Abstract
Minimal change disease (MCD) is a well-known benign primary glomerulonephritis because of its distinct rare tendency to progress to end-stage renal disease. However, factors associated with relapse in adults are not well known. We aimed to identify predictors of relapse in adult-onset MCD patients.
A retrospective cohort of 195 patients with adult-onset primary MCD with nephritic syndrome and disease onset between 1979 and 2013 was followed up for >12 months. The number of relapses was counted and predictors of relapse were analyzed.
A total of 195 patients were included. Median age at diagnosis was 38 years (IQR, 23–53 years) and 113 (57.9%) were men. During 81 months (IQR, 44–153 months) of follow-up, 92% of patients achieved remission after initial treatment. However, only 60 (32.8%) did not experience a relapse and 11 patients failed to remit. Among the remaining 124 patients, 65 experienced a relapse once or twice and 59 experienced a relapse more than twice. Younger onset age, increased severity of nephrotic features such as lower serum albumin levels and higher cholesterol level were associated with relapse. Interestingly, the grade of mesangial proliferation was lower in patients who experienced a relapse. Initial combined treatment with corticosteroids (CS) and cyclophosphamide reduced the number of relapses. In addition, patients with shorter treatment duration tended to experience relapse more often. Multivariate analysis showed that younger onset age, combined mesangial proliferation, initial treatment regimen, and treatment duration were independent risk factors for relapse. Progression to end-stage renal disease was developed in only a patient.
In conclusion, more than two-thirds of adult-onset nephrotic MCD patients experienced relapse, although their renal progression was rare. Younger onset age, CS without cyclophosphamide treatment, and shorter treatment duration were independent risk factors for relapse in adult-onset MCD patients.
INTRODUCTION
Minimal change disease (MCD) is a common cause of nephrotic syndrome (NS) in about 90% of children younger than 10 years, 50% to 70% of older children, and 10% to 15% of adults.1 Because of its high prevalence in children, most studies on the natural course, treatment, and prognosis of MCD have focused on children. In children, MCD is a benign condition because almost all patients achieve remission with corticosteroid (CS) treatment within 4 weeks, and rarely experience progression to renal failure, although they experience frequent relapse.2
Compared to pediatric MCD, adult-onset nephrotic MCD has been studied less extensively in terms of clinical features, treatment options and adequate durations, and relapse. Previous studies on adult-onset MCD patients have reported a higher risk of acute kidney injury3–5 and delayed response to treatment with CS compared to pediatric MCD patients.6,7 Although most previous studies have shown that 60% to 90% of adults with MCD achieve complete remission (CR) with CS alone,4–6,8–10 a recent study showed that only 30% of adult Chinese MCD patients achieved CR after initial treatment.11 In addition, long-term prognosis may not be favorable, as indicated in a previous study in which a considerable number of adult-onset MCD patients who were found to have focal segmental glomerulosclerosis (FSGS) on a second kidney biopsy experienced progression to end-stage renal disease (ESRD) or death.11 These findings indicate that adult-onset MCD patients may have more severe clinical features than pediatric MCD patients.
Another important morbidity associated with adult-onset MCD is relapse of NS. Comparable to pediatric MCD patients, relapse following initial treatment commonly occurs in adult patients.3,6,7,11 However, data on relapse in adult-onset MCD patients are very scarce. A recent study on Japanese MCD patients showed that 74.1% of patients experienced at least one relapse and initial methylprednisolone treatment was associated with a lower incidence of relapse in adult-onset MCD patients.12 A small study reported a 39.3-month period of freedom from relapse following treatment.13
We previously showed that most adult-onset MCD patients who did not develop FSGS rarely experienced progression to ESRD, and had a mortality rate that was not different from that in age- and sex-matched general population.14 However, it is unclear whether they experienced frequent relapse and factors affecting relapse. Therefore, we explored characteristics and predictors of relapse in adult-onset nephrotic MCD patients.
METHODS
Subjects
From 1979 to 2013, data were extracted on adult (age ≥18 years) patients with biopsy-proven primary MCD at the Seoul National University Hospital, Korea. We excluded patients who were followed up less than 12 months or those with incomplete medical records. Patients with secondary MCD were excluded to avoid any effect on treatment response, relapse, and long-term prognosis. Ethical approval was obtained from the Seoul National University Hospital Institutional Review Board (H-1206-127-416), and this study was conducted in accordance with the principles of the Declaration of Helsinki. As the study was retrospective in design and did not involve any interventions, the Institutional Review Board waived informed consent for this study.
Clinical and Pathohistological Assessment
Clinical information was collected by electrical medical record review. Demographic factors at the time of renal biopsy were obtained. Blood and urinalysis were extensively reviewed from the time of MCD diagnosis to the last follow-up. The estimated glomerular filtration rate (eGFR) was calculated using the assay traceable to isotope dilution mass spectrometry and 4-variable Modification of Diet in Renal Disease Equation.15 Proteinuria was measured using random urine protein–creatinine ratio (UPCR) or 24-hour urinary protein measurement. NS was defined as proteinuria more than 3.5 g/day, serum albumin levels less than 3.0 g/dL, generalized edema and hyperlipidemia.
All native renal biopsies were processed according to standard techniques for light microscopy, immunofluorescence, and electron microscopy. To evaluate histopathological changes, 2 independent pathologists reviewed the renal biopsy slides. On light microscopy, the proportions of global sclerosis, segmental sclerosis, and crescent formation in the glomerular area were calculated as the previous report.16 Mesangial proliferation was also graded as none or minimal (<10%), mild (10–25%), moderate (25–50%), and severe (more than 50%). In the tubulointerstitial area, the degree of tubular atrophy, interstitial fibrosis, and interstitial inflammation was graded semi-quantitatively as follows: none or minimal (<10%), mild (10–25%), moderate (25–50%), and severe (more than 50%). Combined acute tubular necrosis (ATN) was also evaluated.
Treatment and Response Evaluation
Information on treatments prescribed during the follow-up period was collected. Immunosuppressive treatment included CS alone, CS with cyclophosphamide, CS with calcineurin inhibitors, and high-dose CS pulse therapy. CS treatment was initiated with 1 mg/kg of oral prednisolone. The use of cyclophosphamide and cyclosporine was determined by the primary physicians. Adjunctive information on albumin use was also obtained. Treatment response was analyzed as CR and persistent proteinuria. CR was defined as 24-hour urine protein level < 0.3 g/day, random UPCR <0.3 g/g creatinine, or trace or negative urine dipstick albumin results. Persistent proteinuria was defined as proteinuria more than 0.3 g/day or UPCR >0.3 g/g creatinine. Steroid dependence was defined as relapse upon tapering steroid therapy or within 4 weeks of discontinuing steroid therapy. Time to remission was defined as time from therapy initiation to the first day on which remission was observed. Because longer follow-up duration was critical in relapse, we also assessed follow-up duration. We investigated a progression to ESRD and death after diagnosis of MCD.
Outcome Measures
The primary outcome was the number of relapses. Relapse was defined as increased protein excretion of >3 g/day or random UPCR >3 g/g creatinine with generalized edema. We divided study subjects into three groups as follows: nonrelapse group (Group 1), relapse once or twice group (Group 2), and relapse thrice or more group (Group 3).
Statistical Analysis
Categorical variables were presented as frequencies with percentages. Continuous variables were examined for normality by the Kolmogorov–Smirnov test. Nonnormally distributed variables were analyzed by Mann–Whitney U test. Normal distributed variables were summarized as means ± SD, and nonnormally distributed variables as medians and interquartile ranges (IQR). Differences in demographic and pathologic characteristics across the relapse groups were compared using the χ2 test (linear-by-linear association). One-way analysis of variance (ANOVA) and Kruskal–Wallis test were used to analyze the linearity of continuous variables across the relapse groups by P for trend.
To assess the effectiveness of intravenous CS pulse therapy for the relapse of proteinuria in the adult MCD patients, we used propensity scores to parsimoniously adjust for confounding factors. We calculated the propensity score of patients who did not receive intravenous CS pulse therapy after fitting the multivariate logistic regression model into the data of the patients who received the therapy. Data that could affect the intravenous CS pulse therapy were used to calculate the propensity score and included age, serum creatinine or eGFR, number of glomeruli, degree of ATN, and grade of tubulointerstitial atrophy and fibrosis. We matched 1 well-matched patient to 2 nonwell-matched patients by their propensity score +0.005 (0.1 × standard deviation of propensity score) to generate a subcohort of 87 well-matched patients.
To determine predictors of relapse, univariate and multivariate logistic regression analyses were performed. We included variables with significant association (P < 0.10) in the univariate analysis or those of considerable theoretical relevance in the multivariate logistic regression models. The SPSS software package (version 21.0, Chicago, IL) was used in statistical analyses. All tests were 2-tailed, with P < 0.05 considered statistically significant.
RESULTS
Baseline Characteristics
Among a total of 319 patients, patients who experienced progression to FSGS (n = 7), did not present with nephrotic features (n = 21) were excluded. In addition, patients who did not have follow-up data for more than 12 months (n = 41), or had inadequate data on treatment or response (n = 22) were also excluded. Drug-induced MCD was caused by nonsteroidal antiinflammatory drugs including penicillamine in 4 patients and herbal medicine in 1 patient. MCD was associated with thymoma and invasive thymic carcinoma in two patients each. Two patients were found to have amyloidosis on a second biopsy. One patient developed MCD with NS associated with advanced gastric cancer. Four patients developed rheumatic disease associated MCD, including 2 with rheumatic arthritis and 1 each with Sjögren syndrome and polymyositis. Finally, 195 primary adult-onset MCD patients were included in the analyses (Figure 1).
FIGURE 1.
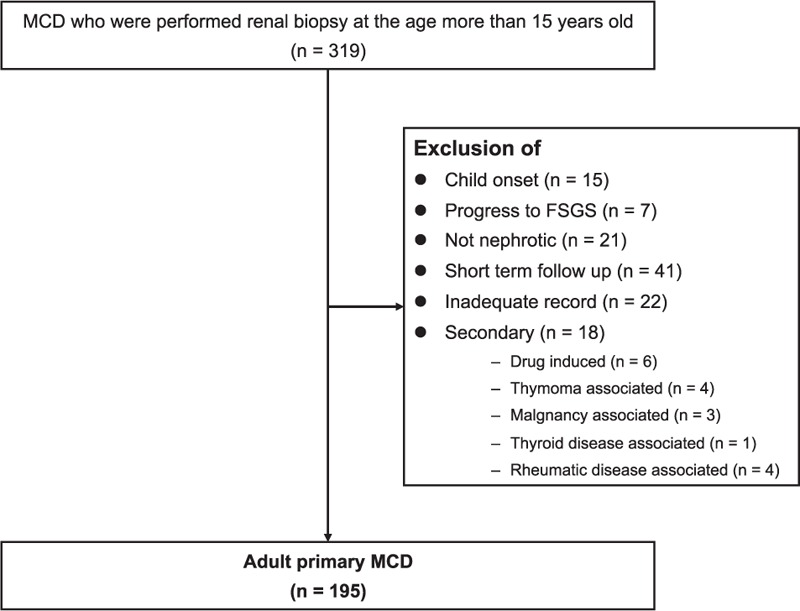
Study flowchart.
Clinical and pathohistological characteristics of the 195 adult primary MCD patients are summarized in Table 1. Median age was 38 years (IQR, 23–53 years), and a higher number of patients were men (57.9%). Only 8 (4.1%) patients had diabetes and 26 (13.3%) had hypertension at the time of diagnosis. At the time of renal biopsy, renal function and blood pressure were within normal range in most patients. The median serum creatinine level was 1.0 mg/dL (IQR, 0.8–1.4 mg/dL) and eGFR was 76.0 ± 31.1 mL/min/1.73 m2. Median serum albumin level was 2.0 g/dL (IQR, 1.6–2.3 g/dL), mean cholesterol level was 420 ± 134 mg/dL, and mean urinary protein excretion was 10.9 ± 6.7 g/day. Microscopic hematuria was detected in 89 (45.6%) patients. Pathohistological findings indicated relatively preserved glomeruli, except for a small proportion of combined global sclerosis. Eighty-four (43.1%) patients showed combined mesangial proliferation and 23 (11.8%) patients had combined ATN on histopathological examination. More than half of the patients had varying degree of interstitial fibrosis.
TABLE 1.
Baseline Characteristics
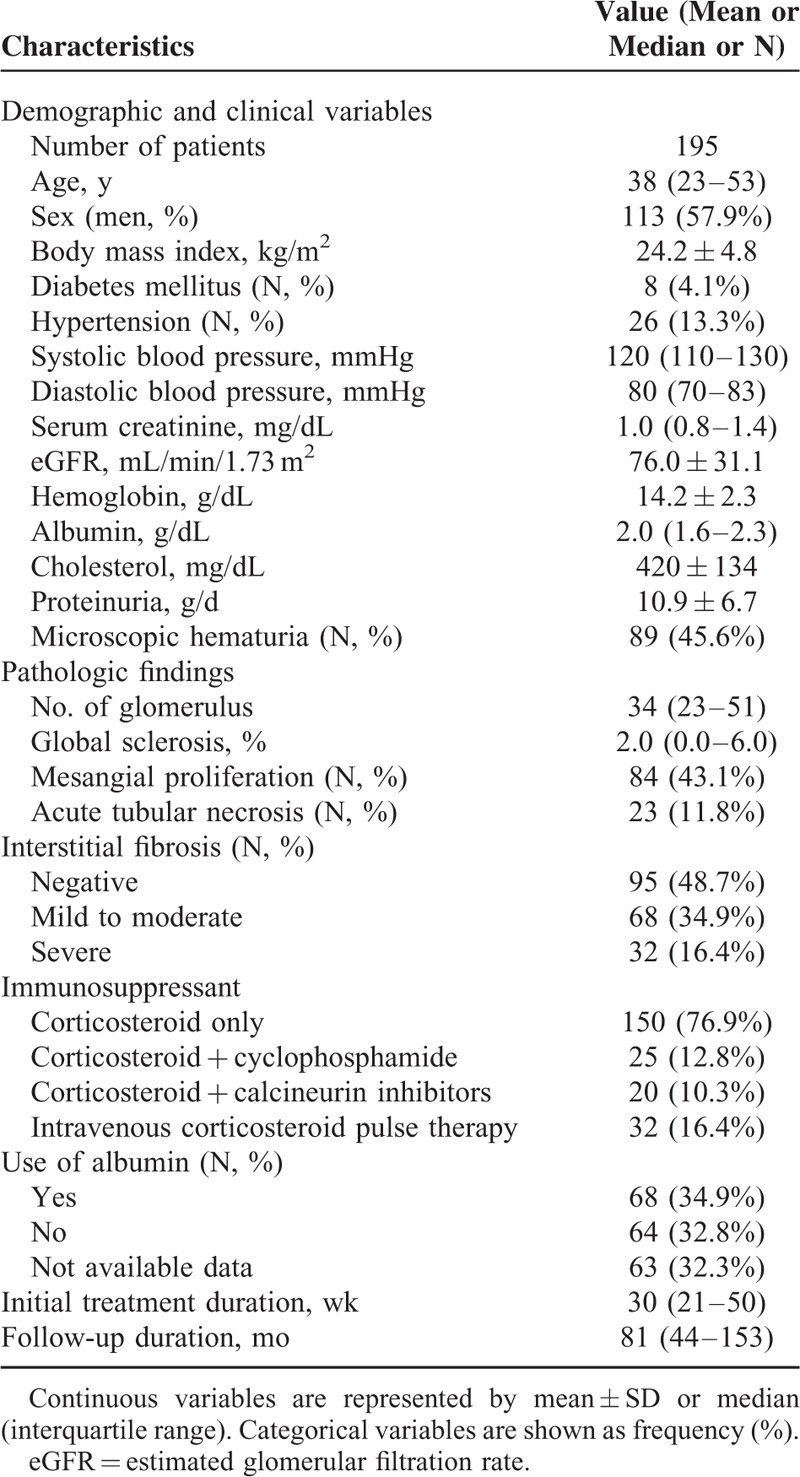
The majority (76.9%) of patients received CS only as a initial treatment. The remaining patients received a combined treatment from initial presentation with CS and cyclophosphamide (25 patients, 12.8%) or CS and calcineurin inhibitors (20 patients, 10.3%). There was no patient who received mycophenolate mofetil, rituximab, or leflunomide as an initial immunosuppressive treatment. Overall, 32 (16.4%) patients received high-dose intravenous CS pulse therapy. Ten patients did not achieve CR. The remaining 185 (94.9%) patients achieved CR within 22 days (IQR 14–53 days). Median treatment duration was 30 weeks (21–50 weeks), and 92% of patients achieved CR after initial treatment. About 33% of patients were dependent on CS, and 29 (14.9%) were refractory to CS after initial response.
Relapse Characteristics
Relapse of NS occurred in 131 (67.2%) initial responders. The number of total relapse is shown in Figure 2A. About one-fourth of patients experienced relapse only once after MCD diagnosis, another two-fifth of patients experienced relapse 2 or 3 times, and one-third experienced relapse more than 3 times. The maximum number of relapses was 33 in a patient with extremely poor treatment compliance that resulted in a relapse every 3 to 6 months. The median annual number of relapses was 0.20 (IQR 0–0.52). The median time to first relapse was 14 weeks (IQR 0–55 weeks). In almost half of relapse patients, first relapse occurred within the first 3 months of diagnosis, as shown in Figure 2B, although in some cases, relapse occurred 5 years after diagnosis. Thirty-nine patients experienced relapse of NS during tapering of immunosuppressant dose. Twenty-one patients experienced relapse within 3 months after cessation of immunosuppressant therapy.
FIGURE 2.
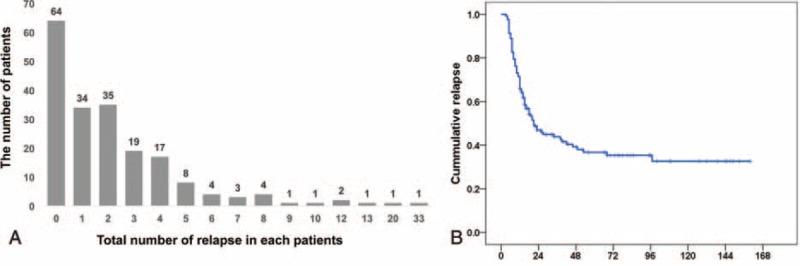
Distribution of the number of relapse (A) and time to relapse (B). X-bar means the number of relapse and y-bar means the number of patients (A). Label B shows the relapse-free survival in adult MCD patients. Time to relapse is categorized at regular intervals of 24 weeks, and the numbers of patients remaining are shown at the bottom.
Even after relapse, more than half of patients (72, 57.6%) received treatment with CS alone. The other 53 (42.4%) patients were treated with combination therapy with CS, as follows: 32 with cyclosporin A, 4 with tacrolimus, and 17 with cyclophosphamide. During a median follow-up period of 82 months (IQR 44–153 months), only 1 patient experienced progression to ESRD. The cause of ESRD was unclear because ESRD was diagnosed 17 years after the last follow-up. One woman died of colon cancer 8 years after MCD diagnosis.
Comparisons According to the Relapse Frequency
Table 2 shows the comparison of the clinical and pathologic characteristics of patients according to the number of relapses. The age of the patients in Group 1, who did not experience relapse after MCD diagnosis, was significantly higher than that of patients in Groups 2 and 3. Moreover, the distribution of relapse groups assumed an ascending stair pattern in Group 1, contrary to the descending stair pattern in Groups 2 and 3 (Figure 3, P for trend = 0.019). The frequency of relapse was associated with the severity of nephrotic features. Group 3 patients showed significantly lower serum albumin and higher cholesterol levels than Group 1 and 2 patients. Interestingly, the proteinuria amount did not differ among the 3 groups. Sex, body mass index, underlying disease, and blood pressure did not differ according to the number of relapses. There were no differences among the three relapse groups in the histopathologic features, except in the mesangial proliferation grade, which was higher in Group 1 than in Groups 2 and 3 (P for trend = 0.018).
TABLE 2.
Comparisons of Clinical and Pathologic Characteristics According to the Frequency of Relapse
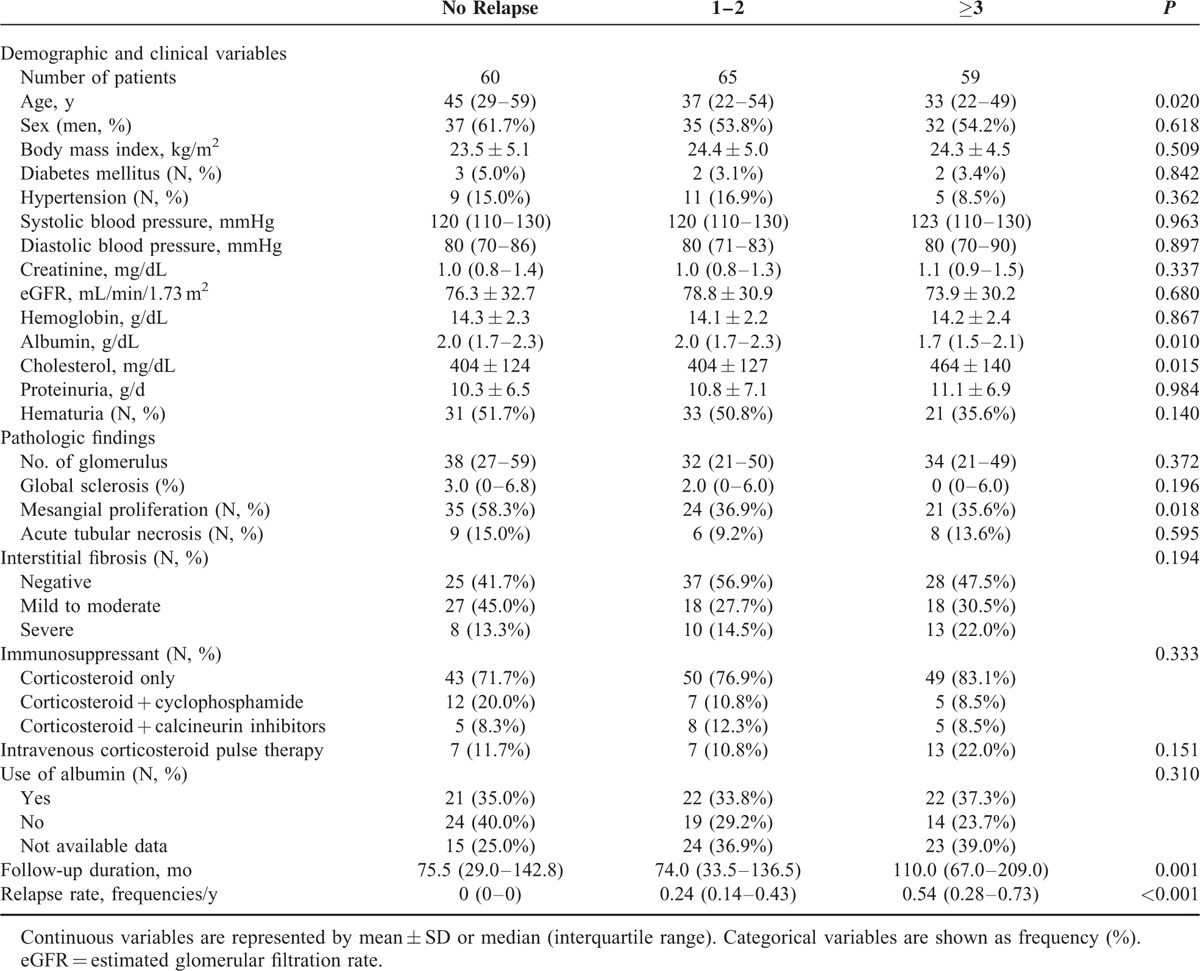
FIGURE 3.
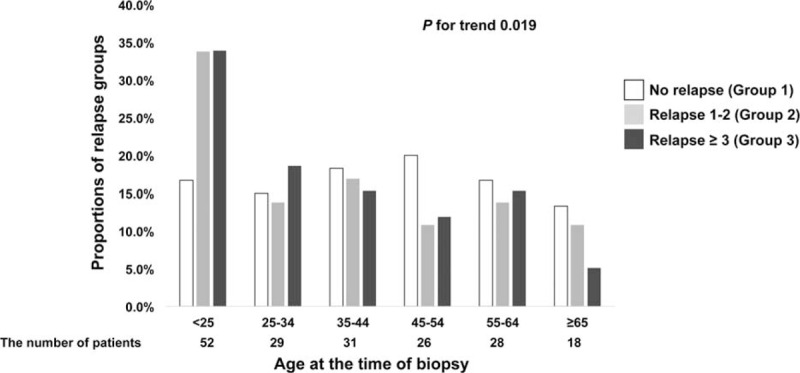
Distribution of relapse groups in 10-year age groups. X-bar means 10-year age groups at the time of initial diagnosis. The number of patients in each age groups are as follows: age < 25 years, 56; 25 to 34 years, 31; 35 to 44 years, 32; 45 to 54 years, 28; 55 to 64 years, 28; and ≥65 years, 20. Y-bar means proportions of relapse groups in each age groups.
There were a total of 55 steroid-dependent MCD patients. In Group 1, there were no steroid-dependent cases. Nineteen Group 2 patients and 36 Group 3 patients were considered steroid dependent. Regarding the use of immunosuppressive drugs, CS pulse therapy tended to be used more frequently in Group 3 than in Groups 1 and 2. This finding was parallel with the relationship between the severity of NS and relapse. Initial choice of immunosuppressant was not associated with the total number of relapses. However, combined treatment with CS and cyclophosphamide significantly lowered the average annual number of relapses as follows (P = 0.015): CS only, 0.20 (IQR, 0–0.51); CS with cyclophosphamide, 0.02 (IQR, 0–0.23); CS with calcineurin inhibitors, 0.44 (IQR, 0.04–0.72). Furthermore, shorter treatment duration was associated with an increased frequency of relapse with marginal significance (P for trend 0.054). Because treatment regimen might affect treatment duration, we compared treatment duration according to treatment regimen. Interestingly, patients receiving a combined initial treatment with CS and cyclophosphamide had a shorter treatment duration (22.6 weeks [IQR, 12.3–45.8 weeks]) than those receiving CS alone (29.9 weeks, [IQR, 22.1–48.6 weeks]) or CS and calcineurin inhibitors (42.7 weeks [IQR, 24.9–72.7 weeks]) (P = 0.042). As our expectation, follow-up duration was longer (P = 0.001) and relapse rate was higher (P < 0.001) in Group 3 than Groups 1 and 2.
Predictors of Relapse
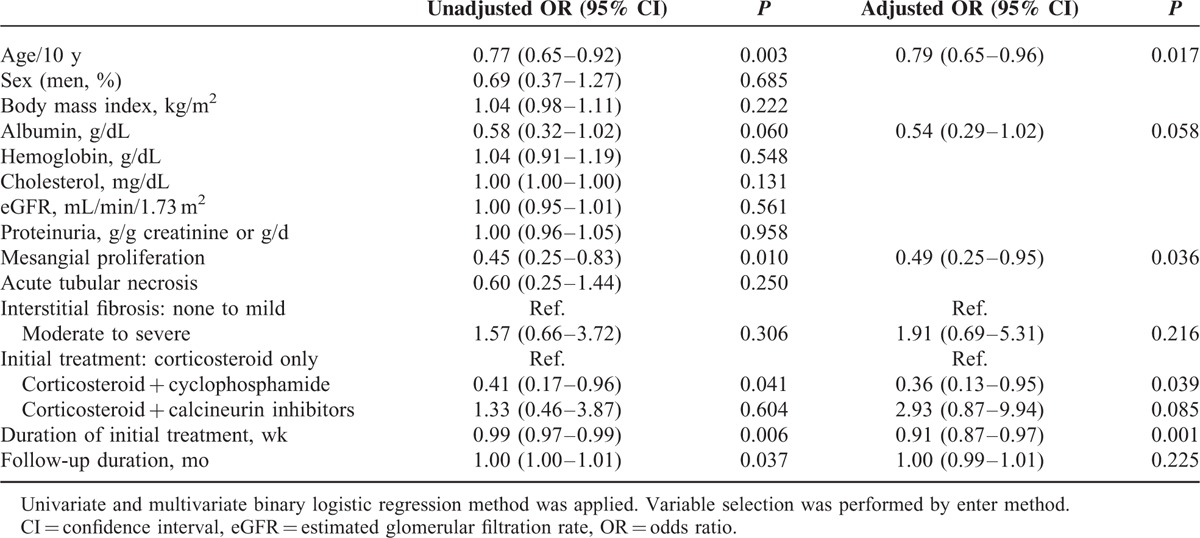
On univariate analysis, younger onset age, lower serum albumin level and mesangial proliferation grade, initial treatment without cyclophosphamide, shorter treatment duration, and longer follow-up duration were associated with relapse. Intravenous CS pulse therapy was not associated with the relapse of NS. On multivariate logistic regression analysis, we found that the risk of relapse reduced by 24% for every 10-year increase in age (adjusted odds ratio [OR], 0.79; 95% confidence interval [CI], 0.65–0.96; P = 0.017). In addition, mesangial proliferation was a protective factor against relapse in adult MCD patients (adjusted OR 0.49, 95% CI, 0.25–0.95, P = 0.036). Shorter treatment duration proved to be an independent risk factor for relapse (adjusted OR 0.91, 95% CI, 0.87–0.97, P = 0.001). Initial treatment with a combination of CS and cyclophosphamide was associated with a 64% lower probability of relapse (adjusted OR 0.36, 95% CI, 0.13–0.95, P = 0.039) compared with treatment with CS alone or in combination with calcineurin inhibitors. Severity of NS, represented by serum albumin level, was not significantly associated with relapse (Table 3).
TABLE 3.
Predictors for Relapse
Initial Intravenous CS Pulse Therapy and Relapse of MCD
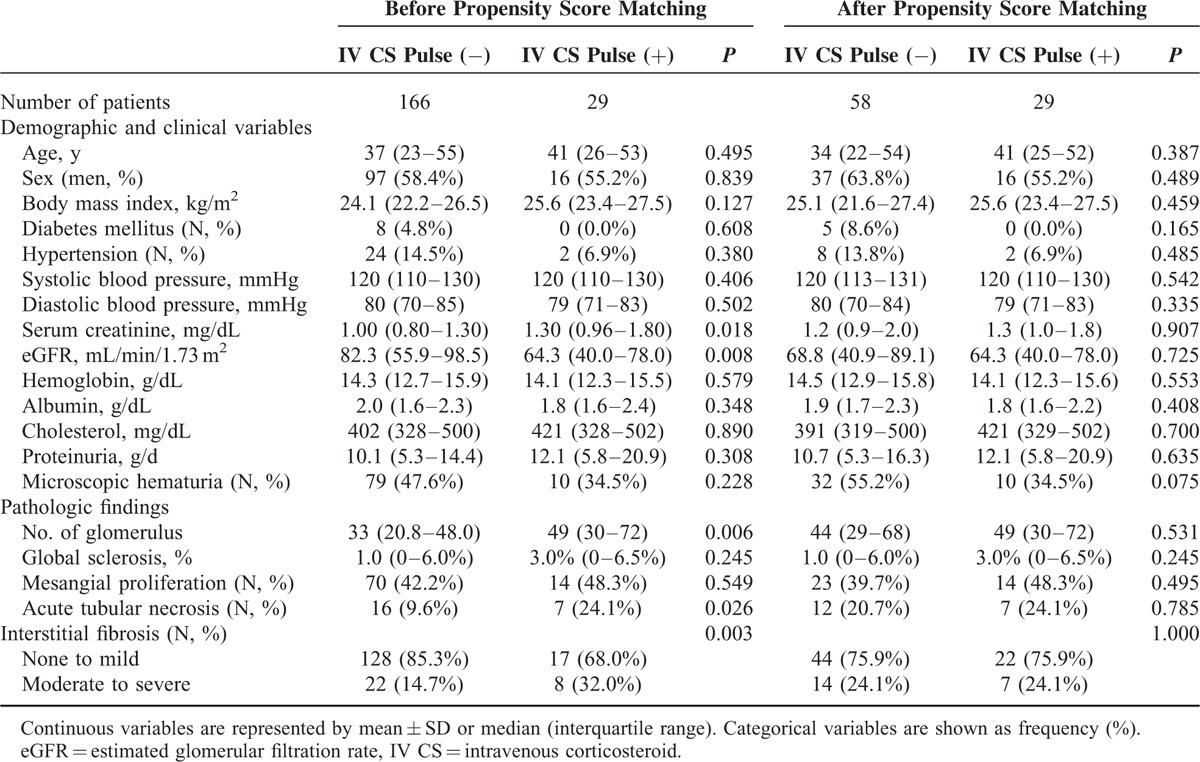
Table 4 shows a comparison between patients who were, at the time of the initial attack, treated with intravenous CS pulse therapy and those who were not. Patients treated with intravenous CS pulse therapy had lower eGFR, higher number of glomeruli, and more severe ATN and tubulointerstitial fibrosis. After matching, these differences were no longer significant between the 2 groups. Intravenous CS pulse therapy was not associated with relapse of NS in adult onset MCD patients even after matching (OR 1.28, 95% CI 0.48–3.413, P = 0.623).
TABLE 4.
Comparisons Between Patients With and Without Intravenous Corticosteroid Pulse Therapy
DISCUSSION
In this study, we examined the clinical characteristics of relapse and its predictors in adult-onset MCD patients. Our results reconfirmed that adult-onset MCD is a relatively benign disease in terms of long-term renal and patient outcome. However, we also found that more than two-thirds of adult-onset MCD patients experienced relapse more than once after diagnosis. In addition, younger onset age, lower grade of mesangial proliferation, initial treatment without cyclophosphamide, and shorter initial immunosuppressive treatment duration were found to be independent risk factors for relapse in adult-onset MCD patients. Calcineurin inhibitors and intravenous CS pulse therapy did not show any protective effect on relapse in this retrospective cohort. To our knowledge, our study is the first to examine relapse and its predictors in adult-onset MCD patients.
Similar to other glomerulonephritis, MCD is diagnosed using both clinical and pathologic features. Primary MCD is diagnosed based on clinical presentation of NS and pathohistological findings. One of the important clinical features that should be considered in the diagnosis of primary MCD is treatment response. According to the KDIGO guidelines for glomerulonephritis, in MCD patients refractory to initial CS treatment, a second kidney biopsy should be considered to exclude FSGS or other glomerulonephritis conditions.17 A recent Chinese study supported this recommendation.11 Among the 38 treatment-resistant MCD patients, 30 progressed to ESRD. Among them, 12 repeated kidney biopsies proved 5 FSGS. In patients responsive to treatment, ESRD progression was found in only 2 of 302 patients. In this study, we excluded such patients from the study to focus on pure MCD; hence, only one adult-onset MCD patient progressed to ESRD.
MCD is considered a relatively benign primary glomerulonephritis because of its excellent responsiveness to CS treatment and a rare tendency to progress to renal dysfunction. However, the clinical factors associated with relapse, which is the most distinctive feature of MCD, remain obscure. Our study showed that more than two-thirds of patients experienced relapse despite immunosuppressant treatment for >7 months. A treatment duration of 3 to 6 months with CS in pediatric MCD patients18,19 and a longer treatment duration of >6 months in adult-onset MCD patients6 due to their delayed response6,11 are recommended.
In this study, we found that younger onset age was associated with an elevated risk of relapse of NS, and this finding is consistent with those of previous studies.4,9,10,20 Initial combined treatment of CS with cyclophosphamide can reduce the risk of a relapse, and has been shown to be associated with a generally good response in CS-resistant MCD.9 However, studies on the effects of cyclophosphamide in reducing relapse are scarce, except for small observational studies showing that patients achieved CR with cyclophosphamide alone21 or CS–cyclophosphamide combination9 showed a lower frequency of relapse. The addition of cyclophosphamide was shown to reduce the frequency of relapse in multivariate logistic regression analysis despite shorter treatment duration than other treatment regimen in our study.
In pediatric MCD patients, longer duration of treatment with CS had a heterogeneous effect on relapse. Several previous studies suggested that longer duration of CS use can reduce the frequency of relapse,22 however, recent studies did not show any difference in relapse.18 In adult patients, our study is the first to show the effect of treatment duration on relapse frequency. Therefore, to confirm the impact of longer duration of CS on relapse frequency reduction, further balanced studies on risk factors of relapse in adult-onset MCD patients, including treatment duration, are needed.
Impact of intravenous CS pulse therapy on relapse frequency remains controversial. Two small randomized controlled trials showed that intravenous CS pulse therapy had no additive gain on relapse frequency reduction compared with oral steroid only.23,24 On contrary, a recent retrospective study reported that initial use of intravenous CS was associated with a lower incidence of the first relapse than steroid single treatment in adult MCD patients.12 In our study, we tried to prove the effect of CS pulse therapy on relapse prevention using matched control, however, the effect was not observed. Different from the Japanese cohort in which near half of patients were treated with CS pulse therapy, only about 20% of patients received it. In addition, we investigated the role of CS pulse therapy compared with combination treatment, not with CS alone. These discrepancies might bring about the different results. Further controlled prospective studies are warranted to clarify the efficacy of CS pulse therapy.
Our findings are controversial to the Hoyer study, which suggested that even short-term add-on use of cyclosporine might protect against relapse in idiopathic pediatric NS patients. However, this effect attenuated from 1-year after treatment, and also by aging, therefore it remains difficult to apply to adult MCD patients.25 Further studies need to be performed to verify our results. In addition, the impact of longer treatment duration on relapse has not been clarified.18,26
In spite of this study's novelty, some limitations should be noted. First, this study is a retrospective single center study, and, there was no specific treatment protocol. The selection of immunosuppressant, dose, and duration was completely depended on each attending physician, which could have affected the treatment-associated predictors for relapse. Moreover, information on supportive care for NS, including anticoagulant, renin-angiotensin system blockade, and lipid-lowering agent use, was limited. Second, this study takes place over a long large time period over 34 years, and therefore there are some secular changes in demographic factors, biopsy practices, and treatment patterns. Although the severity of NS at the time of biopsy did not changed much, mean age increased recently. The improved percutaneous renal biopsy safety and general health status in elderly may make renal biopsy more active. In terms of treatment, the use of calcineurin inhibitors and intravenous pulse therapy is increasing in recent years, whereas the combined use of cyclophosphamide is decreasing. These temporal trends should be considered when interpreting our study results.
In summary, we found that more than two-thirds of adult MCD patients experienced more than one relapse after diagnosis. Younger onset age, lower combined mesangial proliferation on histological evaluation, initial treatment without cyclophosphamide, and shorter duration of initial treatment can lead to relapse in adult MCD patients. To determine whether relapse is associated with NS- or treatment-related complications or not, further studies are needed.
Footnotes
Abbreviations: ATN = acute tubular necrosis, CR = complete remission, CS = corticosteroid, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, FSGS = focal segmental glomerulosclerosis, MCD = minimal change disease, NS = nephrotic syndrome, UPCR = urine protein–creatinine ratio.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Johnson RJ, Feehally J, Floege J. Comprehensive Clinical Nephrology. 5th ed.Philadelphia: Elsevier Saunders; 2014. [Google Scholar]
- 2.Tarshish P, Tobin JN, Bernstein J, et al. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997; 8:769–776. [DOI] [PubMed] [Google Scholar]
- 3.Huang JJ, Hsu SC, Chen FF, et al. Adult-onset minimal change disease among Taiwanese: clinical features, therapeutic response, and prognosis. Am J Nephrol 2001; 21:28–34. [DOI] [PubMed] [Google Scholar]
- 4.Mak SK, Short CD, Mallick NP. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant 1996; 11:2192–2201. [DOI] [PubMed] [Google Scholar]
- 5.Keskar V, Jamale TE, Kulkarni MJ, et al. Minimal-change disease in adolescents and adults: epidemiology and therapeutic response. Clin Kidney J 2013; 6:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2007; 2:445–453. [DOI] [PubMed] [Google Scholar]
- 7.Korbet SM, Schwartz MM, Lewis EJ. Minimal-change glomerulopathy of adulthood. Am J Nephrol 1988; 8:291–297. [DOI] [PubMed] [Google Scholar]
- 8.Tse KC, Lam MF, Yip PS, et al. Idiopathic minimal change nephrotic syndrome in older adults: steroid responsiveness and pattern of relapses. Nephrol Dial Transplant 2003; 18:1316–1320. [DOI] [PubMed] [Google Scholar]
- 9.Nolasco F, Cameron JS, Heywood EF, et al. Adult-onset minimal change nephrotic syndrome: a long-term follow-up. Kidney Int 1986; 29:1215–1223. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama M, Katafuchi R, Yanase T, et al. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis 2002; 39:503–512. [DOI] [PubMed] [Google Scholar]
- 11.Szeto C-C, Lai FM-M, Chow K-M, et al. Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis 2015; 65:710–718. [DOI] [PubMed] [Google Scholar]
- 12.Shinzawa M, Yamamoto R, Nagasawa Y, et al. Comparison of methylprednisolone plus prednisolone with prednisolone alone as initial treatment in adult-onset minimal change disease: a retrospective cohort study. Clin J Am Soc Nephrol 2014; 9:1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kida H, Iida H, Dohi K, et al. Period of freedom from relapse as an indication of cure in minimal change nephrotic syndrome in adults. Nephron 1977; 19:153–157. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Kim DK, Oh KH, et al. Mortality and renal outcome of primary glomerulonephritis in Korea: observation in 1,943 biopsied cases. Am J Nephrol 2013; 37:74–83. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145:247–254. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Hwang JH, Paik JH, et al. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol 2014; 15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glomerulonephritis KCPGf. Chapter 5: minimal-change disease in adults. Kidney Int Suppl 2012; 2:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn D, Hodson EM, Willis NS, et al. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev (Online) 2015; 3:CD001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nephrologie ∗∗∗AfP. Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children∗∗∗. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Lancet 1988; 1:380–383. [PubMed] [Google Scholar]
- 20.Shinzawa M, Yamamoto R, Nagasawa Y, et al. Age and prediction of remission and relapse of proteinuria and corticosteroid-related adverse events in adult-onset minimal-change disease: a retrospective cohort study. Clin Exp Nephrol 2013; 17:839–847. [DOI] [PubMed] [Google Scholar]
- 21.Al-Khader AA, Lien JW, Aber GM. Cyclophosphamide alone in the treatment of adult patients with minimal change glomerulonephritis. Clin Nephrol 1979; 11:26–30. [PubMed] [Google Scholar]
- 22.Srivastava RN, Vasudev AS, Bagga A, et al. Long-term, low-dose prednisolone therapy in frequently relapsing nephrotic syndrome. Pediatr Nephrol 1992; 6:247–250. [DOI] [PubMed] [Google Scholar]
- 23.Imbasciati E, Gusmano R, Edefonti A, et al. Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. Br Med J (Clin Res Ed) 1985; 291:1305–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung CK, Wong KL, Ng WL. Intravenous methylprednisolone pulse therapy in minimal change nephrotic syndrome. Aust N Zeal J Med 1983; 13:349–351. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer PF, Brodeh J. Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. J Am Soc Nephrol 2006; 17:1151–1157. [DOI] [PubMed] [Google Scholar]
- 26.Teeninga N, Kist-van Holthe JE, van Rijswijk N, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol 2013; 24:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]


