Abstract
The prevalence of diabetes in Jordan has been increasing. The early diagnosis of diabetes is vital to slow its progression. The Arab Risk (ARABRISK) screening tool is a self-administered questionnaire used to determine people who are at high risk for developing diabetes. This study aimed to identify people at high risk for developing type 2 diabetes by using the ARABRISK in the capital of Jordan.
A cross-sectional study was conducted with a convenience sample of people in the capital of Jordan. The ARABRISK screening tool was administered to identify the participants’ risk for developing diabetes. In addition to descriptive statistics, percentages of the ARABRISK categories were represented, and an independent samples t test was used to explore the differences between men and women. A total of 513 participants with a mean age of 51.94 (SD = 10.33) were recruited; 64.9% of the participants were men (n = 333).
The total ARABRISK score ranged from 0 to 25 with a mean score of 12.30 (SD = 4.76). Using the independent samples t test, women (mean = 13.25, SE = 0.10) had significantly higher ARABRISK total scores than men did (mean = 12.95, SE = 0.09), t(141) = −2.23, P = 0.03 in the “moderate risk” category. All of the items in the ARABRISK questionnaire were found to be good predictors of the ARABRISK total scores. Among them, age, body mass index (BMI), and high blood glucose (HBG) were the best predictors as indicated by the standardized regression coefficient (β). Older age, obesity, elevated weight circumference, absence of daily physical activity, daily consumption of fruits/vegetables, presence of high blood pressure (HBP), and HBG were significantly associated with increased odds of high ARABRISK total scores. Neither a history of gestational diabetes nor a positive family history was associated with an increased odds of high ARABRISK total scores.
By identifying risk factors in these participants, interventions and lifestyle changes can be suggested and implemented to reduce the risk and incidence of diabetes.
INTRODUCTION
Diabetes mellitus is a chronic disease with debilitating complications that contribute to morbidity and mortality. The worldwide prevalence of diabetes has been increasing at a noteworthy rate. It has been estimated that the total number of people with diabetes would inflate from 171 million in 2000 to 366 million in 2030.1 Healthcare costs from diabetes impose a global economic burden. The healthcare costs from diabetes alone were $376 billion USD in 2010 and have been estimated to increase to $490 billion USD in 2030.2 Presently, the Middle East region is among the most impacted countries.1
As noted in multiple research studies, the ability to recognize members of a population who are at risk for diabetes is critical for multiple reasons. Among these is that at the time of initial diagnosis, many patients are already demonstrating signs of small and large vessel complications, which indicate that diabetes may have gone undetected from 4 to 7 years before the patients’ diagnoses.3,4 Additionally, patients who are found to have prediabetes, as indicated by impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT), demonstrate a 10 to 20 times greater risk of developing of type 2 diabetes (T2D) compared with people with normal glycemic levels.5,6 Presently, there is a notable lack of assessment tools available to identify persons of Middle-Eastern origin who are prediabetic or have undiagnosed diabetics, despite the high numbers of both types of patients in the population.
Hence, there is an urgent need to apply applicable screening tools to facilitate diabetes prediction and support the global prevention effort. Previous randomized experimental studies on the prevention of diabetes have reported the effectiveness of lifestyle intervention to reduce the incidence of diabetes among those with prediabetes.7
The use of risk-scoring questionnaires may be helpful to upgrade the individual risk assessments.
Many risk-scoring models for T2D need specific blood test outcomes that assume that a clinical examination or diagnostic assessment has effectively occurred.8–15 This limits the widespread use of these models in a public health system. The Finnish Diabetes Risk Score (FINDRISC), developed in Finland, is a participant-rated diabetes risk assessment that does not require any knowledge of particular laboratory test values.16 Based on the FINDRISC, Canadian researchers developed the Canadian Risk (CANRISK), which considers the variability of the ethnicity of the Canadian population as well as the subject's sex and educational level.17 Both tools were developed to identify people who are at high risk for developing diabetes. An Arabic version of the CANRISK was adapted and validated to enable use with Arab-speaking people in Jordan and Saudi Arabia.18 The Arab Diabetes Risk Assessment Questionnaire (ARABRISK) represents an Arabic questionnaire designed to screen a person's risk of developing T2D or prediabetes in an Arab population.
The World Health Organization (WHO) has noted the threat of an increased prevalence of overweight and obesity as a detriment to the health of the worldwide population.19 Presently, the trend of sedentary work has increased20; thus, the risk of obesity has been increased because of long sedentary working hours.21 More recently, Leischik et al22 reported a high risk of cardiovascular disease, high prevalence of metabolic disease, increased waist circumference, and higher carotid intima media thickness in sedentary clerks compared with firefighters. Furthermore, Leischik et al23 reported an association of sedentary occupations with obesity and metabolic syndrome in middle-aged men. Therefore, the workplace is a good setting for the implementation of health programs.24 Ramli et al25 reported a significant improvement in physical fitness and body fat percentage following the implementation of health programs at a worksite. The prevalence of diabetes is the highest in Jordan among the world, which makes it an alarming public health burden.26 The prevalence of diabetes increased from 6.3% in 2002 to 7.4% in 2004 in Jordan's population.27 A study published in 2008 revealed a 31.5% increase in the prevalence of diabetes in Jordanians aged 25 years or older compared with a similar survey conducted in 1994.28 A previous study suggested that at the end of 2050, approximately 1 to 3 million people in Jordan will have diabetes, hypertension, or increased blood cholesterol based on the changes in disease prevalence and the growth of the population.29 In developing countries, for example, Palestine, the prevalence of diabetes is projected to be 20.8% in 2020 and 23.4% in 2030, as estimated by model forecasts.30
In Jordan, the self-reported diagnosed prevalence of obesity, high blood pressure, high blood cholesterol, and asthma in adults aged 18 years or older was 12.8%, 22.2%, 20.9%, and 5.1%, respectively.31 In a previous study, the presence of obesity compared with normal weight was significantly associated with diabetes (odds ratio [OR] 3.27), high blood pressure (OR 3.69), high cholesterol (OR 3.45), and asthma (OR 5.12) in the Jordanian population.32 According to the behavioral risk factor survey conducted in the Jordanian population, approximately 50% of the surveyed individuals were not participating in any physical activity.33 Obesity is a worldwide epidemic, particularly in the Middle East, where the rate of obesity is high (38%–44%) compared with Canada (23%) and the United States (21%).34 Another study reported that the prevalence of obesity would be 21.1% and 40.5% in men and women, respectively, in the year 2027.35 Furthermore, in a previous report, it was found that obese men who had a moderate to high fitness level had less than half the risk of death compared to normal weight men who had a poor fitness level.36 Recently, Al-Nsour et al37 reported a high prevalence of diabetes, hypertension, overweight, and obesity in the Jordanian male and female population. Therefore, the present study utilized the ARABRISK to identify and discuss the risk of diabetes among participants in the capital of Jordan (Amman).
METHODS
A cross-sectional questionnaire (ARABRISK)-based survey was used to identify the risk of developing type 2 diabetes (T2D) in a convenience sample of healthy participants in Amman, the capital of Jordan.
Procedures
After obtaining ethical approval from the ethical committee, the faculty of Rehabilitation Sciences in the University of Jordan and the participants gave informed consent. The ARABRISK was completed by healthy participants between June and September 2014. Participants for this study were recruited from public gathering areas such as malls and parks in Amman, Jordan. Both men and women subjects between the ages of 40 and 74 years of age were included in the study. Participants with a known diagnosis of diabetes were excluded.
Statistical Analysis
Descriptive statistics, including measures of central tendency and variability, were calculated to describe participants’ characteristics. Percentages of the ARABRISK categories were represented and compared between sexes. The independent samples t test was also performed between males and females to explore significant differences between sexes on the ARABRISK total score among categories. Stepwise multiple regression analysis was done to identify the best predictors for the outcome. In addition, a multivariate logistic regression analysis was done to determine the OR with the corresponding 95% confidence intervals (95% CI) for all risk factors. All of the statistics were significant when P < 0.05. Statistical analysis was conducted using SPSS statistics for Windows version 21 (SPSS Inc, Chicago, IL).
RESULTS
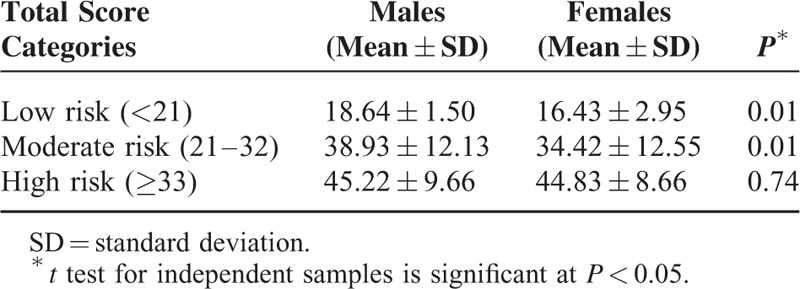
Five hundred thirteen participants were recruited from public areas of Amman, Jordan. The total ARABRISK score ranged from 8 to 76 with a mean total score of 37.35 (SD = 12.46). Sixty-one percent of the participants had a high risk for developing diabetes (Table 1). The comparison between males and females demonstrated that the male participants had significantly higher ARABRISK total scores than did women in both the low- and moderate-risk categories (Table 2), signifying that male participants had a higher risk of developing T2D than did females.
TABLE 1.
Results of ARABRISK Specific Items and Total Score Between Males and Females
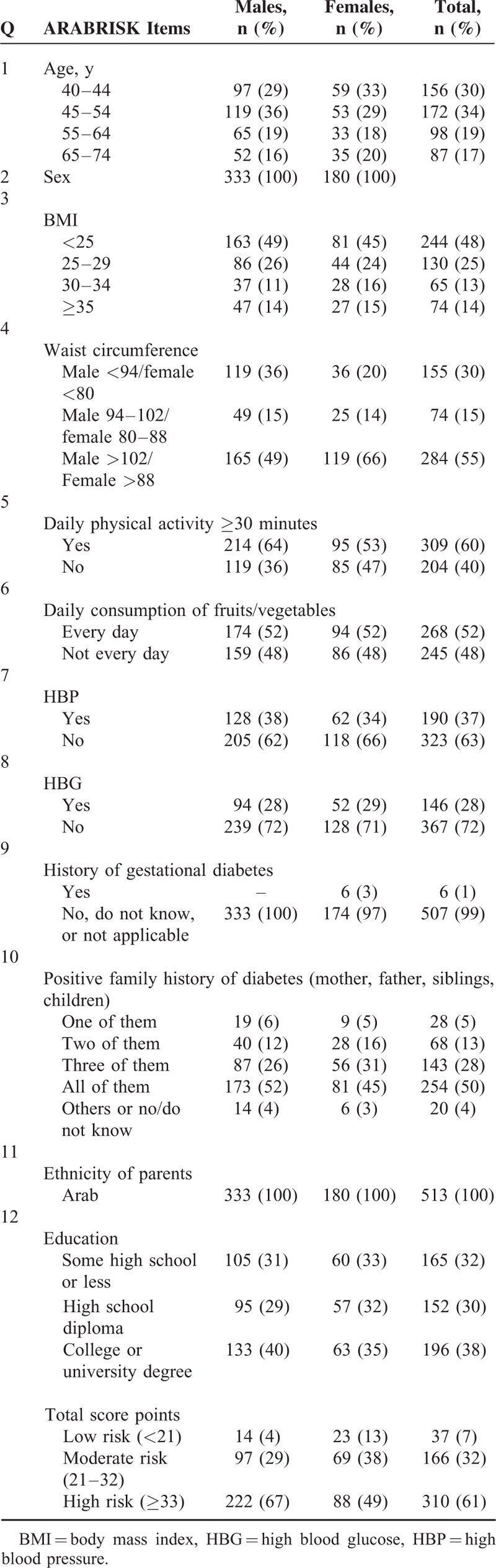
TABLE 2.
Mean and SD of ARABRISK Total Score Between Males and Females
The age of participants ranged from 40 to 74 years, with a mean age of 51.94 (SD = 10.33). The study included 65% males (n = 333) and 35% females (n = 180). The mean BMI score was 27.85 (SD = 7.02), with a low of 17 and a high of 53. The mean waist circumference was 58.93 cm (SD = 22.77). In addition to these characteristics, both the maternal and paternal ethnicity for all participants was Middle Eastern descent (Arab). Only 3% of the women who had given birth to a large baby weighing at least 9 pounds had developed gestational diabetes.
Table 3 details the mean, SD, and 95% CI of the mean ARABRISK total score for the categories of each item. The older participants scored higher in ARABRISK total score compared with younger age groups. Greater BMI and waist circumference values were associated with a higher ARABRISK total score. Physically active participants and who consumed vegetables or fruits daily had a lower ARABRISK total score. In addition, a history of high blood pressure, high blood sugar, and gestational diabetes resulted in a higher ARABRISK total score compared with no such history. Similarly, a high educational status was associated with a lower ARABRISK total score compared with low educational status. There was no association between number of relatives with T2D and ARABRISK total score. Participants who had direct relatives (mother, father, sibling, child) with T2D were not associated with a higher ARABRISK total score compared to having 1, 2, or 3 of them with T2D.
TABLE 3.
Mean and SD With 95% CI of the Mean ARABRISK Total Score for Each Item Category

Table 4 details the best model of regression analysis. The value of R2 in this model was 0.99 or 99% of the variance in ARABRISK total scores. All of the items on the ARABRISK questionnaire were found to be good predictors of the ARABRISK total scores. Among them, age, body mass index (BMI), and high blood glucose (HBG) were the best predictors, as indicated by the standardized regression coefficients values (β). These predictors were positively associated with the ARABRISK total scores. The shared and unique contributions of the predictors age, BMI, and HBG predictors were 99% (each) and 19.8%, 14.4%, 21.9%, respectively, as indicated by the partial correlations in Table 4. The regression plot of standardized residuals versus standardized predicted values indicates that the points are randomly and evenly dispersed throughout the plot, as shown in Figure 1.
TABLE 4.
Stepwise Multiple Regression Analysis for Risk Factors Predicting ARABRISK Total Scores in Study Participants
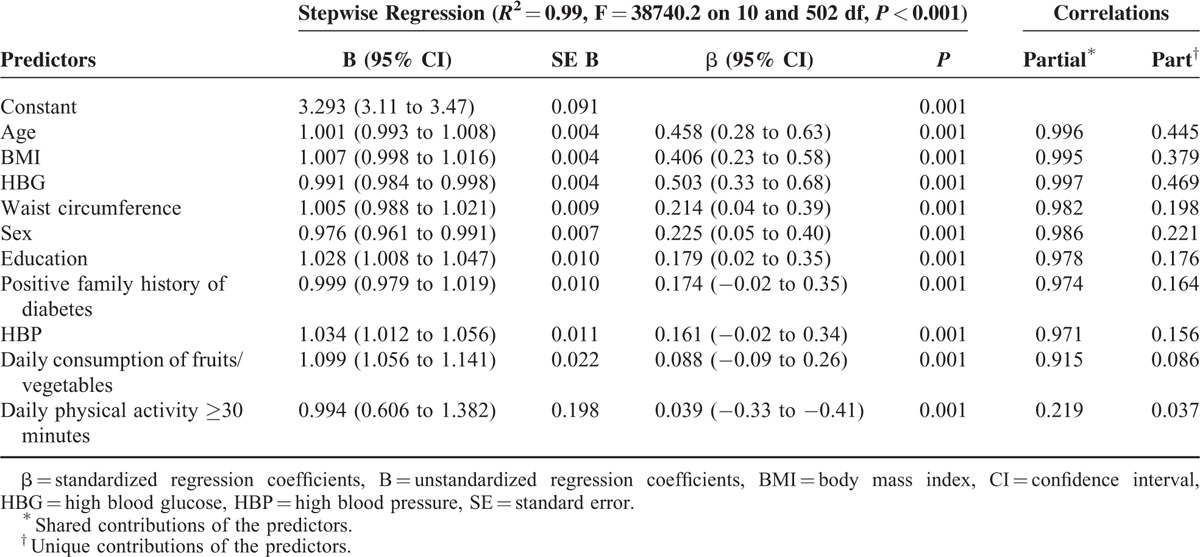
FIGURE 1.
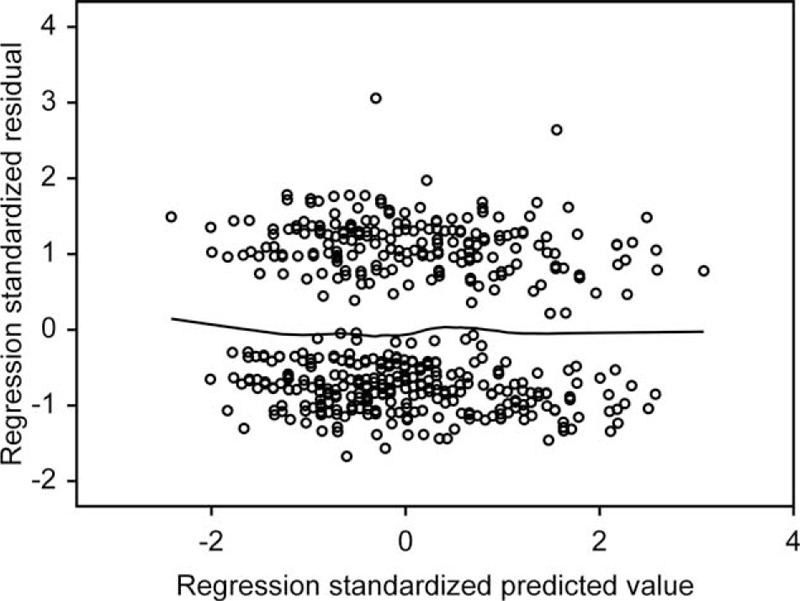
Regression plot of standardized residuals vs standardized predicted values (dependent variable, ARABRISK total score).
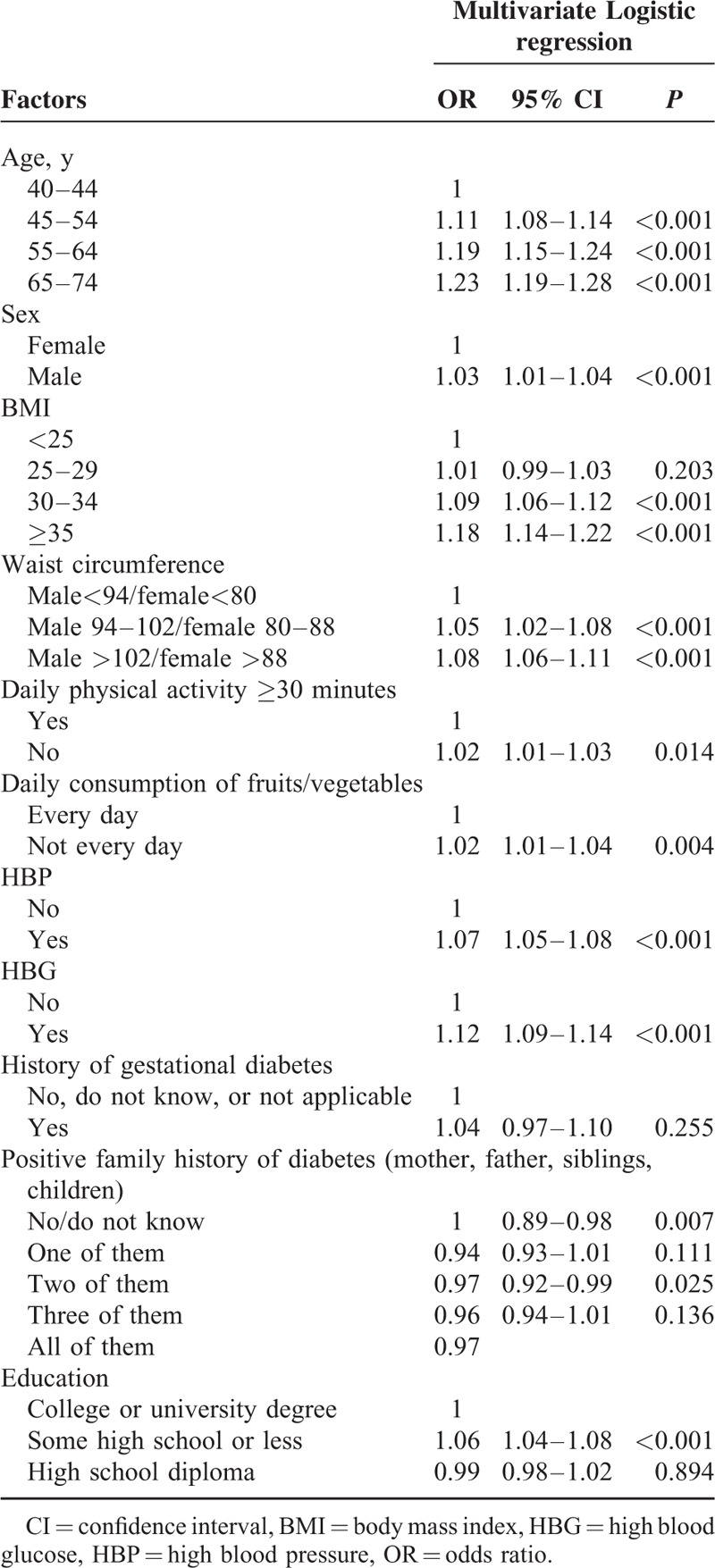
Table 5 details the results of the multivariate logistic regression analyses for the risk factors for the ARABRISK total scores. Older males were significantly associated with increased odds of higher ARABRISK total scores. Being obese and having an elevated weight circumference were significantly associated with increased odds of higher ARABRISK total scores. Additionally, absence of daily physical activity, daily consumption of fruits/vegetables, presence of HBP, and HBG were significantly associated with increased odds of high ARABRISK total scores. Neither a history of gestational diabetes nor a positive family history was associated with increased odds of high ARABRISK total scores. Positive family history was not associated with increased odds of high ARABRISK total scores. In addition, a high school education or less was associated with increased odds of high ARABRISK total scores.
TABLE 5.
Multivariate Logistic Regression Analyses for Risk Factors for ARABRISK Total Scores
DISCUSSION
The results of the ARABRISK questionnaire–based survey were helpful in identifying the risk of diabetes in residents of Amman, Jordan. This study investigated the presence of high-risk factors and the degree to which modifiable risk factors affect the present results. Previous studies have reported relationships between various risk factors and the risk of developing T2D. BMI, hypertension, smoking, lipids, physical inactivity, low education, dietary patterns, family history, and specific genes are also reported as risk factors for T2D.38–42
In the present study, age and sex were associated with the ARABRISK total scores. Older males were associated with increased odds of high ARABRISK total scores. A previous study reported an association of T2D prevalence with age and sex.43 The prevalence of T2D was 4.2% and 2.7% in men and women, respectively. In the present study, a higher BMI and waist circumference were associated with higher ARABRISK scores. In addition, obesity and waist circumference were significantly associated with increased odds of high ARABRISK total scores. Such an outcome is strongly supported by many longitudinal studies that have reported BMI as a strong risk factor for T2D.39,43–45 Similarly, Li et al46 reported an association of obesity measures and waist circumference with T2D and abnormal glucose metabolism. In the present study, approximately 37% and 28% of the participants suffered from hypertension and high blood glucose levels, respectively, which were associated with increased odds of high ARABRISK scores. Similarly, studies have reported the progression of hypertension and glucose impairment as an important predictor of T2D.46–49 The study participants were active; approximately two-thirds of the male and more than half of the female participants reported that they were physically active. In the present study, a lack of daily physical activities was associated with increased odds of high ARABRISK total scores. Additionally, approximately half of the women and men participants reported eating vegetables or fruits; eating fruits and vegetables was associated with increased odds of high ARABRISK scores. However, ARABRISK does not consider the type or frequency of physical activity or types of vegetables and fruits. Previous studies have reported a strong association between physical inactivity and the risk of developing T2D.45,50–53 In addition, prolonged television watching, which is a sedentary lifestyle marker, was strongly associated with the risk of developing diabetes in both men and women.54–56 Moderate and heavy physical activity were associated with a low risk of developing T2D.50,57 With regard to fruits and vegetables, an important life style factor associated with the development of T2D is dietary habits. Food intake pattern is associated with the risk of developing T2D.58–60 In contrast, another study reported no association between the consumption of fruits and vegetables and T2D.43
In the present study, more than two-thirds of the participants had a direct relative who was diagnosed with diabetes. Such results would help in knowing the role of this factor in increasing their risk in developing diabetes because previous studies have reported that genetic factors play a vital role in the pathogenesis of T2D.61–63 A positive family history among first-degree relatives was associated with an increased risk of T2D; the risk was greater if both parents are affected.61–65 Moreover, diabetes prevalence varies considerably among different ethnic groups.66 Nonetheless, there was no difference in ethnicity among Amman population because our participants were all Arabs.
CONCLUSIONS
The present study demonstrates a high risk of developing T2D in the Jordanian population. Participants with higher scores in all risk factors for developing T2D including age, BMI, waist circumference, high blood pressure, high blood glucose, and gestational diabetes, had higher ARABRISK total scores. Participants with higher scores in protective factors from developing T2D including regular physical activity and daily vegetable or fruit consumption had lower ARABRISK total scores.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research through the research group NO. RGP-VPP-209.
Footnotes
Abbreviations: ARABRISK = Arab Diabetes Risk Assessment Questionnaire, BMI = body mass index, CANRISK = Canadian Diabetes Risk Assessment Questionnaire, FINDRISC = the Finnish Diabetes Risk Score, IFG = impaired fasting glucose, IGT = impaired glucose tolerance, T2D = type 2 diabetes.
The authors report no conflicts of interest.
REFERENCES
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87:293–301. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Klein R, Welborn TA, et al. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 1992; 15:815–819. [DOI] [PubMed] [Google Scholar]
- 4.Hypertension in Diabetes Study (HDS). Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens 1993; 11:309–317. [DOI] [PubMed] [Google Scholar]
- 5.Unwin N, Shaw J, Zimmet P, et al. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002; 19:708–723. [DOI] [PubMed] [Google Scholar]
- 6.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001; 285:2109–2113. [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 8.Buijsse B, Simmons RK, Griffin SJ, et al. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev 2011; 33:46–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin SJ, Little PS, Hales CN, et al. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev 2000; 16:164–171. [DOI] [PubMed] [Google Scholar]
- 10.He G, Sentell T, Schillinger D. A new public health tool for risk assessment of abnormal glucose levels. Prev Chronic Dis 2010; 7:A34. [PMC free article] [PubMed] [Google Scholar]
- 11.Heikes KE, Eddy DM, Arondekar B, et al. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care 2008; 31:1040–1045. [DOI] [PubMed] [Google Scholar]
- 12.Koopman RJ, Mainous AG, 3rd, Everett CJ, et al. Tool to assess likelihood of fasting glucose impairment (TAG-IT). Ann Fam Med 2008; 6:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson KM, Boyko EJ. Predicting impaired glucose tolerance using common clinical information: data from the Third National Health and Nutrition Examination Survey. Diabetes Care 2003; 26:2058–2062. [DOI] [PubMed] [Google Scholar]
- 14.Park PJ, Griffin SJ, Sargeant L, et al. The performance of a risk score in predicting undiagnosed hyperglycemia. Diabetes Care 2002; 25:984–988. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt MI, Duncan BB, Vigo A, et al. Detection of undiagnosed diabetes and other hyperglycemia states: the Atherosclerosis Risk in Communities Study. Diabetes Care 2003; 26:1338–1343. [DOI] [PubMed] [Google Scholar]
- 16.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003; 26:725–731. [DOI] [PubMed] [Google Scholar]
- 17.Kaczorowski J, Robinson C, Nerenberg K. Development of the CANRISK questionnaire to screen for prediabetes and undiagnosed type 2 diabetes. Can J Diabetes 2009; 33:381–385. [Google Scholar]
- 18.Alghwiri A, Alghadir A, Awad H. The Arab Risk (ARABRISK): Translation and Validation. Biomed Res 2014; 25:271–275. [Google Scholar]
- 19.Organization WH. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894:1–253. [PubMed] [Google Scholar]
- 20.Ramachandran A, Snehalatha C. Rising burden of obesity in Asia. J Obes 2010; 2010:868573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulte PA, Wagner GR, Ostry A, et al. Work, obesity, and occupational safety and health. Am J Public Health 2007; 97:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leischik R, Foshag P, Strauss M, et al. Physical activity, cardiorespiratory fitness and carotid intima thickness: sedentary occupation as risk factor for atherosclerosis and obesity. Eur Rev Med Pharmacol Sci 2015; 19:3157–3168. [PubMed] [Google Scholar]
- 23.Leischik R, Foshag P, Strauss M, et al. Aerobic capacity, physical activity and metabolic risk factors in firefighters compared with police officers and sedentary clerks. PloS One 2015; 10:e0133113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atlantis E, Chow CM, Kirby A, et al. Worksite intervention effects on physical health: a randomized controlled trial. Health Promot Int 2006; 21:191–200.Epub 2006/04/06. [DOI] [PubMed] [Google Scholar]
- 25.Ramli A, Henry LJ, Liang YF, et al. Effects of a worksite health programme on the improvement of physical health among overweight and obese civil servants: a pilot study. Malays J Med Sci 2013; 20:54–60. [PMC free article] [PubMed] [Google Scholar]
- 26.Ajlouni K, Jaddou H, Batieha A. Diabetes and impaired glucose tolerance in Jordan: prevalence and associated risk factors. J Intern Med 1998; 244:317–323. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control, Prevention (CDC). Assessing risk factors for chronic disease–Jordan, 2004. MMWR Morb Mortal Wkly Rep 2006; 55:653–655. [PubMed] [Google Scholar]
- 28.Ajlouni K, Khader YS, Batieha A, et al. An increase in prevalence of diabetes mellitus in Jordan over 10 years. J Diabetes Complications 2008; 22:317–324. [DOI] [PubMed] [Google Scholar]
- 29.Brown DW, Mokdad AH, Walke H, et al. Projected burden of chronic, noncommunicable diseases in Jordan. Prev Chronic Dis 2009; 6:A78. [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Rmeileh NM, Husseini A, Capewell S, et al. Preventing type 2 diabetes among Palestinians: comparing five future policy scenarios. BMJ Open 2013; 3:e003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control Prevention (CDC). Prevalence of selected risk factors for chronic disease — Jordan, 2002. MMWR Morb Mortal Wkly Rep 2003; 52:1042–1044. [PubMed] [Google Scholar]
- 32.Zindah M, Belbeisi A, Walke H, et al. Obesity and diabetes in Jordan: findings from the behavioral risk factor surveillance system, 2004. Prev Chronic Dis 2008; 5:A17. [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control Prevention (CDC). State specific prevalence of obesity among adults — United States, 2005. MMWR Morb Mortal Wkly Rep 2006; 55:985–988. [PubMed] [Google Scholar]
- 34.Beltaifa L, Traissac P, El Ati J, et al. Prevalence of obesity and associated socioeconomic factors among Tunisian women from different living environments. Obes Rev 2009; 10:145–153. [DOI] [PubMed] [Google Scholar]
- 35.Saidi O, O’Flaherty M, Mansour NB, et al. Forecasting Tunisian type 2 diabetes prevalence to 2027: validation of a simple model. BMC Public Health 2015; 15:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 2009; 43:1–2. [PubMed] [Google Scholar]
- 37.Al-Nsour M, Zindah M, Belbeisi A, et al. Prevalence of selected chronic, noncommunicable disease risk factors in Jordan: results of the 2007 Jordan Behavioral Risk Factor Surveillance Survey. Prev Chronic Dis 2012; 9:E25. [PMC free article] [PubMed] [Google Scholar]
- 38.Valdes S, Botas P, Delgado E, et al. Population-based incidence of type 2 diabetes in northern Spain: the Asturias Study. Diabetes Care 2007; 30:2258–2263. [DOI] [PubMed] [Google Scholar]
- 39.Meisinger C, Thorand B, Schneider A, et al. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med 2002; 162:82–89. [DOI] [PubMed] [Google Scholar]
- 40.Bassuk SS, Manson JE. Lifestyle and risk of cardiovascular disease and type 2 diabetes in women: a review of the epidemiologic evidence. Am J Lifestyle Med 2008; 2:191–213. [Google Scholar]
- 41.Gadsby R. Epidemiology of diabetes. Adv Drug Deliv Rev 2002; 54:1165–1172. [DOI] [PubMed] [Google Scholar]
- 42.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301:2129–2140. [DOI] [PubMed] [Google Scholar]
- 43.Kufe CN, Klipstein-Grobusch K, Leopold F, et al. Risk factors of impaired fasting glucose and type 2 diabetes in Yaounde, Cameroon: a cross sectional study. BMC Public Health 2015; 15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowler WC, Pettitt DJ, Saad MF, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutr 1991; 53 (6 Suppl):1543S–1551S. [DOI] [PubMed] [Google Scholar]
- 45.Almdal T, Scharling H, Jensen JS, et al. Higher prevalence of risk factors for type 2 diabetes mellitus and subsequent higher incidence in men. Eur J Intern Med 2008; 19:40–45. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Xiao J, Ji L, et al. BMI and waist circumference are associated with impaired glucose metabolism and type 2 diabetes in normal weight Chinese adults. J Diabetes Complications 2014; 28:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med 2004; 164:1873–1880. [DOI] [PubMed] [Google Scholar]
- 48.Conen D, Ridker PM, Mora S, et al. Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J 2007; 28:2937–2943. [DOI] [PubMed] [Google Scholar]
- 49.Movahed MR, Sattur S, Hashemzadeh M. Independent association between type 2 diabetes mellitus and hypertension over a period of 10 years in a large inpatient population. Clin Exp Hypertens 2010; 32:198–201. [DOI] [PubMed] [Google Scholar]
- 50.Fretts AM, Howard BV, Kriska AM, et al. Physical activity and incident diabetes in American Indians: the Strong Heart Study. Am J Epidemiol 2009; 170:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gimeno D, Elovainio M, Jokela M, et al. Association between passive jobs and low levels of leisure-time physical activity: the Whitehall II cohort study. Occup Environ Med 2009; 66:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villegas R, Shu XO, Li H, et al. Physical activity and the incidence of type 2 diabetes in the Shanghai women's health study. Int J Epidemiol 2006; 35:1553–1562. [DOI] [PubMed] [Google Scholar]
- 53.Jeon CY, Lokken RP, Hu FB, et al. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007; 30:744–752. [DOI] [PubMed] [Google Scholar]
- 54.Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003; 289:1785–1791. [DOI] [PubMed] [Google Scholar]
- 55.Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001; 161:1542–1548. [DOI] [PubMed] [Google Scholar]
- 56.Krishnan S, Rosenberg L, Palmer JR. Physical activity and television watching in relation to risk of type 2 diabetes: the Black Women's Health Study. Am J Epidemiol 2009; 169:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA 2004; 292:1188–1194. [DOI] [PubMed] [Google Scholar]
- 58.Liese AD, Weis KE, Schulz M, et al. Food intake patterns associated with incident type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes care 2009; 32:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010; 170:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulze MB, Liu S, Rimm EB, et al. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004; 80:348–356. [DOI] [PubMed] [Google Scholar]
- 61.Amini M, Janghorbani M. Diabetes and impaired glucose regulation in first-degree relatives of patients with type 2 diabetes in isfahan, iran: prevalence and risk factors. Rev Diabet Stud 2007; 4:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000; 49:2201–2207. [DOI] [PubMed] [Google Scholar]
- 63.Harrison TA, Hindorff LA, Kim H, et al. Family history of diabetes as a potential public health tool. Am J Prev Med 2003; 24:152–159. [DOI] [PubMed] [Google Scholar]
- 64.Ma XJ, Jia WP, Hu C, et al. Genetic characteristics of familial type 2 diabetes pedigrees: a preliminary analysis of 4468 persons from 715 pedigrees. Zhonghua Yi Xue Za Zhi 2008; 88:2541–2543. [PubMed] [Google Scholar]
- 65.Bjornholt JV, Erikssen G, Liestol K, et al. Type 2 diabetes and maternal family history: an impact beyond slow glucose removal rate and fasting hyperglycemia in low-risk individuals? Results from 22.5 years of follow-up of healthy nondiabetic men. Diabetes Care 2000; 23:1255–1259. [DOI] [PubMed] [Google Scholar]
- 66.Diamond J. The double puzzle of diabetes. Nature 2003; 423:599–602. [DOI] [PubMed] [Google Scholar]


