Abstract
Some epidemiologic surveillance studies have recorded adverse drug reactions to radiocontrast agents. We aimed to investigate the incidence and management of acute adverse reactions (AARs) to Ultravist-370 and Isovue-370 in patients who underwent contrast-enhanced computed tomography (CT) scanning.
Data from 137,473 patients were analyzed. They had undergone enhanced CT scanning with intravenous injection of Ultravist-370 or Isovue-370 during the period of January 1, 2006 to December 31, 2012 in our hospital. We investigated and classified AARs according to the American College of Radiology and the Chinese Society of Radiology (CSR) guidelines for iodinated contrast media. We analyzed risk factors for AARs and compared the AARs induced by Ultravist-370 and Isovue-370.
Four hundred and twenty-eight (0.31%) patients experienced AARs, which included 330 (0.24%) patients with mild AARs, 82 (0.06%) patients with moderate AARs, and 16 (0.01%) patients with severe AARs (including 3 cases of cardiac arrest and one case of death). The incidence of AARs was higher with Ultravist-370 than with Isovue-370 (0.38% vs 0.24%, P < 0.001), but only for mild AARs (0.32% vs 0.16%, P < 0.001). Analyses on risk factors indicated that female patients (n = 221, 0.43%, P < 0.001), emergency patients (n = 11, 0.51%, P < 0.001), elderly patients aged 50 to 60 years (n = 135, 0.43%, P < 0.001), and patients who underwent coronary computed tomography angiography (CTA) (n = 55, 0.51%, P < 0.001) had a higher risk of AARs. Cutaneous manifestations (50.52%)—especially rash (59.74%)—were the most frequent mild AARs. Cardiovascular manifestations accounted for most moderate and severe AARs (62.91% and 48.28%, respectively). After proper management, the symptoms and signs of 96.5% of the AARs resolved within 24 hours without sequelae.
Ultravist-370 and Isovue-370 are safe for patients undergoing enhanced CT scanning. The incidence of AARs is higher with Ultravist-370 than with Isovue-370, but this difference is limited only to the mild AARs. The incidence of AARs could be affected by multiple factors.
INTRODUCTION
Iodine-based contrast media Ultravist-370 (generic name, iopromide; Bayer Healthcare, Berlin, Germany) and Isovue-370 (generic name, iopamidol; Bracco Imaging, Milan, Italy) are both the most commonly used media contrast in improving the computed tomography (CT) diagnosis.
The AARs (i.e., nonrenal adverse reactions such as nausea, itching, vomiting, urticaria, dyspnea, vasovagal attack, and even fatal anaphylactic reactions) caused by iodine-based contrast medium occur within 1 hour after the contrast medium injection.1 However, late adverse reactions (e.g., nausea, vomiting, headache, musculoskeletal pain, and fever) are not explicit and have not been analyzed because they appear unrelated to the contrast media, although the reactions actually are related to the media. Renal adverse reactions such as contrast medium nephrotoxicity are rare and usually occur in patients with a high risk, particularly in patients with renal insufficiency.2 Patients who have renal insufficiency should not be injected with iodine-based contrast agents, unless they are undergoing dialysis or it is clinically required.
As a new generation of iodine-based contrast media, Isovue-370 is presumed to cause less AARs according to preobservations in more and more institutions, and some institutions had restricted the wide use of Ultravist-370. However, no previous studies had been conducted to investigate and compare the AARs induced by these 2 contrast agents in a large population to support the presume, though there is no lack of surveillance studies performed to prove the safety and tolerability of iodinated contrast media.3–7
Real-life studies are observational studies that do not alter the clinical routine and treatment of the patients, and therefore the true incidence of AARs could have been observed. In this real-life study, we retrospectively analyzed patients who underwent enhanced CT scanning during the period of January 1, 2006 to December 31, 2012, to investigate the incidence and management of AARs to Ultravist-370 and Isovue-370.
METHODS
Devices and Drugs
Computed Tomography Devices
A 64-slice scanner (GE LightSpeed VCT scanner; GE Healthcare, Milwaukee, WI) and a 256-slice scanner (Philips Brilliance iCT scanner; Royal Dutch Philips Electronics Ltd, Amsterdam, The Netherlands) were used for CT scanning.
Contrast Agents
Ultravist-370 and Isovue-370 were stored in thermostats at a constant temperature of 36°C to 37°C before use.
Devices and Drugs for AAR Treatment
All CT rooms were equipped with basic first aid facilities and monitoring devices (e.g., monitoring instruments, defibrillator, oxygen therapy devices, sputum suction devices, sphygmomanometers, laryngoscopes, endotracheal intubation packages, and different airway tubes). Sufficient rescue drugs were also provided such as promethazine (Phenergan), epinephrine, dexamethasone, chlorpheniramine (Chlor-Trimeton), saline, metoprolol, salbutamol, and nitroglycerin. Once an AAR occurred, the emergency department physicians, radiologists, and nurses could use the devices and drugs to preliminarily treat the patients until they were stable. The patients would then be sent to the emergency department for further treatment and observation.
Treatment Strategy for AARs
No patients received premedication prior to the CT scanning. The basic treatment strategies for AARs were the following: for patients with mild AARs, the symptoms of AARs resolved spontaneously only after immediately stopping the injection of Ultravist-370 or Isovue-370 and without any further treatment. Some patients may need to take chlorpheniramine orally, drink a substantial amount of water, have an injection of dexamethasone, and have oxygen supplied. For patients with moderate AARs, aggressive treatments should be considered and physicians from the emergency department should be called for help. After the preliminary treatments, patients should be transferred to the emergency department for further treatment and observation. For patients with severe AARs, immediate treatments should be performed. Clinicians from the emergency department, anesthesia department, and other related departments are required to treat a patient cooperatively. Once cardiopulmonary arrest occurs, cardiopulmonary resuscitation based on Basic Life Support or Advanced Cardiovascular Life Support should be immediately started. After being rescued, patients should be transferred to the emergency department or intensive care unit for further treatment and observation.
Inclusion Criteria for Analysis
To avoid any influences on the routine enhanced CT scan, only patients who had an indication for the scan and use of the contrast medium Ultravist-370 or Isovue-370 were eligible for analysis. All patients agreed to the procedure and signed informed consent forms before the examinations. All procedures were conducted by radiologists, technicians, and nurses in accordance with the guidelines for the use of iodinated contrast media (version 2) issued by the Chinese Society of Radiology (CSR).8 For patients with any risk factors for AARs (e.g., history of AAR, allergy, or asthma),10 dynamic observations were performed during and after the CT scan and treatments for AARs, if necessary. No patient was permitted to leave the CT waiting room within 30 minutes after the injection and patients with AARs should be followed up for 24 hours or more. All important data of the patients were collected.
Data Collection
Data collection was based on the inclusion criteria. We used self-designed case report forms (CRFs) to collect data from the communication system (i.e., picture archiving and communication system) and the documents recorded by nurses in the radiology department during the period of January 1, 2006 to December 3, 2012. The CRF included the demographics of the patients (e.g., sex, age), type and dosage of the contrast media, scanning regions, symptoms of the AARs, treatment of the AARs, and outcomes of the AARs. We had a meeting to ensure that everyone fully understood and agreed with the CRF. Data collected by an investigator were required to be checked by another investigator. Discrepancies in the data collection were resolved through a discussion between the investigators.
Classification Criteria of the Iodinated Contrast Media-Related AARs
The contrast media-related AARs were classified according to the American College of Radiology (ACR) criteria (version 7)10 and CSR criteria (version 2)8 (Table 1).
TABLE 1.
The ACR Criteria (Version 7) and CSR Criteria (Version 2)

The ACR criteria and CSR criteria have some differences. Vomiting and urticaria are classified as mild AARs in the ACR criteria, whereas severe vomiting and urticaria are classified as moderate AARs in the CSR criteria. Shaking and chills are classified as mild AARs in the ACR criteria, but trembling (i.e., shaking or chills) was classified as a severe AAR in the CSR criteria. Facial swelling is classified as a mild AAR in the ACR criteria but as a moderate AAR in the CSR criteria. Laryngeal edema was classified as a severe AAR in the CSR criteria, but it was divided as a function of severity into moderate AAR or severe AAR in the ACR criteria.
In this study, we classified mild/moderate vomiting, mild/moderate urticaria, mild shaking (i.e., chills or trembling), and mild or localized facial swelling as mild AARs; severe vomiting or urticaria, systemic shaking (i.e., chills/trembling) without systemic symptoms, and substantial facial swelling as moderate AARs; and systemic shaking (i.e., chills or trembling) that accompanies systemic symptoms as severe AARs.
Several symptoms such as transient chest pain, abdominal pain, transient anesthesia (lip and/or limbs), urinary incontinence, foaming at the mouth, tears, transient blurred vision, hoarseness, conjunctival congestion were not included in either of the 2 criteria. In this study, transient chest pain, single abdominal pain, transient anesthesia (of the lip and/or limbs), blurred vision, tears, and conjunctival congestion were classified as mild AARs. Foaming at the mouth, single urinary incontinence, and hoarseness were classified as moderate AARs. Urinary incontinence with a disturbance in consciousness was classified as severe AAR.
Statistical Analysis
The data were analyzed using Statistical Package for Social Sciences (SPSS) software version 20.0 (SPSS Inc, Chicago, IL). The chi-square test was applied for comparisons. Two tailed P < 0.05 was considered statistically significant.
Informed Consent and Ethical Approval
The ethics committees granted exempt status for this study and waived the need for informed consent.
RESULTS
Patient Demographics
A total of 137,473 patients (mean age, 54.40 ± 18.76 years) were included who underwent enhanced CT scanning using Ultravist-370 (67, 923 patients) or Isovue-370 (69, 550 patients) during the period of January 1, 2006 to December 31, 2012. Among these patients, 53,614 (39%) patients were female (39%) and 83,859 (61%) were male (61%). Four hundred twenty-eight patients experienced AARs. They had a mean age of 51.6 ± 18.0 years (range, 5 months–105 years). The mean dose of the contrast media was 69.5 ± 18.3 mL (Table 2).
TABLE 2.
Patient Demographics and Clinical Characteristics
The Incidence of AARs
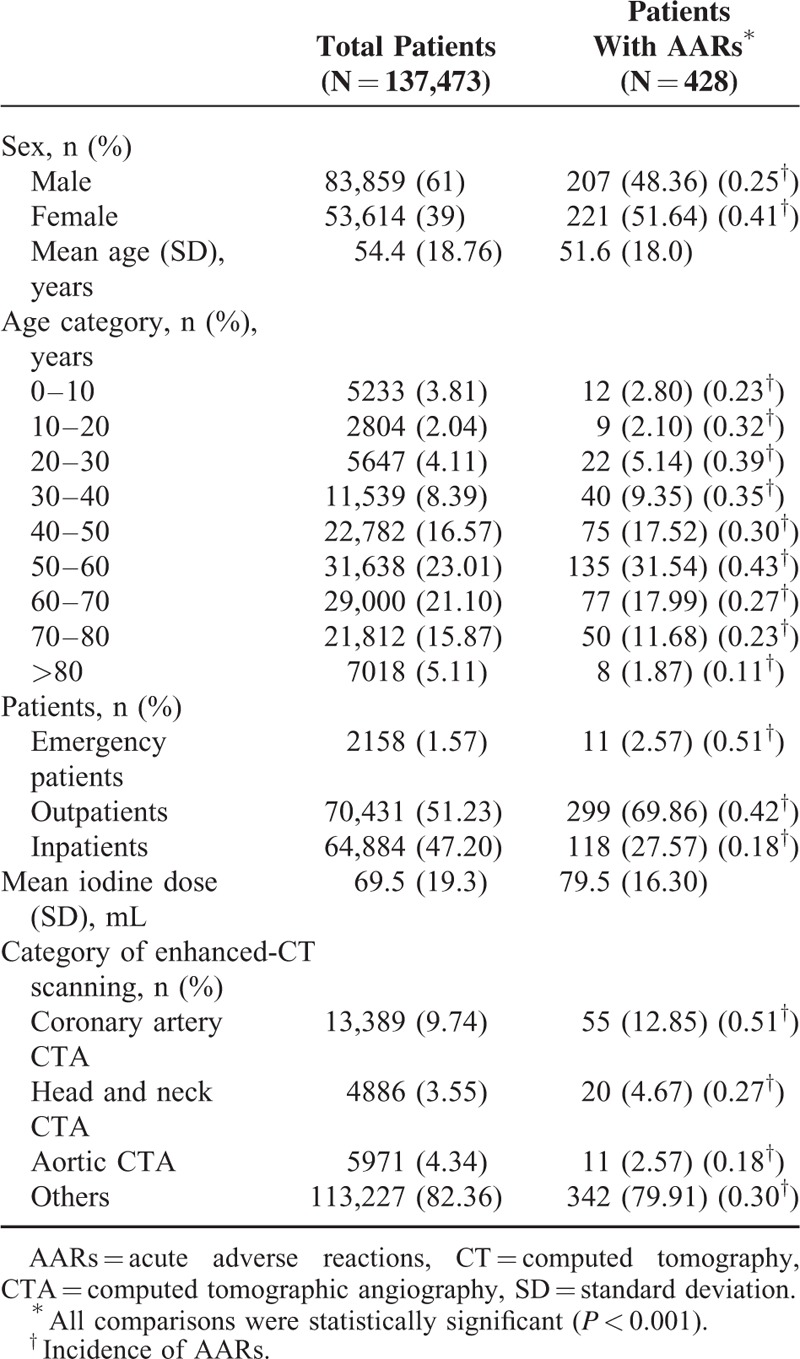
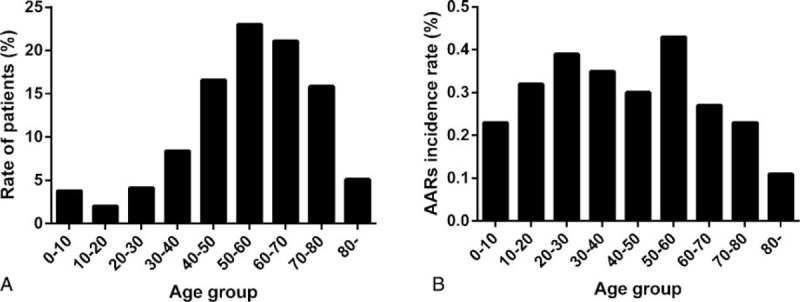
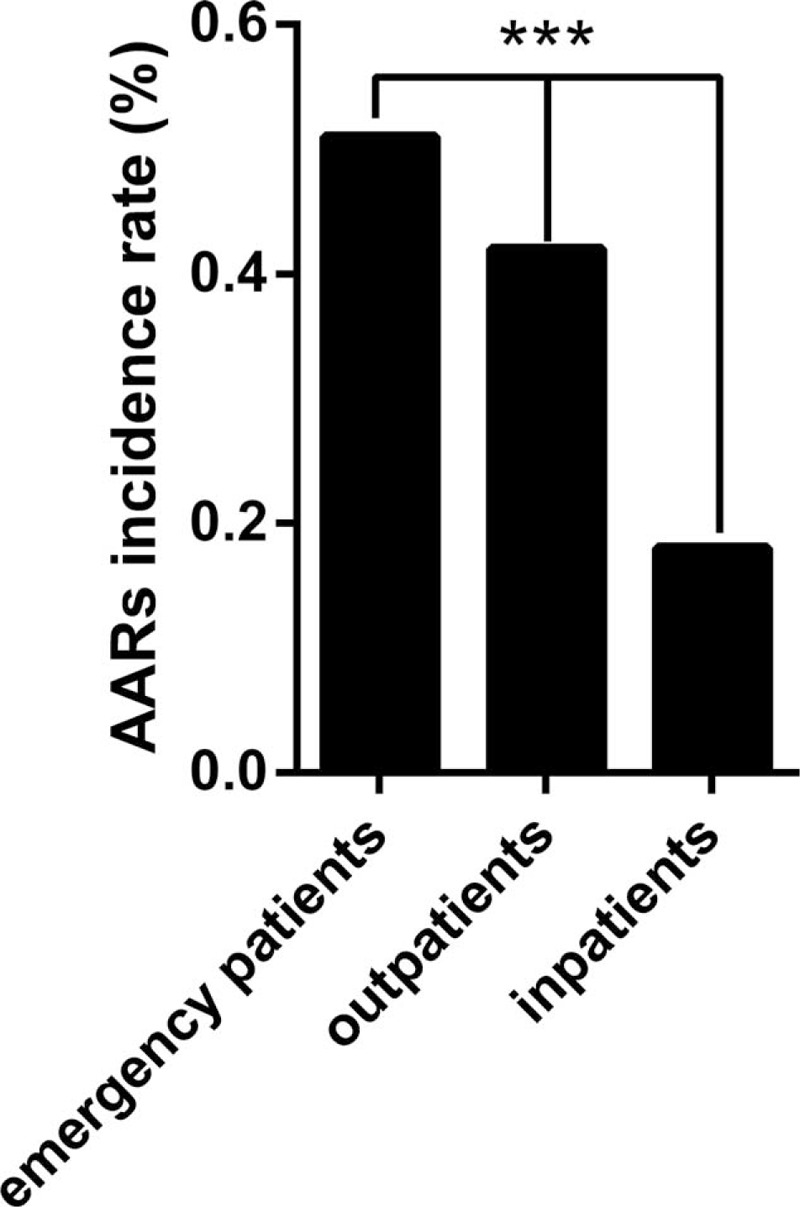
The overall incidence of AARs was 0.31% (n = 428). There were 330 (0.24%) mild AARs, 82 (0.06%) moderate AARs, 16 (0.01%) severe AARs (including 3 cases of cardiac arrest and 1 case of death). The mild, moderate, and severe AARs accounted for 77.1%, 19.2%, and 3.7% of all AARs, respectively. The overall incidence of AARs was higher with Ultravist-370 than with Isovue-370 (0.38% vs 0.24%, P < 0.001). We observed a statistical significance between Ultravist-370 and Isovue-370 only for mild AARs (0.32% vs 0.16%, P < 0.001), but not for moderate AARs (0.07% vs 0.05%, P = 0.152) or severe AARs (0.01% vs 0.01%, P = 0.584) (Figure 1). Analyses of the risk factors revealed that female patients (n = 221, 0.41%) had a higher incidence of AARs than male patients (n = 207, 0.25%), and the difference was statistically significant (P < 0.001) (Table 2). For patients in the different age groups, the incidence of AARs was highest in the age group of 50 to 60 years (0.43%, n = 135, P < 0.001), followed by the age group of 20 to 30 years (0.39%, n = 22, P < 0.001), whereas patients >80 years had the lowest incidence (n = 8, 0.11%, P < 0.001) (Table 2 and Figure 2). The incidence of AARs was 0.51% (n = 11), 0.42% (n = 299), and 0.18% (n = 118) for emergency patients, outpatients, and inpatients, respectively (Table 2 and Figure 3). The incidence of AARs was 0.51% (n = 55), 0.27% (n = 20), 0.18% (n = 11), and 0.30% (n = 342) for patients who underwent coronary computed tomography angiography (CTA), head and neck CTA, aortic CTA, and other enhanced CT examinations, respectively. The differences reached statistical significance (P < 0.001) (Table 2 and Figure 4).
FIGURE 1.
A, The rate of patients in different age groups; B, The incidence of AARs in different age groups (P < 0.001). AARs = acute adverse reactions.
FIGURE 2.
The incidence of AARs in emergency patients, outpatients, and inpatients (P < 0.001). AARs = acute adverse reactions.
FIGURE 3.
The incidence of AARs in patients underwent coronary artery CTA, head and neck CTA, aortic CTA, and other enhanced CT examinations (P < 0.001). AARs = acute adverse reactions, CT = computed tomography, CTA = computed tomography angiography.
FIGURE 4.
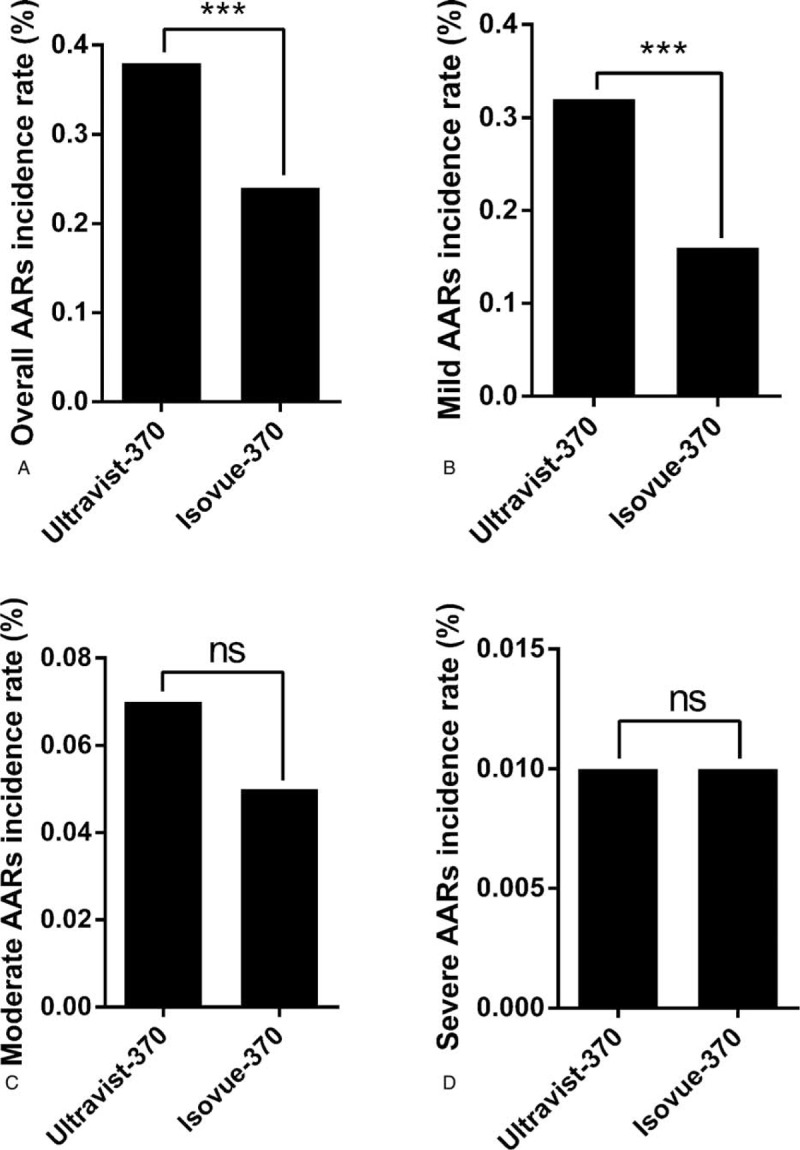
Comparison of incidence of AARs between Ultravist-370 and Isovue-370. A, Overall incidence (P < 0.001); B, Incidence of mild AARs (P < 0.001); C, Incidence of moderate AARs (P = 0.152); D, Incidence of severe AARs (P = 0.584). AARs = acute adverse reactions.
Manifestations of AARs
Mild AARs
Three hundred thirty patients (representing 772 AARs) experienced mild AARs (Table 3). The symptoms and signs of mild AARs were classified into 7 categories. Most patients had 2 or more reactions. Cutaneous manifestations were the most frequent mild AAR and accounted for 50.52% of AARs. Among all cutaneous presentations, rash ranked highest at approximately 59.74%.
TABLE 3.
The Symptoms and Signs of Mild AARs
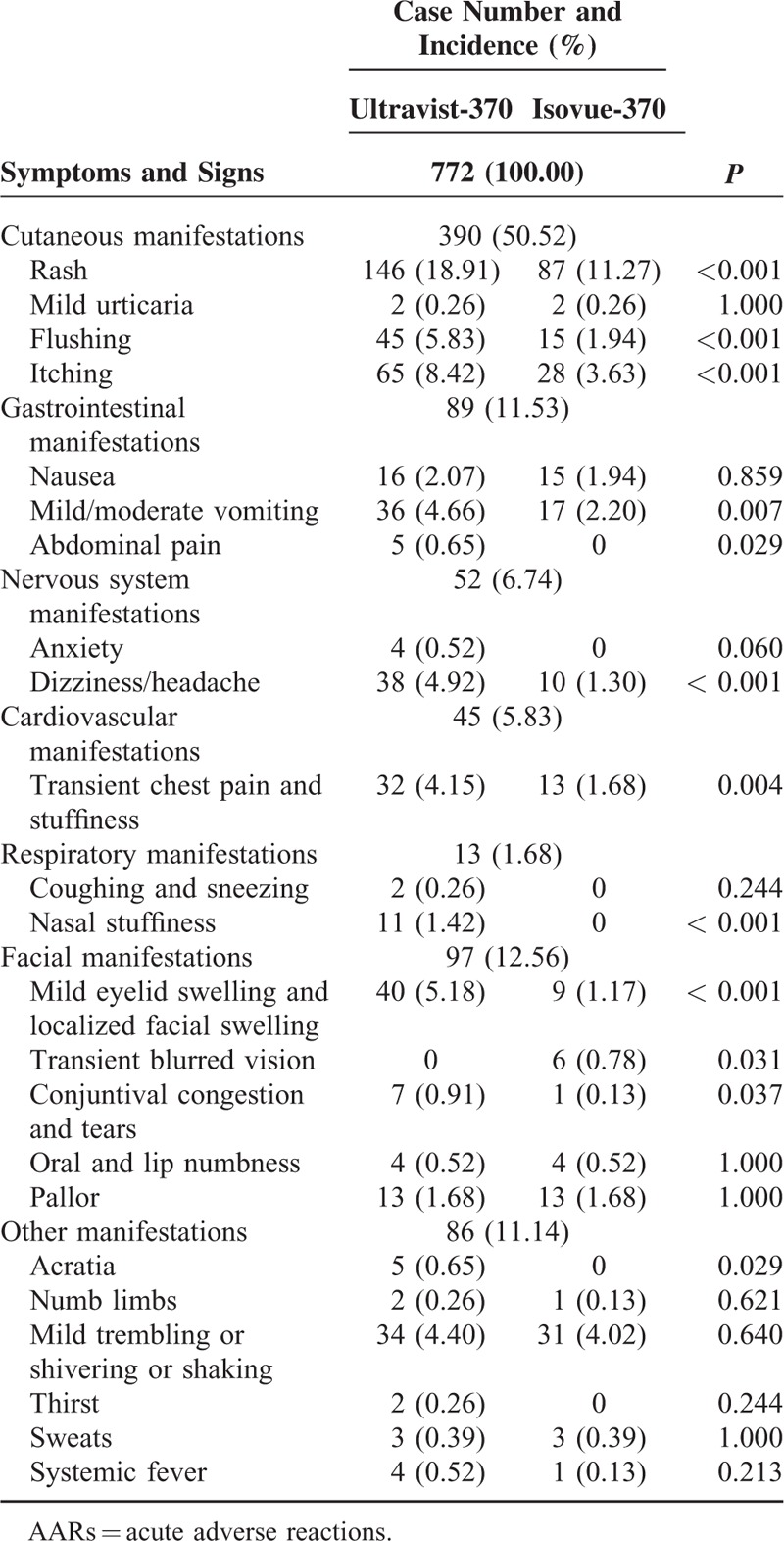
For cutaneous manifestations, the incidence of rash, flushing, and itching were higher with Ultravist-370 than with Isovue-370 (for all, P < 0.001). For gastrointestinal manifestations, the incidence of mild/moderate vomiting and abdominal pain were higher with Ultravist-370 than with Isovue-370 (P = 0.007 and P = 0.029, respectively). For nervous system manifestations, the incidence of dizziness/headache was higher with Ultravist-370 than with Isovue-370 (P < 0.001). For respiratory manifestations, the incidence of nasal stuffiness was higher with Ultravist-370 than with Isovue-370 (P < 0.001). For facial manifestations, the incidence of mild eyelid swelling and localized facial swelling, conjunctival congestion, and tears were higher with Ultravist-370 than with Isovue-370 (P < 0.001 and P = 0.037, respectively), but the incidence of transient blurred vision was higher with Isovue-370 (P = 0.031). For facial manifestations, the incidence of mild eyelid swelling and localized facial swelling, transient blurred vision, and conjunctival congestion and tears were higher with Ultravist-370 than with Isovue-370 (P = 0.000, P = 0.031, and P = 0.037, respectively).
Moderate AARs
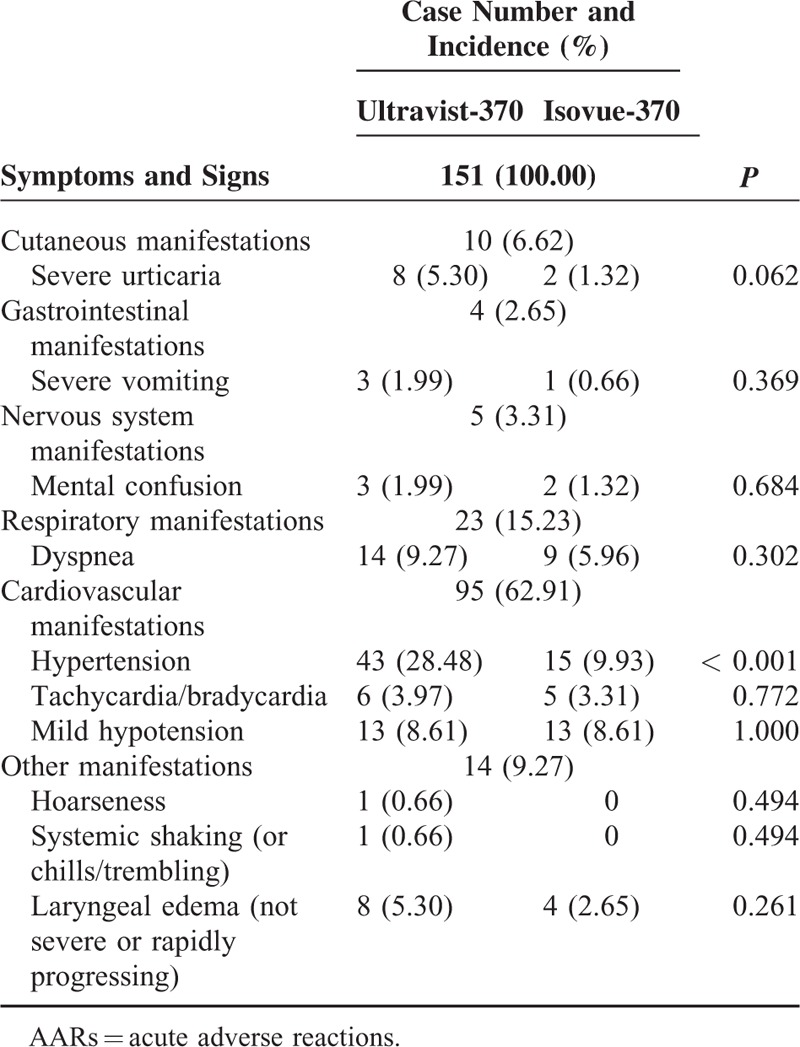
Eighty-two patients (representing 151 AARs) experienced moderate AARs (Table 4). The symptoms of moderate AARs were classified into 6 categories. Nearly all patients had 2 or more reactions. Cardiovascular manifestations were the most frequent moderate AARs and accounted for 62.91% of AARs. Of all cardiovascular presentations, hypertension ranked highest at approximately 61.05%. For moderate AARs, only the incidence of hypertension was higher with Ultravist-370 than with Isovue-370 (P < 0.001).
TABLE 4.
The Symptoms and Signs of Moderate AARs
Severe AARs
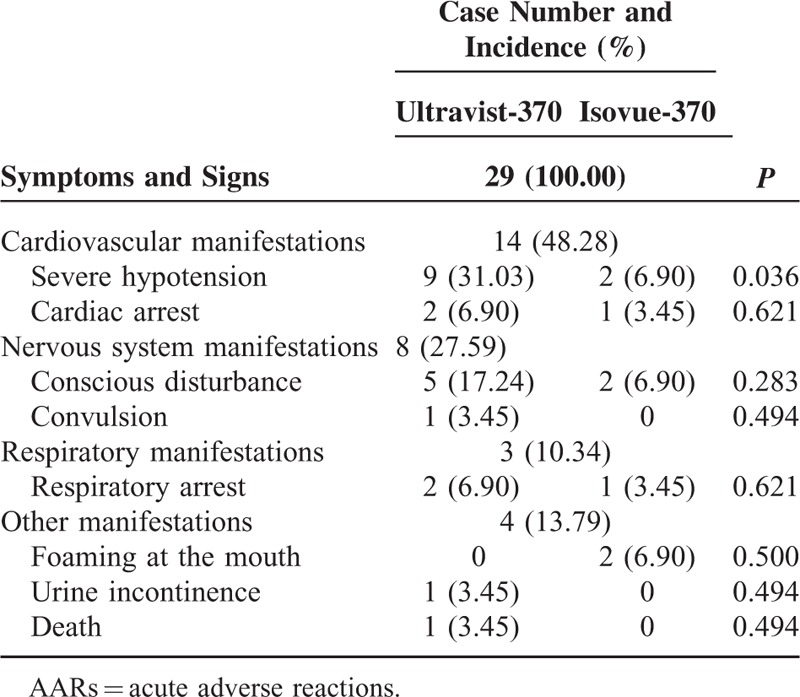
Sixteen patients (representing 29 AARs) experienced severe AARs (Table 5). The symptoms and signs of severe AARs were classified into 4 categories. Most patients had >2 reactions. Cardiovascular manifestations were the most frequent severe AARs and accounted for 48.28% of AARs. Among all cardiovascular presentations, severe hypotension ranked the highest at approximately 78.57%. For severe AARs, only the incidence of severe hypotension was higher with Ultravist-370 than with Isovue-370 (P = 0.036).
TABLE 5.
The Symptoms and Signs of Severe AARs
Treatment for AARs
Two hundred eight (48.60%) patients with AARs recovered spontaneously without specific treatment, whereas the remaining 220 patients required further treatments. The treatments were as follows:
Hydration
All patients (except those with a contraindication for water drinking) who underwent enhanced CT scanning were required to drink 300 to 500 mL of water before and after the examination. Oral hydration was first administered to patients with mild AARs; however, 13 patients also received 0.9% normal saline (NS). For patients with moderate AARs, intravenous hydration (0.9% NS [n = 17], Ringer's solution [n = 2], or 5% glucose solution [n = 2]) were administered. The remaining 65 patients received oral hydration. For patients with severe AARs, intravenous hydration (0.9% NS [n = 8], Ringer's solution [n = 4], or 5% glucose solution [n = 3]) was administered.
Oxygen Inhalation
One hundred twenty-eight patients were administered oxygen. Of these patients, 54 patients with mild AARs underwent nasal catheter oxygen inhalation (2–4 L/min), 53 patients with moderate AARs underwent mask oxygen inhalation (6–10 L/min), and 21 patients with severe AARs underwent nasal catheter oxygen inhalation (4–8 L/min).
Hexadecadrol
Two hundred sixteen patients were injected intravenously with dexamethasone (10 mg; Hexadecadrol), which included 126 patients with mild AARs, 72 patients with moderate AARs, and 18 patients with severe AARs.
Chlor-Trimeton
One hundred fifty-six patients (which included 145 patients with mild AARs and 11 patients with moderate AARs) orally took Chlor-Trimeton (2–8 mg).
Other Drugs
Some other drugs were also applied by clinicians. For instance, promethazine (Phenergan) (usually 50 mg) was orally administered to 4 patients with mild AARs, to 3 patients with moderate AARs, and to 1 patient with severe AARs. Epinephrine (0.2–1.0 mg) was intramuscularly injected into 26 patients with mild hypotension, whereas intravenously injected into 14 patients with cardiac arrest and severe hypotension. Dopamine (40 mg) was intravenously dripped in 1 patient with a weak pulse. Sublingual nitroglycerin was administered to 1 patient who experienced angina. After the preliminary treatments, the patients were transferred to the emergency department for further treatment and observation.
Outcomes of AARs
The symptoms and signs of 96.5% of AARs resolved without sequelae and approximately 3.5% of AARs resolved within 24 hours after proper treatment. One death occurred, even after the rescue.
DISCUSSION
Primary Findings and Analysis
This real-life study included 137,473 patients who received an intravenous injection of Ultravist-370 or Isovue-370 for contrast-enhanced CT scanning. The overall incidence of AARs was 0.31%, which was much lower than the rates of 2.49%, 2.8%, and 2% reported in 3 previous studies3,5,11 that aimed to determine the safety and tolerability of Ultravist-370. There are several possible reasons for our findings. First, all patients had a strict screening before they underwent enhanced CT scanning. Those patients who had a history of renal insufficiency, bronchial asthma, allergies, and/or contrast media reactions were not injected with iodine-based contrast agents unless necessary. All patients had to be accompanied by their clinicians before and after the examination. Second, very mild AARs were easy to underestimate or neglect because of the absence of very obvious discomfort. Third, before use, Ultravist-370 and Isovue-370 were stored in thermostats with a constant temperature of 36°C to 37°C to warrant a perfectly matched physiological temperature of the body when injected. To our knowledge, no previous studies have reported or applied this method to reduce the incidence of AARs, although this method was recommended in 2014 by European Society of Urogenital Radiology Guidelines on contrast media (version 9.0). Fourth, a standard protocol was performed by experienced doctors (which included emergency department doctors) and specially trained nurses to prevent and treat AARs. Fifth, differences may exist in the constitution and educational background between Chinese and Western patients.
The analysis of 428 patients with AARs revealed that the overall incidence of mild, moderate, and severe AARs was 0.24%, 0.06%, and 0.01%, respectively. Most AARs in this study were mild or moderate, and severe AARs were rather rare. The incidence of mild AARs may be underestimated. However, moderate and severe AARs were relatively easy to observe. Therefore, the records of these AARs were objective.
We observed that the incidence of AARs was higher with Ultravist-370 than with Isovue-370 (0.38% vs 0.24%, P < 0.001), which may be ascribed to the different molecular structures of the contrast agents and to the different mixtures added to the contrast agents. However, the difference in incidence between Ultravist-370 and Isovue-370 was limited only to mild AARs (0.32% vs 0.16%, P < 0.001).
Among all mild AARs, cutaneous manifestations was far more frequent than other types of AARs (e.g., gastrointestinal manifestations, facial manifestations) and accounted for 50.52% of AARs. Among the cutaneous manifestations, rashes ranked highest (59.74%), which was inconsistent with the results of Subathra et al12 who reported vomiting (33.7%) as the most common adverse drug reaction and rashes (30.6%) as the second most common adverse drug reaction. For moderate AARs, cardiovascular manifestations (62.91%) were the most frequent and hypertension accounted for 61.05%. For severe AARs, cardiovascular manifestations (48.28%) were similarly the most common symptoms and severe hypotension accounted for 78.57% of AARs. These results suggest that if a patient experiences moderate or severe AARs, his or her blood pressure more commonly will be affected.
Unlike in most previous studies, 1 death occurred in our study. An 80-year-old woman was suspected of having hilar cholangiocarcinoma and pancreatic head carcinoma. She first presented with cyanosis and then respiratory arrest after the examination. Timely cardiopulmonary resuscitation was performed; however, the elderly woman eventually died. We did not know the exact causes of her death, but speculated that it may be related to her poor condition caused by the carcinomas and AARs to the contrast medium. This finding indicated that AARs sometimes can be fatal, which was in line with the findings of Leone et al.13
Previous studies have shown that the incidence of AARs is associated with a patient's age. Our study demonstrated that the mean age of the 428 patients was 51.6 ± 18.0 years (range 5 months–105 years). Of these, the patients with the age of 50 to 60 years had the highest incidence of AARs (0.43%), which was not in accordance with the highest incidence for patients 20 to 30 years old reported by Khachman et al9 or for patients younger than 18 years old reported by Palkowitsch et al.5 However, patients 20 to 30 years old had the second highest incidence of AARs in our study. Of note, this study showed that AARs can also occur in infants. To date, no studies have reported AARs in patients younger than 1 year.
According to some studies, sex can also affect the development of adverse drug reaction to a certain degree.14,15 Among 428 patients, 207 (48.4%) patients were male and 221 (51.6%) patients were female. The incidence of AARs was higher in female patients (0.41%) than in male patients (0.25%), which was consistent with the findings of most studies. However, our finding was inconsistent with that of Katayama et al.16 We speculated that female patients tended to express their discomfort more easily than male patients, and differences in constitution could also contribute to this interesting phenomenon.
In this study, we also found that the incidence (0.51%) of AARs was higher in patients who underwent coronary artery CTA. These patients may have certain underlying diseases such as coronary heart disease, hypertension, hemadostenosis, and cerebral infarction. These underlying diseases could increase the odds of AARs.17 In addition, we compared the incidence of AARs in emergency patients, outpatients, and inpatients, and found that the incidence of AARs was higher in emergency patients (0.51%), followed by outpatients (0.42%). The reasons are unclear.
The treatment for AARs varies, although hydration (i.e., oral and intravenous hydration, which can accelerate the excretion of contrast media) is a major prevention and treatment strategy for contrast media-related AARs. In this study, all patients (except those with a contraindication for water drinking) who underwent enhanced CT scanning were required to drink 300 to 500 mL of water before and after the examination. Intravenous hydration was administered to patients with mild (i.e., very few), moderate, or severe AARs, which can contribute to lowering the incidence and alleviating the symptoms of AARs.18–20 In the present study, 128 patients underwent oxygen inhalation (e.g., nasal catheter oxygen inhalation [n = 75] and mask oxygen inhalation [n = 53]) and this therapy was proven as effective. For patients with allergy symptoms, Hexadecadrol (n = 216) and Chlor-Trimeton (n = 156) were administered, which effectively alleviated the symptoms. Epinephrine (0.2–1.0 mg) was intramuscularly injected into 26 patients with mild hypotension, whereas intravenously injected into 14 patients with cardiac arrest and severe hypotension. It effectively controlled the hypotension. Some other drugs such as dopamine and nitroglycerin were also administered to patients with severe AARs or with a history of cardiovascular disease. As a result, the symptoms and signs of 96.5% AARs resolved without sequelae and approximately 3.5% AARs resolved within 24 hours after proper treatment.
Limitations
Our study had some limitations. First, very mild AARs were easily neglected because of the absence of very obvious discomfort, which could have resulted in an underestimation of mild AARs. Second, no data were available to explore the association between the incidence of AARs and other factors such as the underlying diseases of patients.
CONCLUSIONS
In summary, the incidence of AARs after the injection of Ultravist-370 and Isovue-370 was rather low, and most of AARs were mild, whereas moderate and severe AARs were very rare, which suggests that Ultravist-370 and Isovue-370 are safe for patients undergoing enhanced CT scanning. The incidence of AARs is higher with Ultravist-370 than with Isovue-370, but this difference is only limited to mild AARs. The recognition and classification of AARs will improve the treatment of AARs. Strict screening, using a standard protocol, and keeping the contrast agent within a constant temperature (36°C–37°C) may help to reduce the incidence of AARs.
Footnotes
Abbreviations: AARs = acute adverse reactions, ACR = American College of Radiology, CPR = cardiopulmonary resuscitation, CSR = Chinese Society of Radiology, CTA = computed tomography angiography, GS = glucose solution, NS = normal saline.
This study was supported by grants from the National Scientific Foundation of China (grant number, 81171329) and the Guangdong Science Foundation (grant number, S2011010000790). The study sponsor had no involvement in the conduct of the study or in the writing of the article.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Berner J, Poletti PA, Becker CD, et al. Adverse reactions to iodinated contrast media: how to prevent them? Rev Med Suisse 2009; 5:2016–2018.2020-2021. [PubMed] [Google Scholar]
- 2.ESUR Guidelines on contrast media. Version 9.0, 2014. www.esur.org Accessed September 15, 2015. [Google Scholar]
- 3.Palkowitsch PK, Bostelmann S, Lengsfeld P. Safety and tolerability of iopromide intravascular use: a pooled analysis of three non-interventional studies in 132,012 patients. Acta Radiologica 2014; 55:707–714. [DOI] [PubMed] [Google Scholar]
- 4.Haussler MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol 2010; 51:924–933. [DOI] [PubMed] [Google Scholar]
- 5.Palkowitsch P, Lengsfeld P, Stauch K, et al. Safety and diagnostic image quality of iopromide: results of a large non-interventional observational study of European and Asian patients (IMAGE). Acta Radiol 2012; 53:179–186. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Hou L, Lv B, et al. Post-marketing surveillance study with iodixanol in 20185 Chinese patients from routine clinical practices. Br J Radiol 2014; 87: 20130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endrikat JS, Dohanish S, Balzer T, et al. Safety of gadoxetate disodium: results from the clinical phase II-III development program and postmarketing surveillance. J Magn Reson Imaging 2015; 42:634–643. [DOI] [PubMed] [Google Scholar]
- 8.Iodinated contrast agents application guidelines (2nd Ed). Chinese J Radiol 2013; 47:869–872. [Google Scholar]
- 9.Khachman D, Gandia P, Sallerin F, et al. Immediate and delayed hypersensitivity reactions to iodinated radiographic contrast agents: an update. Therapie 2009; 64:331–339. [DOI] [PubMed] [Google Scholar]
- 10.ACR Manual on Contrast Media. Version 7, 2010. www.acr.org Accessed September 16, 2015. [Google Scholar]
- 11.Kopp AF, Mortele KJ, Cho YD, et al. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiologica 2008; 49:902–911. [DOI] [PubMed] [Google Scholar]
- 12.Subathra A, Sandhiya S, Kesavan R. An analysis of adverse drug reactions to radiographic contrast media reported during a 3 year period in a tertiary care hospital in south India. Indian J Physiol Pharmacol 2014; 58:45–50. [PubMed] [Google Scholar]
- 13.Leone R, Sottosanti L, Luisa IM, et al. Drug-related deaths: an analysis of the Italian spontaneous reporting database. Drug Saf 2008; 31:703–713. [DOI] [PubMed] [Google Scholar]
- 14.Pradubpongsa P, Dhana N, Jongjarearnprasert K, et al. Adverse reactions to iodinated contrast media: prevalence, risk factors and outcome- the results of a 3-year period. Asian Pac J Allergy Immunol 2013; 31:299–306. [DOI] [PubMed] [Google Scholar]
- 15.Wendt-Nordahl G, Rotert H, Trojan L, et al. Intravenous contrast media in uroradiology: evaluation of safety and tolerability in almost 50,000 patients. Med Princ Pract 2006; 15:358–361. [DOI] [PubMed] [Google Scholar]
- 16.Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990; 175:621–628. [DOI] [PubMed] [Google Scholar]
- 17.Vogl TJ, Honold E, Wolf M, et al. Safety of iobitridol in the general population and at-risk patients. Eur Radiol 2006; 16:1288–1297. [DOI] [PubMed] [Google Scholar]
- 18.Meth MJ, Maibach HI. Current understanding of contrast media reactions and implications for clinical management. Drug Saf 2006; 29:133–141. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen HS, Morcos SK. ESUR guidelines on contrast media. Abdom Imaging 2006; 31:131–140. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M, Heine O, Wolf M, et al. Safety and tolerability of iobitridol in general and in patients with risk factors: results in more than 160,000 patients. Eur J Radiol 2011; 80:357–362. [DOI] [PubMed] [Google Scholar]


