare all the variables, and significant prognostic factors identified from the univariate analysis were entered into the multivariate Cox regression analysis of survival to test for independence. Hazard ratios (HRs) estimated from the Cox analysis were reported as relative risks with corresponding 95% confidence intervals (CIs). All statistical analyses were performed using SPSS21.0 software (IBM, Armonk, NY) and MedCalc (MedCalc Software, Ostend, Belgium). All tests were 2-sided and a P value <0.05 was considered statistically significant.
RESULTS
Clinicopathologic Characteristics
A total of 786 enrolled patients were diagnosed with RCC. The majority of the patients enrolled were males (n = 526, 66.90%), and pathological diagnosed as clear cell carcinoma (n = 605, 77.00%). The mean age was 51.27 (standard deviation [SD]: ±13.57) years. Among these, 527 (67.10%), 122 (15.50%), 88 (11.20%), and 49 (5.20%) were staged in I, II, III, and IV, respectively. Most of the enrolled patients (n = 716, 91.10%) received no adjuvant therapy. The baseline characteristics of the 786 patients are shown in Table 1.
TABLE 1.
Baseline Characteristics of All Patients (n = 786)

The distributions of preoperative serum lipid and lipoprotein levels are shown in Table 2. In a majority of the RCC patients, serum lipid and lipoprotein levels, except LDL-C level, were within normal limits before surgery. Below the normal levels of serum cholesterol, HDL-C, LDL-C, ApoA-I, and ApoB were observed in nearly 0.1% (n = 1), 6.2% (n = 49), 14.2% (n = 112), 28.0% (n = 220), and 8.8% (n = 69) of patients, respectively. On the contrary, 9.7% (n = 76), 33.3% (n = 262), 1.0% (n = 8), 36.6% (n = 288), 3.6% (n = 28), and 23.0% (n = 181) of patients had exceed the normal range of serum cholesterol, triglyceride, HDL-C, LDL-C, ApoA-I, and ApoB, respectively.
TABLE 2.
The Distribution of Baseline Serum Lipid and Lipoprotein Levels

The mean and median follow-up time was 81.05 (SD: ±1.40) and 76.19 (interquartile range [IQR]: 50.00–107.47) months. Mean DFS and OS were 149.40 (SD: ±2.38) and 143.51 (SD: ±3.09) months, respectively. The recommended cutoff values of serum cholesterol, triglyceride, HDL-C, LDL-C, ApoA-I, and ApoB, respectively, were based on the most prominent point on the ROC curve for each value based on specificity and sensitivity (Supplementary Table 1). The recommended serum cholesterol, triglyceride, HDL-C, LDL-C, ApoA-I, and ApoB values were defined as 5.30, 1.49, 1.31, 2.67, 1.04, and 0.82, respectively. Based on the pairwise comparisons of significant biomarkers (cholesterol, triglyceride, HDL-C, and ApoA-I), the results revealed there is no significant differences (Supplementary Table 2). According to the cutoff values, all the patients were divided into the low and high groups, respectively (Table 1).
The Relationship Between Clinicpathologic Characteristics and OS
In the univariate Cox proportional hazards regression model analysis, based on the cutoff levels of lipids and lipoproteins, the preoperative high serum cholesterol (P < 0.001), triglyceride (P = 0.004), HDL-C (P = 0.006), LDL-C (P = 0.018), and ApoA-I (P < 0.001) were associated with better OS. In addition, the age, pathological types, Fuhrman-grade, pTNM stage, pT-status, pN-status, pM-status, adjuvant therapy, LDH, and CRE also remained clinically and statistically significant predictors of prognosis (Table 3 ).
TABLE 3.
Univariate and Multivariate Analyses for Variables Considered for Overall Survival (OS) (Cox Proportional Hazard Regression Model) (n = 786)
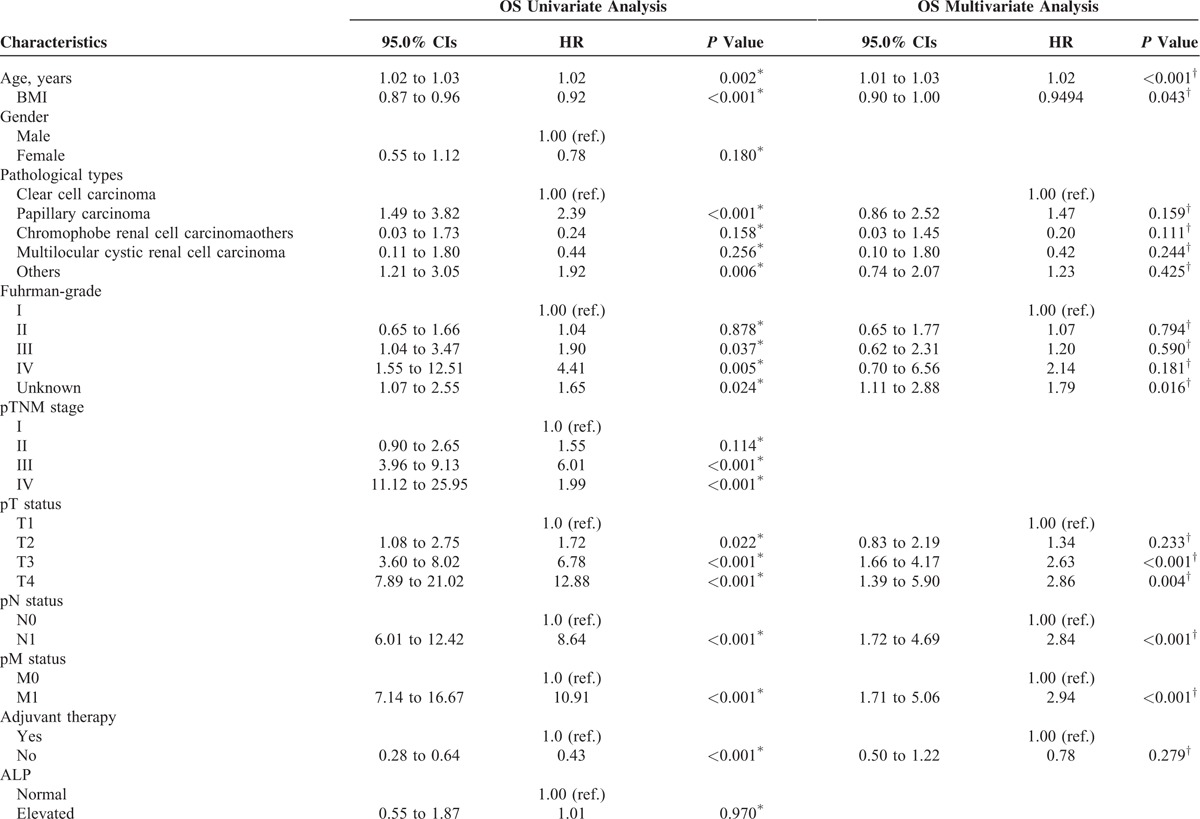
In the multivariate Cox regression model, considering the influence of statistical colinearity between pTNM stage and pT-status, pN-status, pM-status, the multivariate model did not include pTNM stage. The results showed that the high ApoA-I was a significant independent predictors of favorable OS (hazard ratio [HR], 0.57; P = 0.003). In addition, the age, BMI, pT-status, pN-status, and pM-status also remained clinically and statistically significant predictors of prognosis (Table 3 ).
To further investigate the prognostic significance of ApoA-I level in surgical RCC patients, the whole cohort was compared by the Kaplan–Meier method and the log-rank test. Patients with ApoA-I < 1.04 (n = 207) showed a significantly worse OS than the ApoA-I ≥ 1.04 group (n = 579) (ApoA-I < 1.04 vs ≥1.04, mean OS: 126.13 vs 141.78 months, respectively, P < 0.001, Figure 1A). We also evaluated the prognostic influence of the ApoA-I level in the subgroups based on the pT-status, pN-status, pM-status, respectively. Patients with a low ApoA-I level had a significantly shorter OS compared with those patients with a high ApoA-I level in the T1–2 subgroup (n = 676, ApoA-I < 1.04 vs ≥1.04, mean OS: 143.26 vs 149.91 months, respectively, P = 0.002, Figure 2A), N0 subgroup (n = 729, ApoA-I < 1.04 vs ≥1.04, mean OS: 134.03 vs 147.43 months, respectively, P < 0.001, Figure 2C), and M0 subgroup (n = 755, ApoA-I < 1.04 vs ≥1.04, mean OS: 134.68 vs 144.45 months, respectively, P = 0.001, Figure 2E). There was not statistical significance in the T3–4 subgroup (n = 110, ApoA-I < 1.04 vs ≥1.04, mean OS: 60.59 vs 88.33 months, respectively, P = 0.051, Figure 2B), N1 subgroup (n = 57, ApoA-I < 1.04 vs ≥1.04, mean OS: 51.89 vs 52.49 months, respectively, P = 0.254, Figure 2D), or M1 subgroup (n = 31, ApoA-I < 1.04 vs ≥1.04, mean OS: 30.29 vs 38.61 months, respectively, P = 0.384, Figure 2F).
FIGURE 1.

According to the preoperative ApoA-I levels, Kaplan–Meier curves depicting OS (A) in 786 patients and DFS (B) in 755 patients (M0) with renal cell cancer. DFS = disease-free survival, OS = overall survival.
FIGURE 2.

Kaplan–Meier curves depicting OS according to preoperative ApoA-I levels in 786 patients with renal cell cancer. Patients were stratified according to the pT-status, pN-status, and pM-status. (A) Kaplan–Meier analysis of OS in T1-2 subgroup. (B) Kaplan–Meier analysis of OS in T3-4 subgroup. (C) Kaplan–Meier analysis of OS in N0 subgroup. (D) Kaplan–Meier analysis of OS in N1 subgroup. (E) Kaplan–Meier analysis of OS inM0 subgroup. (F) Kaplan–Meier analysis of OS in M1 subgroup. ApoA-I = apolipoprotein A-I, OS = overall survival.
Association of Preoperative ApoA-I Level With Clinicopathologic Characteristics
The preoperative ApoA-I level was significantly correlated with gender (P < 0.001), pTNM (P < 0.001), pT status (P = 0.002), pN status (P = 0.029), pM status (P < 0.001), and LDH (P < 0.001). BMI (P = 0.487), age (P = 0.319), pathological types (P = 0.290), etc., had not influence on the preoperative ApoA-I level (Table 4).
TABLE 3 (Continued).
Univariate and Multivariate Analyses for Variables Considered for Overall Survival (OS) (Cox Proportional Hazard Regression Model) (n = 786)

The Clinicopathologic Characteristics and DFS by Univariate and Multivariate Cox Proportional Hazards Regression Model Analysis
For DFS, we excluded the patients with pM1 classification (n = 31). A total of 755 patients were enrolled to analyze the relationship between lipids and clinicpathologic characteristics. In the univariate Cox proportional hazards regression model analysis, the results showed that high serum cholesterol (P = 0.001), triglyceride (P = 0.011), and ApoA-I (P = 0.003) were significantly independent predictors of favorable DFS. The age, pathological types, pTNM stage, pT-status, pN-status, adjuvant therapy, LDH, and CRE remained a clinically and statistically significant predictors of prognosis (Table 5 ).
TABLE 4.
Clinicopathological Variables of Patients According to the Different Level of ApoA-I

TABLE 5.
Univariate and Multivariate Analyses for Variables Considered for Disease-Free Survival (DFS) (Cox Proportional Hazard Regression Model) (n = 755)

TABLE 5 (Continued).
Univariate and Multivariate Analyses for Variables Considered for Disease-Free Survival (DFS) (Cox Proportional Hazard Regression Model) (n = 755)

Secondly, we used a multivariate model to adjust for the confounders of the association of baseline serum lipid and lipoprotein levels with survival. Considering the mulicolinearity between pTNM stage and pT-status, pN-status, the multivariate model did not include pTNM stage. The results showed that the high ApoA-I was a significantly independent predictor of favorable DFS (HR, 0.65; P = 0.033). In addition, the age, BMI, pT-status, and pN-status also remained clinically and statistically significant predictors of prognosis (Table 5 ).
Thirdly, the Kaplan–Meier method and log-rank test were used to compare the different effects of ApoA-I level on DFS. Patients with ApoA-I < 1.04 (n = 190) showed a significantly worse OS than the ApoA-I ≥ 1.04 group (n = 565) (ApoA-I < 1.04 vs ≥1.04, mean OS: 136.76 vs 143.64 months, respectively, P = 0.002, Figure 1B).
DISCUSSION
This is the 1st large-scale cohort study to analyze the relationship between serum lipids and lipoproteins and RCC. In this research, although we found most of the preoperative serum cholesterol, triglyceride, HDL-C, ApoA-I, and ApoB levels were within the normal range, several studies revealed that a closer relationship between serum lipid profile and the incidence of some cancers has been found in different ethnic groups and a large number of subjects.23–25 Previous studies had also found the different prognostic effects of lipids in some cancers.26–28 However, no studies had been explored to analysis the association the prognostic values of the lipid profile in RCC patients. We used the ROC analysis to determine the optimal cutoff values for the lipids and found that the elevated baseline ApoA-I level was significantly associated with better survival and was independent of other variables predicting the prognosis of RCC patients. For OS, we also found that pretreatment age, BMI, pT-status, pN-status, and pM-status were independent prognostic factors of RCC. Furthermore, for DFS, we found that age, BMI, pT-status, and pN-status were independent prognostic factors.
In the analyses of subgroups between the ApoA-I level and OS according to the pT, pN, pM classification, our study demonstrated the high ApoA-I level was a favorable prognostic factor which associated with pT1–2, pN0, and pM0 subgroups, respectively. Although no statistical significance in the advanced pT, pN, and pM subgroups, the high ApoA-I level also presented a higher mean survival time than the low group. The lack of significance of this finding is probably due to an insufficient number of advanced patients.
ApoA-I, the main protein component of HDL-C, is synthesized predominantly in the liver and the small intestine, and exists transiently in lipid-poor form.29 ApoA-I binding to the extracellular domain of ABCA1 results in the active removal of cellular cholesterol and phospholipids to lipid-poor apolipoproteins from a variety of cells.30 In addition, this process plays crucial roles in both the formation and maintenance of HDL-C levels in plasma and is likely important for the 1st step of the reverse cholesterol transport process from peripheral tissues.31 The HDL-C particle is further matured by lecithin cholesterol acyltransferase (LCAT) binding to ApoA-I on HDL-C and converting cholesterol to cholesteryl ester.32 The chylomicrons secreted from the intestine enterocyte also contain ApoA-I, but it is quickly transferred to HDL-C in the bloodstream. Its function to participate in and promote the reverse transport of cholesterol from tissues to the liver for excretion, and by acting as a cofactor for lectin cholesterol acyltransferase (LCAT), which is responsible for the conversion of cholesterol to cholesteryl ester (CE), and the transfer of fatty acids (linoleic acid and oleic acid) and ethanolamine back to cells for reutilization.33 Nevertheless, the role of ApoA-I in cancer is not well understood. ApoA-I may be mainly attributed to anti-atherogenic, anti-inflammatory, and anti-oxidant properties.34 Recently, researchers used ApoA-I mimetic peptides to observe the association between ApoA-I and cancer cells. ApoA-I mimetic peptides reduced viability and proliferation of ID8 cells and cis-platinum-resistant human ovarian cancer cells, and decreased ID-8 cell-mediated tumor burden in C57BL/6J mice when administered subcutaneously or orally.16 This experimental research can help explain the biological mechanisms for the association between ApoA-I and RCC observed in our epidemiologic study. In addition, decreased ApoA-I levels have also been reported to be an indicator for early epithelial ovarian, pancreatic, nasopharyngeal cancer, and so on. Our results also suggest that the reduction of preoperative ApoA-I level is not only strongly correlated with worse OS and DFS, but also an independent factor for survival in the univariate and multivariate Cox regression analyses.
BMI is one of the nutritional indexes. We found that no significant distribution between the low and high ApoA-I level for BMI. The result revealed that nutritional status was not associated with the ApoA-I concentration. The lower level of ApoA-I was not owing to nutritional deficiency in the RCC patients. One explanation we concluded that the low level of ApoA-I in tumors is dependent on other factors, such as cholesterol concentration or inflammatory reaction. We need further research to support these hypotheses.
Some important clinical implications can be drawn from our findings. Firstly, although the results from our univariate Cox regression analyses showed that serum cholesterol, triglyceride, HDL-C, and LDL-C levels were significantly associated with OS, after adjustment for clinical factors, no statically significance was found. One possible reason is that the changes in the lipids and lipoproteins profiles might be a proxy for changes in some etiological mechanisms for surgical RCC patients. Secondly, in vivo, ApoA-I mimetic peptides also observe the association between ApoA-I and cancer cells. The combination of the ApoA-I treatment and other drugs might be useful in significantly improving the survival of patients. Finally, the cutoff value of ApoA-I might be used to provide further management for clinical trials and high risk group in surgical RCC patients.
However, some limitations exist in our study. Firstly, our outcomes originate from retrospective data. No prospective randomized trials were performed in surgical RCC patients. Hence, we will continue to conduct prospective studies to validate our conclusions. Second, after surgical resection the survival of patients is greatly extended, so our results have not been able to replicate the median survival time. So, we will keep observing these patients were followed to obtain a more reliable result. Lastly, our results still need further validation.
In conclusion, our results revealed that preoperative serum ApoA-I level could function as an independent prognostic factor for surgical RCC patients. This is also confirmed in some subgroups of patients according to the pT, pN, and pM classifications. Serum ApoA-I can be widely used to routinely evaluate pretreatment patients due to a far lower cost and greater convenience than other more complex and expensive evaluative techniques.
Supplementary Material
Acknowledgments
The authors thank National Natural Science Foundation of China (Grant No. 81302224 and 81202013); Medical Scientific Research Foundation of Guangdong Province, China (Grant No. B2012131) for the support.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ApoA-I = apolipoprotein A-I, ApoB = apolipoprotein B, BMI = body mass index, CRE = serum creatinine, HDL-C = high-density lipoprotein–cholesterol, LDH = lactate dehydrogenase, LDL-C = low-density lipoprotein-cholesterol, RCC = renal cell cancer, UA = uric acid.
SG, XH, and QC contributed equally to this work.
This study was supported by grants from National Natural Science Foundation of China (Grant No. 81302224 and 81202013); Medical Scientific Research Foundation of Guangdong Province, China (Grant No. B2012131).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 2000; 89:604–614. [PubMed] [Google Scholar]
- 3.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol 2010; 183:1309–1315. [DOI] [PubMed] [Google Scholar]
- 4.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998; 51:203–205. [DOI] [PubMed] [Google Scholar]
- 5.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997). Urology 2000; 56:58–62. [DOI] [PubMed] [Google Scholar]
- 6.Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology 2005; 236:441–450. [DOI] [PubMed] [Google Scholar]
- 7.Lam JS, Leppert JT, Figlin RA, et al. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep 2005; 6:7–18. [DOI] [PubMed] [Google Scholar]
- 8.Russo P, O’Brien MF. Surgical intervention in patients with metastatic renal cancer: metastasectomy and cytoreductive nephrectomy. Urol Clin North Am 2008; 35:679–686. [DOI] [PubMed] [Google Scholar]
- 9.Shuch BM, Lam JS, Belldegrun AS, et al. Prognostic factors in renal cell carcinoma. Semin Oncol 2006; 33:563–575. [DOI] [PubMed] [Google Scholar]
- 10.Ohno Y, Nakashima J, Ohori M, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol 2010; 184:873–878. [DOI] [PubMed] [Google Scholar]
- 11.Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003; 27:612–624. [DOI] [PubMed] [Google Scholar]
- 12.Permpongkosol S, Colombo JJ, Gill IS, et al. Positive surgical parenchymal margin after laparoscopic partial nephrectomy for renal cell carcinoma: oncological outcomes. J Urol 2006; 176:2401–2404. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis 2006; 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokoglu E, Karaarslan I, Karaarslan HM, et al. Alterations of serum lipids and lipoproteins in breast cancer. Cancer Lett 1994; 82:175–178. [DOI] [PubMed] [Google Scholar]
- 15.The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol 2013; 4:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su F, Kozak KR, Imaizumi S, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci U S A 2010; 107:19997–20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furberg AS, Veierod MB, Wilsgaard T, et al. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst 2004; 96:1152–1160. [DOI] [PubMed] [Google Scholar]
- 18.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011; 60:1094–1102. [DOI] [PubMed] [Google Scholar]
- 19.Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev 2009; 18:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose G, Blackburn H, Keys A, et al. Colon cancer and blood-cholesterol. Lancet 1974; 1:181–183. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Palmieri MR, Sorlie PD, Costas RJ, et al. An apparent inverse relationship between serum cholesterol and cancer mortality in Puerto Rico. Am J Epidemiol 1981; 114:29–40. [DOI] [PubMed] [Google Scholar]
- 22.Jiang R, Yang ZH, Luo DH, et al. Elevated apolipoprotein A-I levels are associated with favorable prognosis in metastatic nasopharyngeal carcinoma. Med Oncol 2014; 31:80. [DOI] [PubMed] [Google Scholar]
- 23.Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev 2009; 18:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallberg-Jonsson S, Dahlen G, Johnson O, et al. Lipoprotein lipase in relation to inflammatory activity in rheumatoid arthritis. J Intern Med 1996; 240:373–380. [DOI] [PubMed] [Google Scholar]
- 25.Mondul AM, Weinstein SJ, Virtamo J, et al. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control 2011; 22:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers 2015; 30:e200–e207. [DOI] [PubMed] [Google Scholar]
- 27.Chi PD, Liu W, Chen H, et al. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One 2014; 9:e91080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo E, Chen L, Xie Q, et al. Serum HDL-C as a potential biomarker for nodal stages in gastric cancer. Ann Surg Oncol 2007; 14:2528–2534. [DOI] [PubMed] [Google Scholar]
- 29.Thomas MJ, Bhat S, Sorci-Thomas MG. Three-dimensional models of HDL apoA-I: implications for its assembly and function. J Lipid Res 2008; 49:1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brewer HJ, Santamarina-Fojo S. New insights into the role of the adenosine triphosphate-binding cassette transporters in high-density lipoprotein metabolism and reverse cholesterol transport. Am J Cardiol 2003; 91:3E–11E. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184. [DOI] [PubMed] [Google Scholar]
- 32.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res 1968; 9:155–167. [PubMed] [Google Scholar]
- 33.Cucuianu M, Coca M, Hancu N. Reverse cholesterol transport and atherosclerosis. A mini review. Rom J Intern Med 2007; 45:17–27. [PubMed] [Google Scholar]
- 34.Tardif JC, Heinonen T, Noble S. High-density lipoprotein/apolipoprotein A-I infusion therapy. Curr Atheroscler Rep 2009; 11:58–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


