Abstract
There is little information on how the change in serum aminotransferase affects mortality. We investigated the association between changes in serum aminotransferase levels and mortality from all causes, cardiovascular disease (CVD), and liver disease.
Three percent of men from the Korean National Health Insurance database were sampled randomly at the end of 2002. After excluding patients with cancer, CVD, CVD risk factors, or liver disease, those who participated in 2 consecutive rounds of the national health screening examination were included (n = 68,431). The primary outcome was CVD mortality. Secondary outcomes were liver disease mortality and all-cause mortality. Change in metabolic profiles was analyzed based on changes in liver enzyme levels. Elevated levels of serum aminotransferase were associated with CVD, liver disease, and all-cause mortality. Men who had sustained elevation of serum aminotransferase during 2 subsequent liver enzyme tests showed a significantly higher risk of CVD mortality (adjusted hazard ratio [aHR] 1.95; 95% confidence interval [CI] 1.07–3.56, 2.29; 1.27–4.12) than the sustained normal group. In contrast, the normalization group (aHR 1.52, 95% CI 0.82–2.81 for aspartate aminotransferase [AST]; aHR 1.35, 95% CI 0.70–2.61 for alanine aminotransferase [ALT]) and the new elevation group (aHR 1.27, 0.66–2.44 for AST; aHR 0.99, 95% CI 0.49–2.20 for ALT) were not different from the sustained normal group in CVD mortality.
Individuals with serum aminotransferase elevation, particularly when sustained, are at higher risk of mortality, and should receive appropriate medical attention.
INTRODUCTION
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are commonly used biomarkers for liver disease1 which have also been linked to metabolic syndrome and cardiovascular disease (CVD).2 Studies have suggested that the elevation of liver enzymes is associated with CVD risk factors,3–6 events,7 and mortality,8,9 as well as liver disease mortality10,11 and all-cause mortality.12 However, other studies found no such associations, and results are inconsistent across studies. These varying findings may be explained by the use of clinic samples,5,6 nonextensive exclusion of those with previous diagnosis,3,4,7,8 and nonexhaustive adjustment of traditional risk factors.12
In addition, previous studies only utilized a single liver enzyme test. However, liver enzyme levels often normalize or fluctuate,13 and only a small proportion of individuals show persistent elevation.14,15 Thus, a single measure may limit the evaluation of the association between elevated liver enzyme levels and mortality. For this reason, previous studies have suggested the incorporation of follow-up laboratory results to refine the understanding of relationships of aminotransferase with morbidity and subsequent mortality.12 However, there is little information on the association between changes in liver enzyme levels and mortality.
Therefore, we carried out a population-based cohort study to investigate the association between liver enzyme changes and cardiovascular mortality, liver disease mortality, and all-cause mortality. We hypothesized that individuals who had sustained liver enzyme elevation would show increased CVD mortality, liver disease mortality, and all-cause mortality, and that their risk would be higher than that of individuals whose liver enzyme levels remained normal or normalized after repeat testing.
METHODS
Study Population
The Korean National Health Insurance (KNHI) program is a mandatory social insurance program that is operated by the government and covers almost the entire Korean population. KNHI provides a free biennial health-screening program for all members. This health-screening program aims to identify CVD risk factors (hypertension, diabetes, dyslipidemia, smoking, alcohol intake, obesity) as well as information on several other chronic conditions including liver disease.
We randomly selected a 3% sample of all male KNHI members as of December 31, 2002. We did not include women in this study because of lower mortality rates for women. As the screening was conducted biennially, we used 2-year windows (e.g., 2003–2004 and 2005–2006) to define participation in screening programs. Men who participated twice in the health screening program during these periods were selected (n = 144,370). We excluded participants who died (n = 304) or were lost to follow-up (n = 21) before 1 year after the second screening. Past medical history was ascertained using diagnoses in KNHI medical service claims data, which is coded according to the International Classification of Diseases, 10th revision (ICD-10). Participants with history of cancer (C00-C99, D00-D09), CVD and CVD related diseases such as hypertension, diabetes, and dyslipidemia (I10-15, I20-25, I34-37, I50, I60-66, I69-I74, E10-14, E78, N18-N19, G590, G632, H280, H360, M142, N083), or liver diseases (K70-K77) (n = 57,597) and missing information on laboratory results or lifestyle at baseline (n = 18,017) were also excluded. Participants with lifestyle risk factors of CVD (smoking, alcohol intake, obesity) were not excluded. The final sample size was 68,431 participants (Figure 1).
FIGURE 1.

Flowchart of study participants.
The study was approved by the institutional review board of the Seoul National University Hospital (IRB No. 1206-055-414).
Data Collection
We obtained baseline information on age, sex, monthly insurance premium (a proxy for economic status), disability status, and disease codes from the KNHI databases. People with disabilities were identified using the National Disability Registry.16 Comorbidities were identified using the ICD-10 diagnoses in the 2002 KNHI medical service claims database. Comorbidities were summarized using the Charlson comorbidity index, a weighted measure of comorbidity.
Laboratory data from health screening including serum aminotransferase, fasting blood glucose (FBS), and total cholesterol level were extracted from the KNHI dataset. The upper limits of normal (ULN) were defined as 30U/L for AST, and 30U/L for ALT according to previous studies.10,11 All participants completed self-questionnaires about their smoking status (nonsmoker, ex-smoker, current smoker), alcohol intake (drinker, nondrinker), and family history of liver disease. Information on height, weight, and blood pressure was also collected.
Study Outcomes
The primary outcome of this study was CVD mortality. Secondary outcomes were liver disease mortality and all-cause mortality. Information on mortality was acquired from the KNHI database which was synchronized with the National Death Registry through the Korean Electronic Government System. All deaths in Korea were registered to the National Death Registry; therefore, precise collection of information on mortality was possible. CVD deaths were defined as deaths with ICD-10 codes I05-I15, I20-28, I30-52, I60-89, and I95-99. Liver disease deaths were defined as deaths with ICD-10 codes of K70-K77. All-cause death included all deaths regardless of causes. To investigate the possible reason for the liver enzyme level changes, metabolic profile changes (body mass index [BMI], systolic blood pressure [SBP], diastolic blood pressure [DBP], FBS, and total cholesterol) and health behaviors (smoking, drinking) were analyzed for the difference between first and second screening results.
Statistical Analysis
The demographic data were reported using descriptive statistics. Values are presented as frequencies (percentages), means ± standard deviations (SD), or median (with 25th percentile, 75th percentile), as appropriate.
To investigate the effect of change in serum aminotransferase, we classified participants according to their baseline liver enzyme levels (<ULN, 1–2ULN, >2ULN) for the initial mortality analysis. Liver enzyme changes were classified into 4 groups: sustained normal (normal–normal), new elevation (normal–elevated), normalization (elevated–normal), and sustained elevation (elevated–elevated). In both analyses, a Cox proportional-hazards regression model was used to estimate the associations between liver enzyme levels and mortality. To assess long-term survival, subjects who died or were lost to follow-up within a year were excluded, a priori, from further analysis.12 Therefore, for the analysis using baseline liver enzyme levels, follow-up was started 1 year after the initial screening, and for analysis using the change in liver enzyme levels, follow-up was started 1 year after the second screening. Study subjects were followed until death or December 31, 2011, whichever came first. CVD mortality and liver disease mortality were adjusted for age, income, BMI, smoking, alcohol, SBP, DBP, FBS, total cholesterol, and family history of liver disease. Family history of liver disease was included as a proxy variable for hepatitis B virus status, as Korea had been an endemic area for vertical hepatitis B transmission. Overall mortality was evaluated after additional adjustment with Charlson comorbidity index and disability. The differences of BMI, SBP, DBP, FBS, and total cholesterol were evaluated using ANCOVA. Smoking and alcohol consumption were evaluated using the χ2 test. All statistical analyses were performed using STATA 12.0 (STATA Corp LP, College Station, TX). Statistical significance was defined as 2-tailed P value less than 0.05.
RESULTS
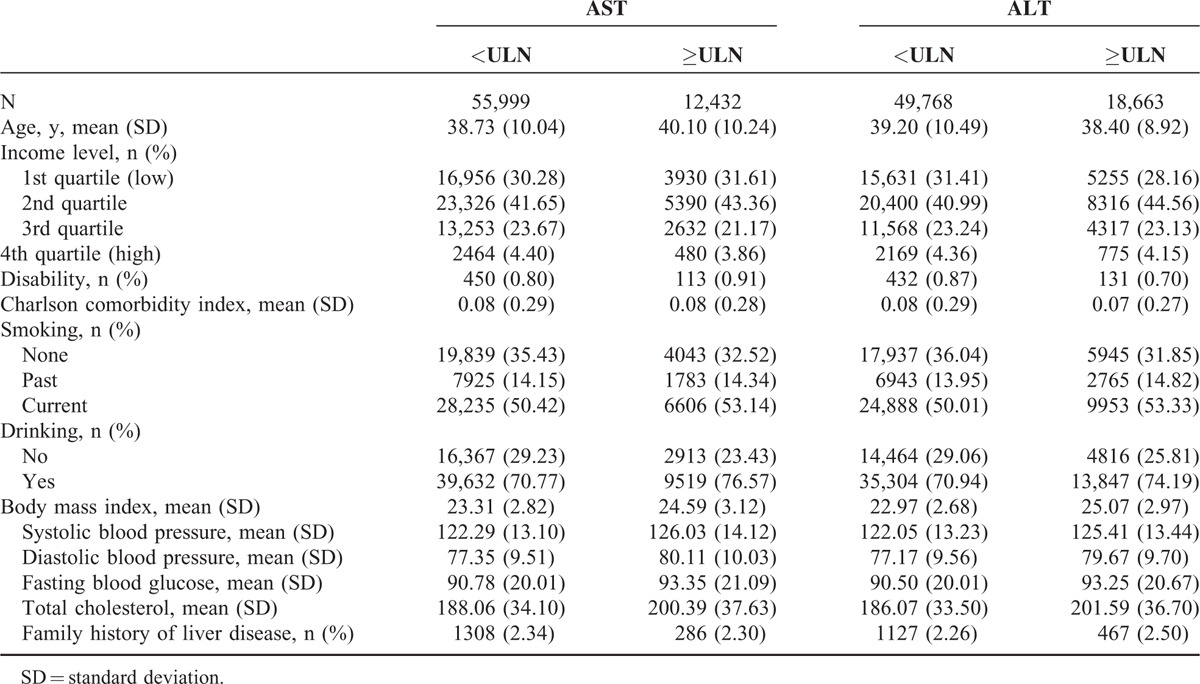
Baseline characteristics of study participants are presented in Table 1. Age was higher in participants with elevated AST. Participants with elevated AST or ALT had higher BMI, SBP, DBP, FBS, and total cholesterol. There were more current smokers and drinkers in the participants with elevated AST or ALT.
TABLE 1.
Baseline Characteristics of Study Participants
At the initial screening, participants with elevated AST or ALT levels had significantly increased risks of CVD mortality and all-cause mortality compared with those with normal AST or ALT levels, even after adjusting for age, income, BMI, smoking, alcohol, total cholesterol, FBS, SBP, DBP, and family history of liver disease. Participants with elevated AST had higher liver mortality. For those whose AST was >2ULN, the adjusted hazard ratios (aHR) and 95% confidence intervals [CI] for CVD mortality, all-cause mortality, and liver mortality were 4.49 (95% CI, 1.61–12.50), 2.10 (95% CI, 1.23–3.59), and 77.23 (95% CI, 11.17–534.15), respectively. For those whose ALT was >2ULN, the aHRs were 4.22 (95% CI, 1.87–9.51), 1.60 (95% CI, 1.02–2.52), and 7.21 (95% CI 0.76–68.28), respectively (Table 2).
TABLE 2.
Hazard Ratios for Mortality by Aspartate Aminotransferase (AST), and Alanine Aminotransferase (ALT) Results
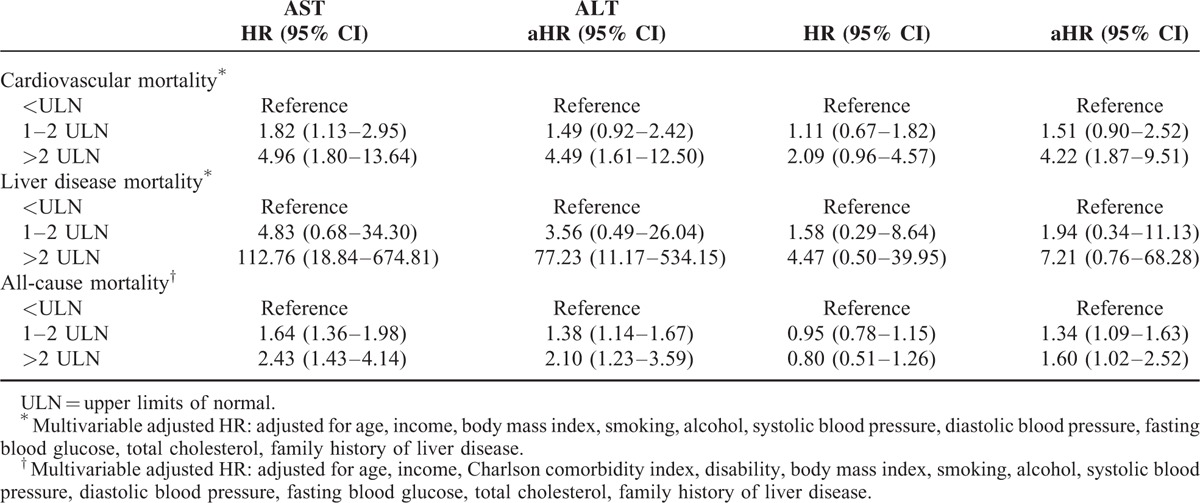
Among those whose AST levels were elevated (n = 12,432) at the first screening, 5489 (44.15%) showed sustained elevation at the second screening. Among those whose ALT levels were elevated (n = 18,663) at the first screening, 11,084 (59.39%) showed sustained elevation at the second screening (Supplementary Table 1).
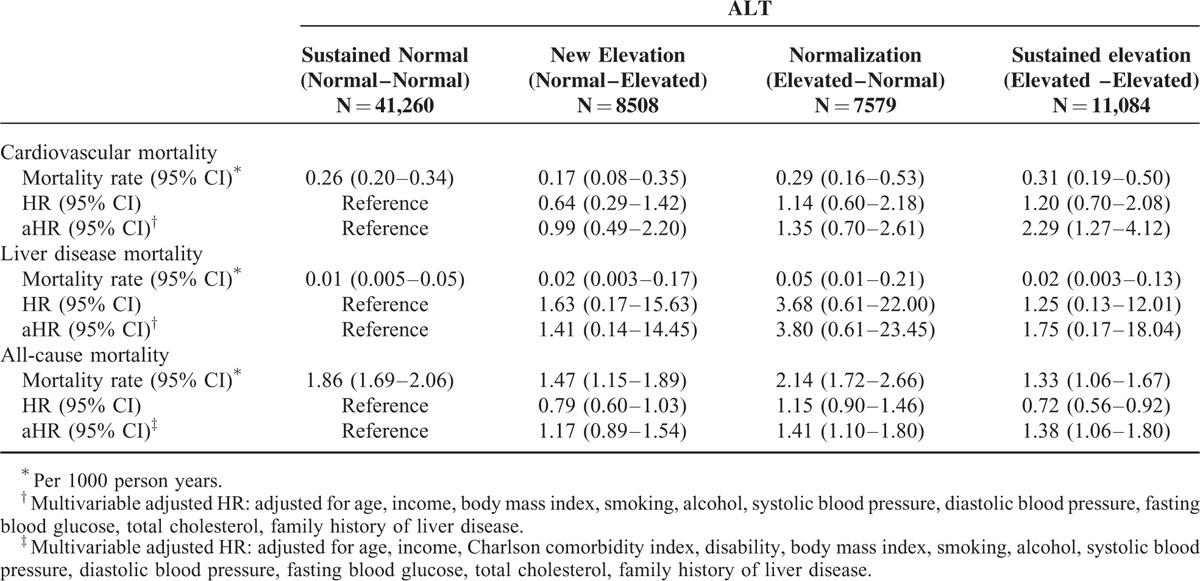
For AST and ALT, the sustained elevation groups showed a significantly higher risk of CVD mortality (aHR 1.95, 95% CI 1.07–3.56 for AST; aHR 2.29, 95% CI 1.27–4.12 for ALT) than those with sustained normal group. On the other hand, the normalization group (aHR 1.52, 95% CI 0.82–2.81 for AST; aHR 1.35, 95% CI 0.70–2.61 for ALT) and the new elevation group (aHR 1.27, 95% CI 0.66–2.44 for AST; aHR 0.99, 95% CI 0.49–2.20 for ALT) did not show significant changes in CVD mortality.
For AST, the sustained elevation group showed higher liver disease mortality (aHR, 10.05; 95% CI, 1.69–59.89) than the sustained normal group, and their risk was also higher than the normalization group (aHR, 2.93; 95% CI, 0.53–16.15). For ALT, the sustained elevation group showed higher liver disease mortality (aHR, 1.75; 95% CI, 0.17–18.04) than the sustained normal group, although not statistically significant.
For AST, the hazard ratio for all-cause mortality was higher in the sustained elevation group (aHR, 1.54; 95% CI, 1.20–1.98) than the sustained normal group, and their risk tended to be higher than the normalization group (aHR, 1.41; 95% CI, 1.11–1.79). The new elevation group (aHR, 1.21; 95% CI, 0.94–1.55) was not different from the sustained normal group. For ALT, a similar trend was observed for the sustained elevation (aHR, 1.38; 95% CI, 1.06–1.80), normalization (aHR, 1.41; 95% CI, 1.10–1.80), and new elevation groups (aHR, 1.17; 95% CI, 0.89–1.54) (Tables 3 and 4).
TABLE 3.
Hazard Ratios for Mortality by Change of Aspartate Aminotransferase (AST)
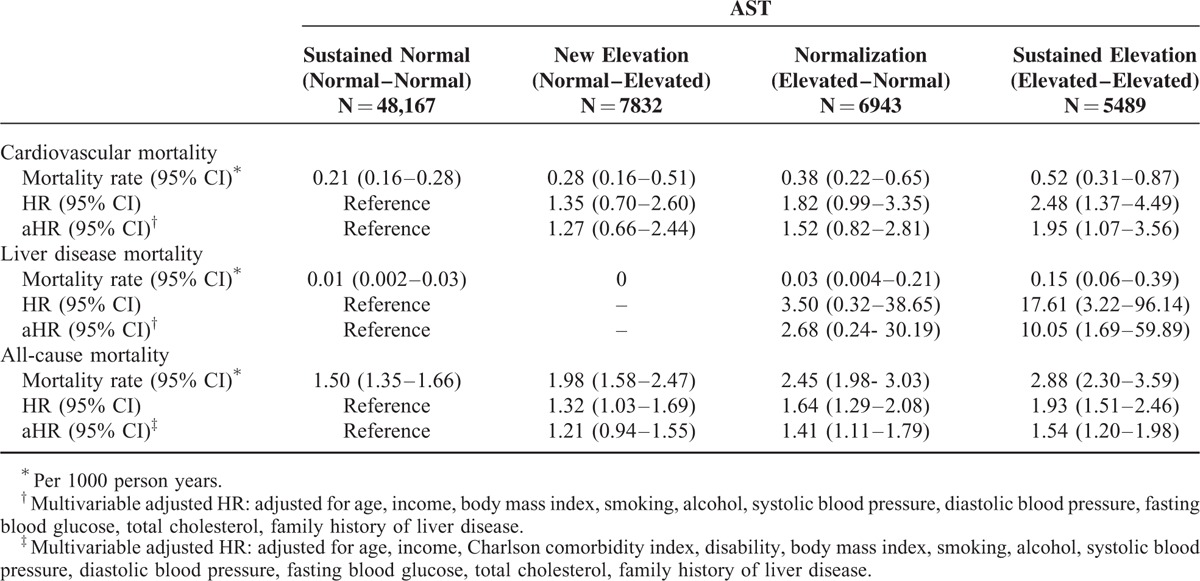
TABLE 4.
Hazard Ratios for Mortality by Change of Alanine Aminotransferase (ALT)
We conducted analyses to evaluate the change of metabolic profiles according to change of AST or ALT. AST and ALT changes were closely linked to the change of metabolic profile, including BMI, blood pressure, fasting blood glucose, and total cholesterol. Men with sustained normal AST showed slight increase in BMI (mean change, 0.16; 95% CI, 0.15–0.17). Participants with normal to increased AST (mean change, 0.44; 95% CI, 0.41–0.47) or sustained elevation of AST (mean change, 0.23; 95% CI, 0.19–0.26) showed higher increase in BMI, while participants with normalized AST showed slight decrease in BMI (mean change, −0.05; 95% CI, −0.08–0.01). Similar results were observed for ALT. Mean changes in BMI, SBP, DBP, FBS, and total cholesterol were as expected. Participants with normal to increased AST or ALT showed the largest change in SBP, DBP, FBS, and total cholesterol (Supplementary Table 2, Supplementary Table 3).
DISCUSSION
In this study of a large, nationally representative cohort, we found that elevated aminotransferase level is associated with cardiovascular, liver, and all-cause mortality, after extensive adjustment for potentially confounding variables. To our knowledge, our study is the first to demonstrate that individuals who have sustained elevation of aminotransferase during 2 subsequent tests show higher mortality than those whose liver enzyme levels are within normal range at least one time. Our study also suggests that the change in aminotransferase levels is linked to changes in weight and other metabolic markers.
There have been inconsistent reports about the association between aminotransferase levels and CVD events or mortality. Regarding AST, several studies reported that AST was associated with cardiometabolic markers.3 However, no significant association was observed between AST level and CVD events and all-cause mortality.4 Regarding ALT, several studies have reported the association between ALT and coronary heart disease7 and mortality.6 However, others found no such significant associations.17,18
Our study adds to the evidence supporting that elevated aminotransferase is linked to CVD and mortality. The fact that such an association remains even after adjustment for other risk factors suggests that the elevation of aminotransferase is not only a marker of metabolic syndromes, but also could reflect other mechanisms that are not covered by traditional risk factors such as BMI, hypertension, diabetes, dyslipidemia, smoking, and alcohol intake.
Although the mechanism is not clear, nonalcoholic fatty liver disease (NAFLD) has been suggested as a key explanation for the correlation between aminotransferase elevation and CVD mortality,4,7,12 as it is considered to be the hepatic manifestation of insulin resistance3,19 and metabolic syndrome.4,20 However, our study shows that elevated aminotransferase levels are still related to CVD mortality after adjusting for known traditional behavioral and metabolic risk factors. Similar associations were found in another study that showed the correlation between aminotransferase elevation and CVD events after adjusting for age, sex, alcohol-intake, smoking, physical activity, waist, triglycerides, systolic blood pressure, fasting glucose, HDL cholesterol.7 Similarly, NAFLD was also reported to be associated with atherosclerosis even after adjusting traditional confounders.21,22 Another possible explanation can be nontraditional risk factors such as persistent organic pollutants, which can link ALT elevation to increased mortality. POPs are lipophilic compounds that accumulate mainly in lipid-containing tissues and cause metabolic disturbance that can result in type 2 diabetes.23 POPs are also associated with elevated serum liver enzymes including ALT and GGT.24 Therefore, other than the traditional risk factors of mortality, relatively new risk factor, such as POPs, can act as a mediator of elevated ALT to mortality. Considering all these studies, people with elevated aminotransferase may need more vigilant attention for CVD prevention, even though the mechanism is still not fully understood.
In addition, our study uniquely showed that the sustained elevation of aminotransferase was associated with greater CVD mortality than those whose liver enzymes were within normal range at least one time. Furthermore, the normalization group and sustained normal group had similar risks of CVD mortality. In our analyses of metabolic profile changes, weight reduction was associated with normalization of aminotransferase, and subsequent reduction of CVD risk. Although the mechanisms of how weight changes, changes in aminotransferase levels, and mortality are interrelated are unclear, our study suggests the possibility that lifestyle modifications, which lead to weight reduction, might result in a favorable change in aminotransferase levels, reducing subsequent CVD risk.
New and sustained elevations of AST were associated with increased mortality from liver disease, even after participants with preexisting liver diseases were excluded.11,25 There are several explanations for the association between elevated aminotransferase and liver disease mortality. First, chronic liver disease and early hepatic cellular carcinoma could not be excluded, although we excluded participants with known liver diseases using claims data.10 Second, nonalcoholic steatohepatitis may contribute to liver mortality.25 Again, sustained elevation of aminotransferase was much more strongly associated with liver disease mortality than those whose liver enzymes were within normal range at least one time. Therefore, as previously established, individuals who show sustained elevation of aminotransferase during follow-up need more attention, and diagnostic tests have to be performed to investigate the reasons for liver enzyme elevation, including testing for the hepatitis B or C serology markers.26
In the present study, elevation of aminotransferase was associated with all-cause mortality. One explanation is that aminotransferase elevation is associated with subclinical inflammation. In 1 study, participants with NAFLD had higher levels of inflammatory markers than participants without NAFLD, regardless of traditional risk factors.27 This subclinical inflammation may increase long-term tissue damage and susceptibility to noninfectious diseases.28 Another possible explanation is that aminotransferase elevation is associated with alcohol consumption; therefore, alcohol-related diseases, such as cancer, depression, substance abuse, and car accidents, can occur more frequently in patients with elevated aminotransferase.29,30
Our study had several potential limitations. First, our study did not exclude all confounding factors including baseline serology results for viral hepatitis. Therefore, it may be possible that asymptomatic viral hepatitis patients were not properly excluded from our study, resulting in increased liver-related and all-cause mortality. However, we tried to minimize this by excluding all patients who carried liver disease in their medical records or had family history of liver disease. Also, we could not exclude people with chronic kidney diseases (CKD), which is a risk factor of increased cardiovascular and all-cause mortality.31 However, a majority of CKD patients have diabetes or hypertension. According to previous studies, 44% of new end stage renal disease patients have diabetes,32 and 80% to 85% of CKD patients have hypertension.33 We gave effort to exclude or adjust well-known risk factors such as hypertension, diabetes, dyslipidemia, obesity, smoking, and alcohol intake; therefore, we think that CKD will have little effect on the results. Second, aminotransferase was measured in different methods by each participating institution, and the normal range of aminotransferase and assay results may be different slightly between institutions. However, it was known that assay results are correlated very well between methods,34 and all institutions were subjected to the mandatory internal and external quality control.10 Another limitation of this study is the possible selection bias. This study was done using patients who participated in the National Screening “voluntarily.” Patients with severe diseases might be less likely to participate in the screening test. Conversely, patients with some health issue or concern might also participate more frequently than do healthy individuals from the population.
In conclusion, our study confirms the association of elevated aminotransferase with CVD mortality, liver disease mortality, and all-cause mortality. Individuals with sustained elevation of aminotransferase are at particularly higher risk of mortality. Individuals with sustained elevation of AST showed higher cardiovascular, liver, and all-cause mortality, and those with sustained elevation of ALT showed only higher cardiovascular and all-cause mortality. Individuals with sustained elevation of aminotransferase should receive appropriate medical attention to identify potentially modifiable risk factors or treatable diseases because they have more metabolic problems. Lifestyle modification, including weight reduction, might lead to normalization of aminotransferase levels and subsequent reduction of risk for cardiovascular, liver, and all-cause mortality.
Supplementary Material
Footnotes
Abbreviations: aHR = adjusted hazard ratio, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CVD = cardiovascular disease, DBP = diastolic blood pressure, FBS = fasting blood glucose, HR = hazard ratio, NAFLD = nonalcoholic fatty liver disease, SBP = systolic blood pressure, ULN = upper limits of normal.
This study was supported by the Ministry of Health and Welfare, Korea (Grant 2012-0897).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002; 123:1367–1384. [DOI] [PubMed] [Google Scholar]
- 2.Oren R. Serum liver enzymes-should we count on them? Liver Int 2014; 34:171–173. [DOI] [PubMed] [Google Scholar]
- 3.Porter SA, Pedley A, Massaro JM, et al. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013; 33:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goessling W, Massaro JM, Vasan RS, et al. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008; 135:1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui MS, Sterling RK, Luketic VA, et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 2013; 145:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun KE, Shin CY, Yoon YS, et al. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis 2009; 205:533–537. [DOI] [PubMed] [Google Scholar]
- 7.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis 2007; 191:391–396. [DOI] [PubMed] [Google Scholar]
- 8.Fraser A, Thinggaard M, Christensen K, et al. Alanine aminotransferase, gamma-glutamyltransferase (GGT) and all-cause mortality: results from a population-based Danish twins study alanine aminotransferase, GGT and mortality in elderly twins. Liver Int 2009; 29:1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DS, Evans JC, Robins SJ, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2007; 27:127–133. [DOI] [PubMed] [Google Scholar]
- 10.Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyl transferase and mortality in the United States population. Gastroenterology 2009; 136:477–485. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Kim WR, Benson JT, 3rd, et al. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008; 47:880–887. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Rhee EJ, Bae JC, et al. Increased risk of type 2 diabetes in subjects with both elevated liver enzymes and ultrasonographically diagnosed nonalcoholic fatty liver disease: a 4-year longitudinal study. Arch Med Res 2013; 44:115–120. [DOI] [PubMed] [Google Scholar]
- 14.Friedman LS, Dienstag JL, Watkins E, et al. Evaluation of blood donors with elevated serum alanine aminotransferase levels. Ann Intern Med 1987; 107:137–144. [DOI] [PubMed] [Google Scholar]
- 15.Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med 2008; 148:348–352. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Park JH, Lee SY, et al. Disparities in antihypertensive medication adherence in persons with disabilities and without disabilities: results of a Korean population-based study. Arch Phys Med Rehabil 2008; 89:1460–1467. [DOI] [PubMed] [Google Scholar]
- 17.Fraser A, Harris R, Sattar N, et al. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol 2007; 27:2729–2735. [DOI] [PubMed] [Google Scholar]
- 18.Kunutsor SK, Apekey TA, Khan H. Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis 2014; 236:7–17. [DOI] [PubMed] [Google Scholar]
- 19.Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002; 51:1889–1895. [DOI] [PubMed] [Google Scholar]
- 20.Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012; 18:3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fracanzani AL, Burdick L, Raselli S, et al. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med 2008; 121:72–78. [DOI] [PubMed] [Google Scholar]
- 22.Choi SY, Kim D, Kim HJ, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol 2009; 104:1953–1960. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Porta M, Jacobs DR, Jr, et al. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 2014; 35:557–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CY, Lin LY, Chiang CK, et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol 2010; 105:1354–1363. [DOI] [PubMed] [Google Scholar]
- 25.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008; 49:608–612. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Kim WR, Poterucha JJ. Evaluation of elevated liver enzymes. Clin Liver Dis 2012; 16:183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigam P, Bhatt SP, Misra A, et al. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: a case-control study on Asian Indians in North India. PLoS One 2013; 8:e49286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmadi-Abhari S, Luben RN, Wareham NJ, et al. Seventeen year risk of all-cause and cause-specific mortality associated with C-reactive protein, fibrinogen and leukocyte count in men and women: the EPIC-Norfolk study. Eur J Epidemiol 2013; 28:541–550. [DOI] [PubMed] [Google Scholar]
- 29.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA 1990; 264:2511–2518. [PubMed] [Google Scholar]
- 30.Giacosa A, Adam-Blondon AF, Baer-Sinnott S, et al. Alcohol and wine in relation to cancer and other diseases. Eur J Cancer Prev 2012; 21:103–108. [DOI] [PubMed] [Google Scholar]
- 31.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 32.Kowalski A, Krikorian A, Lerma EV. Diabetes and chronic kidney disease. Disease-a-month DM 2015; 61:378–386. [DOI] [PubMed] [Google Scholar]
- 33.Gargiulo R, Suhail F, Lerma EV. Hypertension and chronic kidney disease. Disease-a-month DM 2015; 61:387–395. [DOI] [PubMed] [Google Scholar]
- 34.Wroblewski F. The clinical significance of transaminase activities of serum. Am J Med 1959; 27:911–923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


