Abstract
The application of liver stiffness measurement (LSM) by transient elastography (TE) in general population remains to clarify. This cohort study aimed to examine the usefulness of TE and to identify factors associated with significant liver fibrosis in community-based population.
We conducted a hepatitis screening program in 2 remote villages of Southern Taiwan. All residents participated voluntarily and received questionnaire evaluation, blood tests, abdominal sonography, and LSM by TE. Residents with any one of following criteria including hepatitis B virus infection, hepatitis C virus infection, more than moderate alcohol drinking, and failure to obtain valid or reliable LSM were excluded.
There were 831 residents participated in program. The valid and reliable LSM were obtained in 98.3% and 96.3% of residents, respectively. Finally, a total of 559 residents including 283 residents with nonalcoholic steatotic fatty liver disease (NAFLD) were enrolled for analysis. The mean liver stiffness was 4.9 ± 1.9 kPa. The liver stiffness increased in residents with diabetes mellitus (DM), higher body mass index (BMI), hypertension, abnormal waist–hip circumference ration (WHR), higher waist circumference (WC), and presence of fatty liver. Higher body weight, higher BMI, higher WC, abnormal WHR, abnormal aspartate aminotransferase (AST), abnormal alanine aminotransferase (ALT), and DM were the factors associated with significant fibrosis (liver stiffness ≥7 kPa) in either all participants or NAFLD residents. As determined by multivariate analysis, abnormal AST values and DM were the 2 independent factors in all participants (abnormal AST: OR 3.648, 95% CI 1.134–11.740, P = 0.03; DM: OR 2.882, 95% CI 1.282–6.478, P = 0.01) and in residents with NAFLD (abnormal AST: OR 4.197, 95% CI 1.154–15.262, P = 0.03; DM: OR 3.254, 95% CI 1.258–8.413, P = 0.02).
LSM by TE is a useful screening tool in community. In residents, who were absence of chronic hepatitis virus infection or consumed less than moderate alcohol drinking, exhibited DM or abnormal AST values may consider a substantial group with significant fibrosis in community.
INTRODUCTION
In patients with chronic liver diseases, determination of liver fibrosis stages is an important indicator in either prediction of outcomes or consuming treatment.1,2 Traditionally, liver biopsy to obtain liver tissue for histology evaluation is the golden standard of fibrosis staging.3 However, risks accompanied with the invasive procedure such as bleeding, or even death drive the development of noninvasive measurement. Among those noninvasive methods, liver stiffness measurement (LSM) by transient elastography (TE; FibroScan, Echosens, France) is widely used and applied in clinical situations and has been approved to be an alternative and reliable tool in assessing liver fibrosis.4 There are strong correlation between liver histology and TE in various liver diseases including chronic viral hepatitis5–8 or nonalcoholic steatohepatitis.9 In addition, TE also presented a useful tool to predict disease outcomes.10,11
There were few reports addressing the role of TE in healthy subjects.12–15 The participated subjects mainly are those who received medical check-up in hospital, not in community. In addition, many tests accompanied with TE of the studies may not be feasible or available in community. Tests or measurements that can be easily applied in community are preferred. Hence, in addition to TE, factors that obtain from baseline characteristics of participated subjects and those tests that are commonly used in community are analyzed targets of our study.
We conducted a specialist directed screening program in community. Residents participated in program voluntarily and received evaluation of liver diseases. This community-based cohort study aimed to investigate the role of TE measurement in the application of screening of liver diseases and to identify factors that were associated with significant liver fibrosis demonstrated by abnormal LSM values in subjects without overt liver diseases.
METHODS
Screening Activities
This is an observational study. Residents who participated in a hepatology specialist directed screening program for liver disease in 2 remote counties (J-J and C-K) of Tainan City of southern Taiwan from August 2014 to May 2015 were the study targets. The study was approved by the Institutional Review Board of National Cheng Kung University Hospital. All participants signed informed written consent.
Members of screening team composed of hepatology specialists, nursing staffs, assistants, and volunteers. The program aimed to comprehensively evaluate liver disease that included questionnaire to survey knowledge of liver diseases and demography of participated residents, measurement of body weight, body height, and waist/hip/forearm circumferences, fasting blood collections, abdominal sonography survey, and LSM by TE.
Study Subjects
Those residents, who were absence of hepatitis B virus or hepatitis C virus infection and consumed less than moderate alcohol drinking, were the subjects of this study.
Questionnaire
Participated residents were asked to complete a questionnaire addressing issues such as baseline demography and knowledge of chronic liver diseases. We specially focused on the detail history of alcohol and viral hepatitis that included years and amount of alcohol consumption, treatment or follow-up of viral hepatitis, and history of occurrence of complications of liver diseases. Drinking up to 1 drink per day for women and up to 2 drinks per day for men is defined as moderate alcohol drinking according to the Dietary Guidelines for Americans.16
Waist/Hip/Forearm Circumference Measurement
For waist and hip measurements, subject was requested to stand in the manner of feet apart about shoulder-width and body weight equally distributed on both legs. Both waist and hip measurements were taken under normal breath, at the end of a normal expiration, and with the tap parallel to the floor.
For waist circumference (WC), a stretch-resistant tape that provides a constant 100 g tension was gently placed in a horizontal plane at the level of the midpoint between the lower margin of the palpable rib and the top of iliac crest. Measurement of hip circumference was taken at the maximal width of the buttocks. Data were expressed as centimeter (cm). Two consecutive measurements were taken. Average values were obtained if the difference of the 2 measurements was less than 1 cm. If the difference between the 2 measurements was more than 1 cm, 2 measurements were repeated. Abnormal WC was defined as >90 cm in males or >80 cm in females. Abnormal waist–hip circumference ratio (WHR) was defined as ≥0.9 in males or ≥0.85 in females.17
Abdominal Sonography
Abdominal sonography was performed by hepatology specialists who had more than 5-year experience in practicing sonography. Fatty liver on sonography was defined as the presence of liver-renal echo contrast and bright liver.18 Those residents who showed fatty liver on sonography were considered to have nonalcoholic fatty liver disease (NAFLD).
Liver Stiffness Measurement
LSM was performed by the device of FibroScan (Echosens, France). The device generates a vibration to the intercostal spaces of the right lobe of the liver. The propagation velocity of the shear wave subsequently could be measured and expressed as kilopascals (kPa) that indicated and directly related to liver stiffness. All of the LSM were performed by a single experienced operator. M-probe was applied according to manufacturer's instruction. The valid measurement is considered as at least consecutive 10 valid measurements with at least 80% of successful rate at the same examined location. Interquartile range (IQR), an index of intrinsic variability of LSM, corresponds to the interval of LSM results containing 50% of the valid measurements between the 25th and 75th percentiles. The reliable measurement of LSM was defined as those valid measurements with less than 30% of IQR/median. Liver stiffness ≥7 kPa was considered as significant fibrosis according to previous report.19
Laboratory Tests
Blood tests including aspartate aminotransferase (AST, upper limit of normal is 38 U/L), alanine aminotransferase (ALT, upper limit of normal is 40 U/L), hepatitis B surface antigen (HBsAg; Architect HBsAg QT assay, Abbott, Chicago, IL), anti-hepatitis C virus antibody (Anti-HCV; ARCHITECT Anti-HCV, ABBOTT, Diagnostics Division, Germany) were assessed.
Statistical Analysis
Data were expressed as mean plus standard deviation. Groups were compared for distributed data by Student's t-test and for category data by Chi-square test. Different groups were compared by one way ANOVA or Kruskal–Wallis test. Moreover, independent factors associated with higher liver stiffness (defined as ≥7 kPa) were identified using multivariate logistic regression analysis. P values less than 0.05 were considered to be significant. Finally, data handling and analysis were performed with SPSS software for Windows, version 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Selection of Patients
Overall, 831 residents were participated in screening program voluntarily. Figure 1 shows the processes of selection of healthy residents. We excluded residents who exhibited positive HBsAg or positive Anti-HCV, or consumed alcohol exceeded moderate alcohol drinking. A total of 590 residents fulfilled the criteria of inclusion. Among them, 10 (1.7%) residents failed to perform valid LSM and 21 (3.6%) residents obtained unreliable LSM. Finally, 559 (94.7%) residents with valid and reliable LSM were enrolled for analysis.
FIGURE 1.
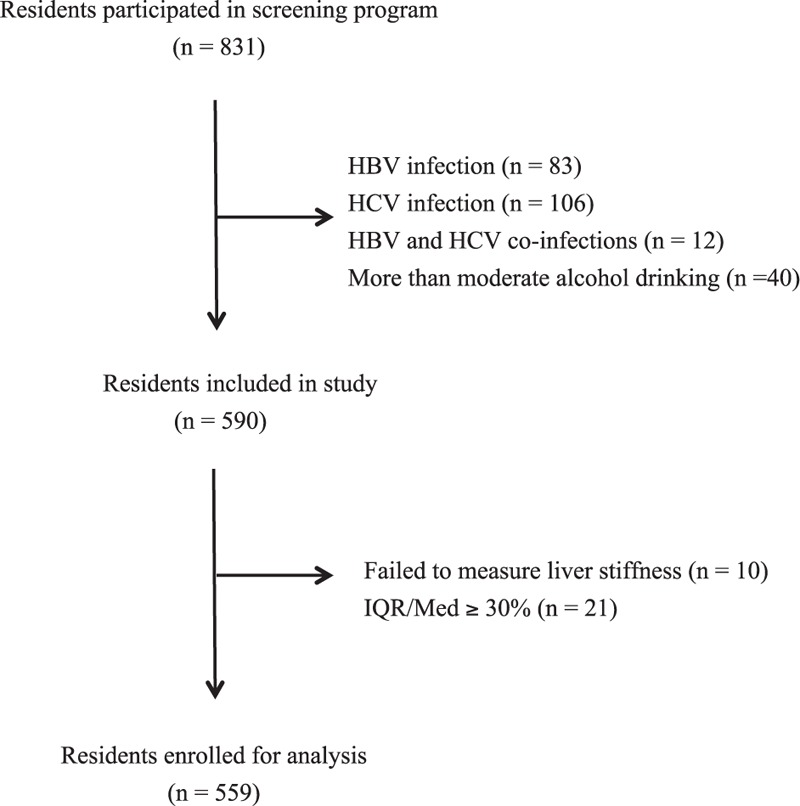
Flow chart of the study. The 1st step was to exclude those residents exhibited hepatitis B or hepatitis C virus infection, or consumed more than moderate amount of alcohol. The 2nd step was to exclude those residents with invalid or unreliable liver stiffness measurement (LSM).
Baseline Characteristics
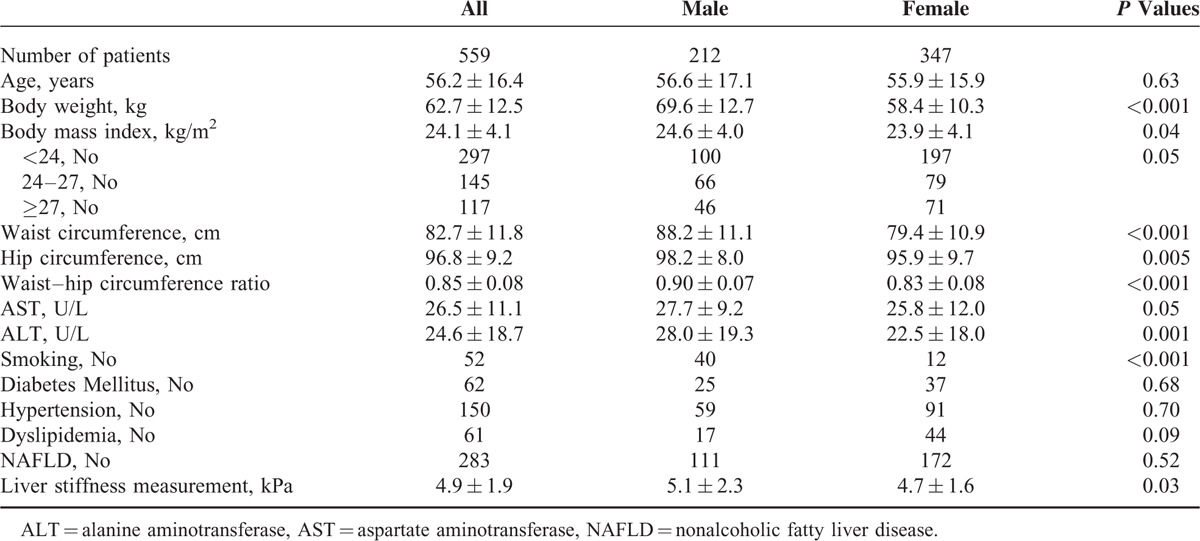
The characteristics of these residents are shown in Table 1. Of the enrolled residents, they were female predominant (62.1%). In addition, obesity (defined as body mass index > 27 kg/m2), diabetes mellitus (DM), dyslipidemia, and hypertension were observed in 21%, 11.1%, 26.8%, and 10.9% of residents, respectively. Normal AST values were present in 514 residents (91.9%) and normal ALT values in 513 residents (91.8%). Females exhibited significant smaller circumferences of waist and hip and WHR, lesser values of AST and ALT, and smaller percentage of having smoke behavior. The percentage of overweight and obesity was higher in males than in females. About half of participated residents had NAFLD with similar distribution between males and females.
TABLE 1.
Baseline Characteristics of Participated Residents
Results of liver Stiffness
The mean liver stiffness was 4.9 ± 1.9 kPa. The LSM were significantly higher in males than in females (5.1 ± 2.3 vs 4.7 ± 1.6 kPa, P = 0.03). There was a weak correlation between age and liver stiffness (r = 0.099, P = 0.02).
The distribution of liver stiffness is depicted in Figure 2. The number of residents in different liver stiffness were 196 (35.1%) exhibited ≤4 kPa, 174 (31.1%) of 4 to 5 kPa, 99 (17.7%) of 5 to 6 kPa, 50 (8.9%) of 6 to 7 kPa, 14 (2.5%) of 7 to 8 kPa, and 26 (4.7%) of ≥8 kPa. Among the 26 residents with liver stiffness ≥8 kPa, 19 exhibited fatty liver under abdominal sonography. Seven residents (1.3%) exhibited liver stiffness values more than 13 kPa that indicated advanced liver fibrosis or even cirrhosis.
FIGURE 2.
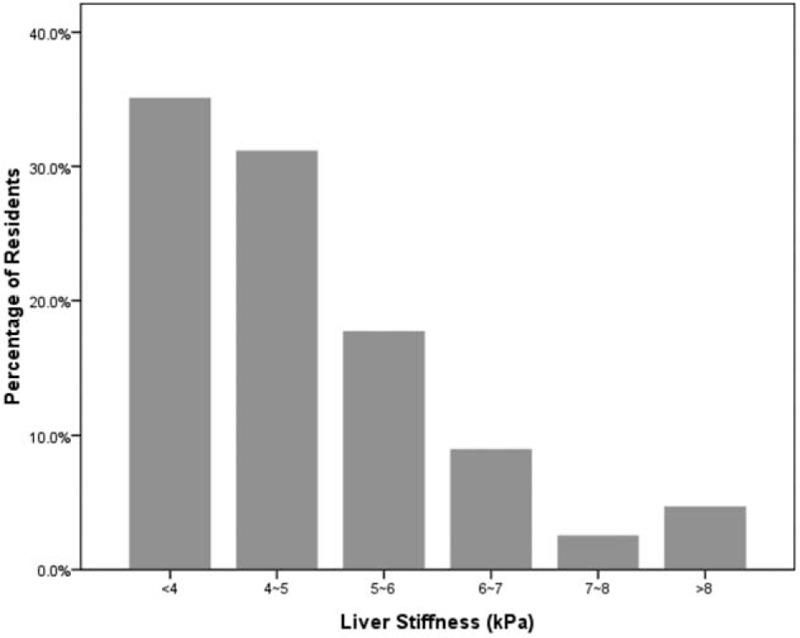
Distribution of liver stiffness measurement (LSM) values.
We compared the liver stiffness between or among residents with different characteristics. Liver stiffness increased significantly as the increment of BMI from 4.6 ± 1.5 kPa of normal BMI (<24 kg/m2), 4.7 ± 1.5 kPa of overweight (BMI between 24 and 27 kg/m2), and 5.8 ± 2.9 kPa of obesity (BMI > 27 kg/m2) (P < 0.001). Those residents with DM also presented significantly higher LSM than those without DM (6.1 ± 3.1 vs 4.7 ± 1.7 kPa, P = 0.001). Hypertension, WHR, and WC also had impact on LSM in either males or females (Figure 3). Interestingly, fatty liver change shown on sonography (NAFLD) increased liver stiffness significantly (P = 0.01). No interaction between residents with smoking versus nonsmoking (4.8 ± 1.8 vs 5.3 ± 2.8 kPa, P = 0.26) and with or without dyslipidemia (4.9 ± 1.9 vs 4.9 ± 2.3 kPa, P = 0.81) was observed for the association with liver stiffness.
FIGURE 3.
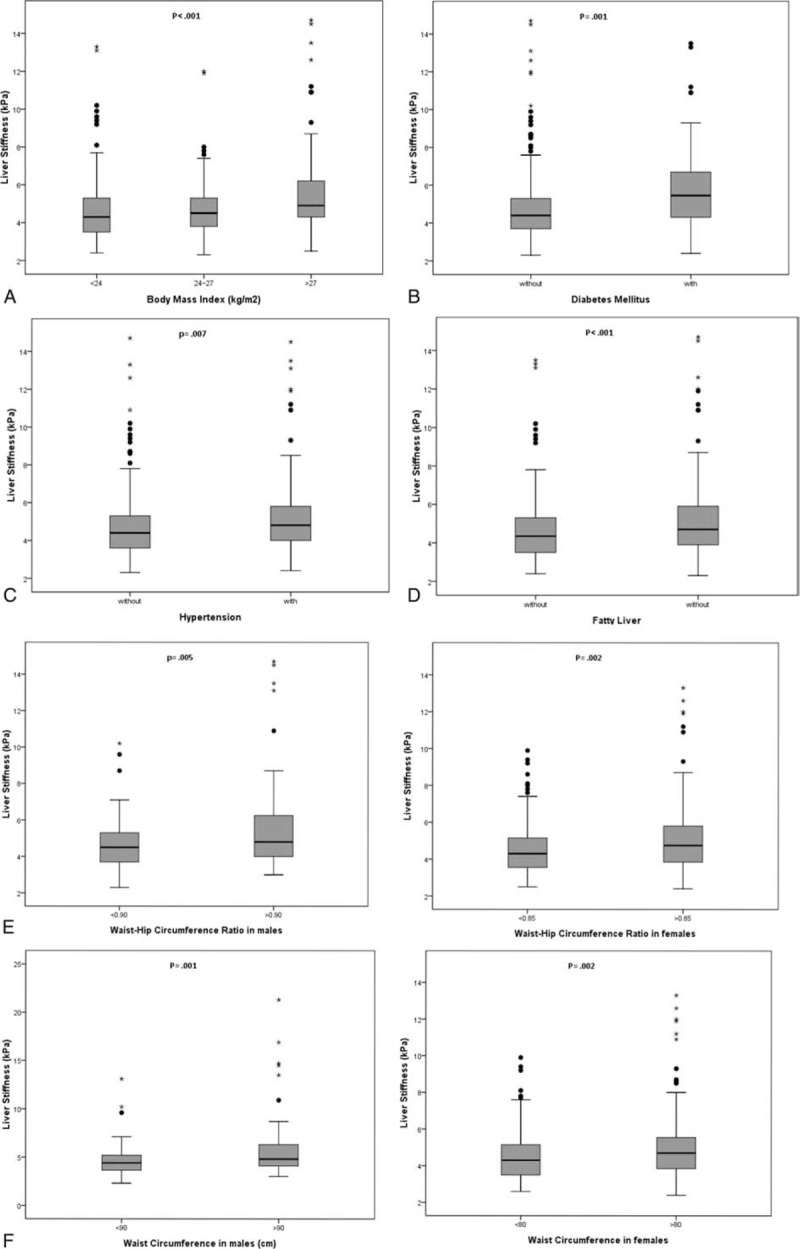
Liver stiffness measurement (LSM) values according to 6 factors. (A) Body mass index, (B) diabetes mellitus, (C) hypertension, (D) fatty liver, (E) waist–hip circumference ratio in males (≥0.9) or females (≥0.85), (F) waist circumference in males (>90 cm) or females (>80 cm).
Predictive Factors Associated With Significant Liver Fibrosis
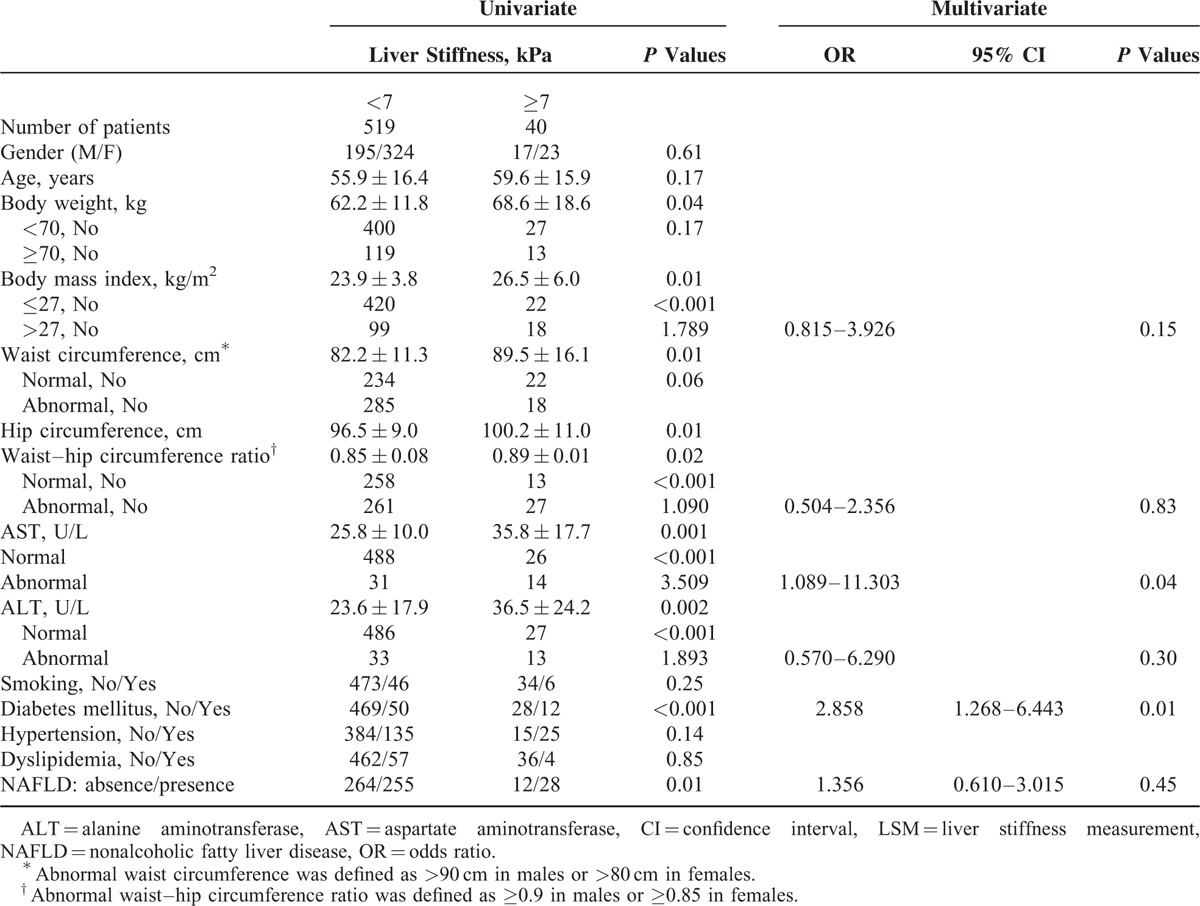
Of the 559 residents, 40 (7.2%) had liver stiffness ≥7 kPa that suggested significant fibrosis. General characteristics and univariate/multivariate analysis of residents according to liver stiffness of 7 kPa are shown in Table 2. In univariate analysis, residents who presented with higher body weight, obesity (BMI > 27 kg/m2), abnormal AST values, abnormal ALT values, abnormal WC or WHR, higher hip circumference, and DM were significantly associated with liver stiffness ≥7 kPa. Furthermore, multivariate logistic regression analysis showed that residents with DM or abnormal AST values at screening were 2 independent factors associated with liver stiffness ≥7 kPa. The odds ratio, 95% confidence interval, and P values were 2.882, 1.282 to 6.478, 0.01 for DM and 3.648, 1.134 to 11.740, 0.03 for abnormal AST values.
TABLE 2.
Univariate and Multivariate Analysis of Factors Associated With Significant Fibrosis by LSM
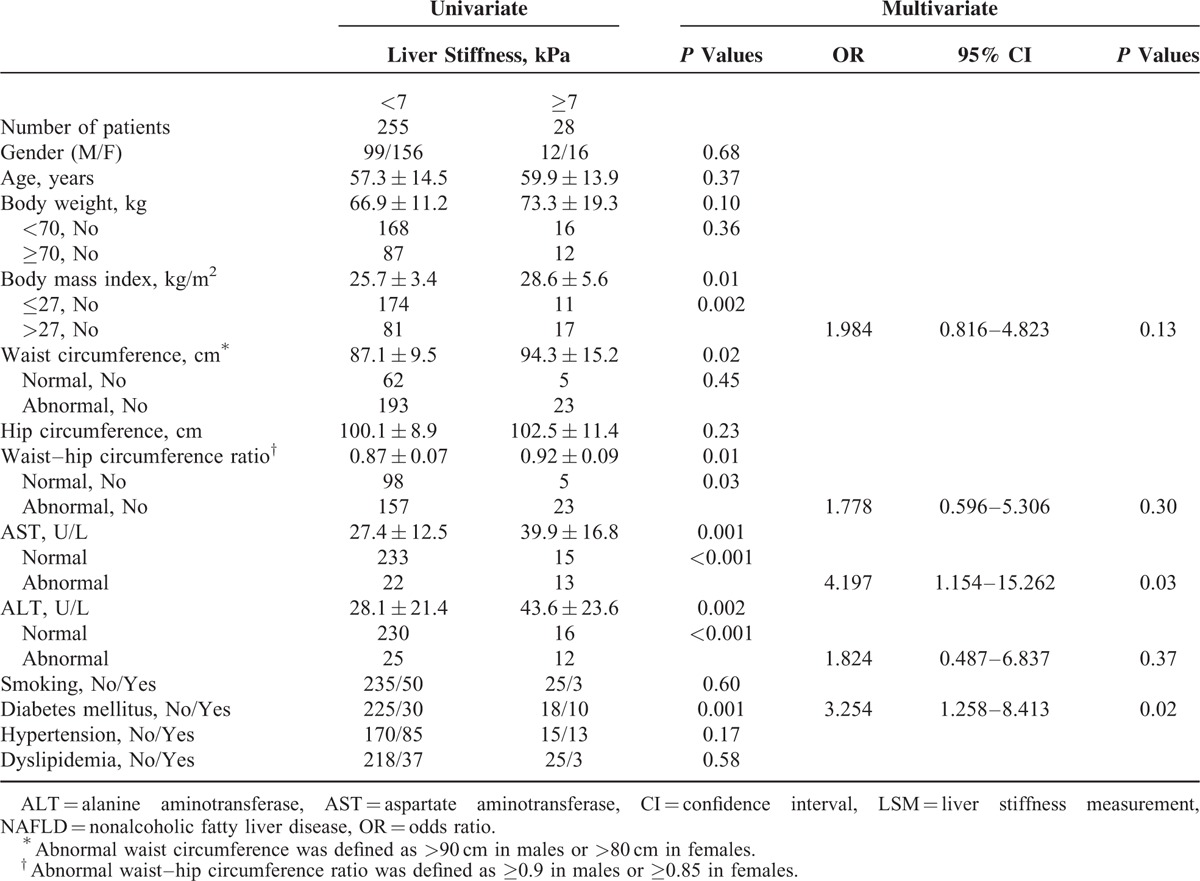
We further analyzed to investigate factors associated with liver stiffness ≥7 kPa in residents with NAFLD shown on sonography. In total of 283 residents, 28 (9.9%) exhibited liver stiffness ≥7 kPa. Similar to the findings that were observed in the 559 residents, higher body weight, obesity, abnormal or higher AST values or ALT values, higher or abnormal WHR, and DM were the factors associated with liver stiffness ≥7 kPa in NAFLD. Abnormal AST values (OR 4.197, 95% CI 1.154–15.262, P = 0.03) and DM (OR 3.254, 95% CI 1.258–8.413, P = 0.02) were also the independent factors that associated with high liver stiffness (≥7 kPa) in resident with NAFLD in community under multivariate logistic regression analysis (Table 3).
TABLE 3.
Univariate and Multivariate Analysis of Factors Associated With Significant Fibrosis by LSM in Residents With NFALD
DISCUSSION
LSM by TE (FibroScan) has been widely used in clinical practice to evaluate liver fibrosis severity in chronic viral hepatitis or nonalcoholic steatotic hepatitis.4–11 The application of LSM in community is less addressed. This cohort study investigated the application of LSM by TE as a screening tool of liver diseases in community. TE exhibits advantages in that it is quite easy to operate, noninvasive, and time-saving. The accuracy of TE mainly based on valid and reliable measurement which could be defined as rate of successful measurement, numbers of consecutive measurement, and IQR/M ratio. An experienced operator is also important to minimize technical variation. In this study, the valid LSM was achieved in 98.3% of enrolled residents and reliable LSM in 96.3% of those residents with valid LSM. The comparable valid and reliable LSM of this study to previous reports15,20 indicated that LSM could be efficiently applied to general population in community.
The 2 counties we screened are well-known high prevalent to hepatitis virus infection. Indeed, there were 201 residents exhibited positive HBsAg and/or positive Anti-HCV. In order to investigate the role of TE in community, we excluded those residents with hepatitis virus infection and consuming moderate amount of alcohol drinking. In the 559 residents enrolled for analysis in this study, the majority of residents had lower LSM values that indicated the presence or absence of mild fibrosis. Similar to previous reports,20 gender could influence the liver stiffness, and age also showed weak association with liver stiffness in this study.
In present study, indicators such as WC or WHR were shown to have association with higher liver stiffness. WC and WHR are considered as indicators of abdominal obesity and are associated with cardiovascular diseases, type 2 DM, hypertension, and overall mortality.21–24 In our study, abnormal WC and higher WHR in either females or males were associated with higher liver stiffness. WC or WHR is frequently linked with metabolic syndrome, insulin resistance, and steatohepatitis and fibrosis and also associated with histology severity of nonalcoholic steatotic hepatitis.25,26 These clinical parameters may indicate the probable linkage among glucose metabolism, insulin resistance, body fat distribution, and fatty liver diseases. Indeed, of those residents with abnormal WC or high WHR, the prevalence of type 2 DM was significantly higher than those with normal WC (15.3% vs 5.7%, P < 0.001) or normal WHR (17.0% vs 4.8%, P < 0.001). Similar observation was obtained between WC or WHR and NAFLD. Abnormal WC (69.2% vs 27.2%, P < 0.001) or high WHR (62.5% vs 38.0%, P < 0.001) were linked to higher prevalence of NAFLD in present study. However, the association of liver stiffness with clinical outcomes in patients with abnormal WC or high WHR is not clear at present and may need long-term observational study to clarify.
Progression of liver fibrosis is associated with poor outcomes regardless of etiology. In this community-based study, there were 7.2% and 4.7% of residents exhibited liver stiffness ≥7 and ≥8 kPa, respectively. Comparable prevalence of liver stiffness ≥8 kPa in our study and other reports were observed.20,27 High prevalence of significant liver fibrosis indicated that the application of LSM by TE could consider as a part of screening program and provide the opportunities to discover a substantial proportion of residents who should receive regular follow-up or further investigate the etiology of liver diseases in community. In other hand, this result also indicated an upcoming important public health issue that needs more emphasizing and addressing.
In accordance with previous studies that used TE in general population, factors that associated with metabolic syndrome, including BMI or obesity and DM, seem to link to significant liver fibrosis.11–15,20 The presence of fibrosis in NAFLD represents a group of residents with progressive liver diseases. Similar to other reports, obesity was associated with significant liver stiffness in residents with NAFLD and may consider a risk factor of disease progression of NAFLD.25,28 In this study, DM and abnormal AST values were the 2 independent factors that associated with high liver stiffness in overall study subjects or in NAFLD subjects. The key features of DM are insulin resistance and hyperinsulinemia. These 2 features of DM were also the most common metabolic features of NAFLD29 and could enhance disease progression through lipogenesis, inflammation, and fibrogenesis.30 Insulin also could stimulate the proliferation and activation of hepatic stellate cells.31 In NAFLD patients, insulin resistance and hyperinsulinemia plus glycemic variation were important predictive factors in glucose impairment for the progression of hepatic fibrosis32 and associated with lower overall survival when compared with general population.28,33 DM also exerts its impact on liver fibrosis in other liver diseases. In chronic hepatitis B, presence of DM could prevent fibrosis regression after antiviral therapy for chronic hepatitis B and also associated with progression of liver fibrosis and complications of liver cirrhosis.34–36 DM appears to be a significant factor of advanced liver fibrosis in NAFLD and chronic hepatitis C.37,38 Taking together of lines of evidence indicated that DM seems to play an universal role in liver fibrosis progression either in general population or in coexisting chronic liver diseases.
AST is an enzyme located in cytoplasmic and mitochondrial of hepatocytes. Insults to hepatocytes could release AST into blood and cause abnormal AST values. However, AST could come from various tissues including heart, muscle, liver, and kidneys. In this study, participated residents all had well and independent performance. Only 1 of 46 the residents reported to have heart disease exhibited abnormal AST values. Four residents reported to have kidney diseases and none of them had abnormal AST values. Therefore, liver-related AST manifestation is a reasonable feature. One recent report demonstrated that AST levels could predict the risk of liver fibrosis progression in HCV/HIV coinfected patients.39 However, the exact mechanisms of AST on liver fibrosis are not fully understood currently. In clinical application, AST measurement is a simple and cheaper test that is able to widely use in large population screening of liver diseases.
There are limitations should be acknowledged in this study. First, we did not check thorough biochemical tests including lipid profiles and insulin resistance for analysis. As this screening program aimed to investigate the application of TE in community, we utilized parameters that are simple, money-saving, easy available such as waist and hip circumferences as instead. The results showed WC or WHR are important factors associated with higher liver stiffness. Second, there is lack of correlation between histology evaluation and LSM. We hope that liver biopsy could be accepted by some of the residents exhibited high liver stiffness after a period of follow-up at outpatient clinic of our hospital. Direct comparison and correlation of fibrosis stages under histology evaluation and LSM values could be possible.
In conclusions, LSM evaluated by TE is feasible and practical in community. A substantial proportion of residents, after exclusion of chronic viral hepatitis and alcoholic liver diseases, exhibited significant liver fibrosis in community. DM and abnormal ALT values are 2 predictive factors of significant fibrosis, defined as liver stiffness ≥7 kPa, in either total enrolled residents or in residents with NAFLD.
Acknowledgement
The authors thank all of the residents who participated in this study and staff including nursing staffs and volunteers. The authors also thank Ms Sandy for her valuable help in performing transient elastography.
Footnotes
Abbreviations: ALT = alanine aminotransferase, Anti-HCV = anti-hepatitis C virus antibody, AST = aspartate aminotransferase, DM = diabetes mellitus, HBsAg = hepatitis B surface antigen, IQR = interquartile range, LSM = liver stiffness measurement, NAFLD = nonalcoholic fatty liver disease, TE = transient elastography, WC = waist circumference, WHR = waist–hip circumference ratio.
The study was financially supported by the CHENG-HSING Medical Foundation of National Cheng Kung University Hospital and Chen Jie-Chen Scholarship Foundation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Xu F, Moorman AC, Tong X, et al. All-cause mortality and progression risks to hepatic decompensation and hepatocellular carcinoma in patients infected with hepatitis C. Clin Infect Dis 2015; pii:civ860. [DOI] [PubMed] [Google Scholar]
- 2.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62:932–954. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Caldwell SH, Goodman ZD, et al. AASLD position paper: liver biopsy. Hepatology 2009; 49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 4.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 5.Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005; 128:343–350. [DOI] [PubMed] [Google Scholar]
- 6.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54. [DOI] [PubMed] [Google Scholar]
- 7.Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 2009; 29:242–247. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974. [DOI] [PubMed] [Google Scholar]
- 9.de Ledinghen V, Beaugrand M, Kelleher TB, et al. Prediction of liver fibrosis in non-alcoholic steatohepatitis (NASH): risk factors and diagnostic potential of liver elasticity using Fibroscan. J Hepatol 2006; 44:S39. [Google Scholar]
- 10.Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011; 140:1970–1979. [DOI] [PubMed] [Google Scholar]
- 11.Robic MA, Procopet B, Metivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol 2011; 55:1017–1024. [DOI] [PubMed] [Google Scholar]
- 12.Roulot D, Czernichow S, Le Clésiau H, et al. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613. [DOI] [PubMed] [Google Scholar]
- 13.Kumar M1, Sharma P, Garg H, et al. Transient elastographic evaluation in adult subjects without overt liver disease: influence of alanine aminotransferase levels. J Gastroenterol Hepatol 2011; 26:1318–1325. [DOI] [PubMed] [Google Scholar]
- 14.You SC, Kim KJ, Kim SU, et al. Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J Gastroenterol 2015; 21:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011; 60:977–984. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition: U.S. Government Printing Office, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva, 8–11 December 2008. 2011. World Health Organization. [Google Scholar]
- 18.Hamaguchi M1, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102:2708–2715. [DOI] [PubMed] [Google Scholar]
- 19.Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013; 57:1182–1191. [DOI] [PubMed] [Google Scholar]
- 20.Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam Study. Hepatology 2016; 63:138–147. [DOI] [PubMed] [Google Scholar]
- 21.de Koning L, Merchant AT, Pogue J, et al. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007; 28:850–856. [DOI] [PubMed] [Google Scholar]
- 22.Delavari A, Forouzanfar MH, Alikhani S, et al. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care 2009; 32:1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huxley R, Mendis S, Zheleznyakov E, et al. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk – a review of the literature. Eur J Clin Nutr 2010; 64:16–22. [DOI] [PubMed] [Google Scholar]
- 24.Huxley R, Barzi F, et al. Obesity in Asia Collaboration. Waist circumference thresholds provide an accurate and widely applicable method for the discrimination of diabetes. Diabetes Care 2007; 30:3116–3118. [DOI] [PubMed] [Google Scholar]
- 25.Rocha R, Cotrim HP, Carvalho FM, et al. Body mass index and waist circumference in non-alcoholic fatty liver disease. J Hum Nutr Diet 2005; 18:365–370. [DOI] [PubMed] [Google Scholar]
- 26.Margariti A1, Kontogianni MD, Tileli N, et al. Increased abdominal fat levels measured by bioelectrical impedance are associated with histological lesions of nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol 2015; 27:907–913. [DOI] [PubMed] [Google Scholar]
- 27.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116:1413–1419. [DOI] [PubMed] [Google Scholar]
- 28.Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol 2007; 13:4669–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bril F, Lomonaco R, Orsak B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014; 59:2178–2187. [DOI] [PubMed] [Google Scholar]
- 30.Ota T, Takamura T, Kurita S, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology 2007; 132:282–293. [DOI] [PubMed] [Google Scholar]
- 31.Kaji K, Yoshiji H, Kitade M, et al. Impact of insulin resistance on the progression of chronic liver diseases. Int J Mol Med 2008; 22:801–808. [PubMed] [Google Scholar]
- 32.Hashiba M, Ono M, Hyogo H, et al. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PLoS One 2013; 8:e76161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver diseases: a population-based cohort study. Gastroenterology 2005; 129:113–121. [DOI] [PubMed] [Google Scholar]
- 34.Kuo YH, Lu SN, Chen CH, et al. The changes of liver stiffness and its associated factors for chronic hepatitis B patients with entecavir therapy. PLoS One 2014; 9:e93160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang YW, Wang TC, Lin SC, et al. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis 2013; 57:1695–1702. [DOI] [PubMed] [Google Scholar]
- 36.Hsiang JC, Gane EJ, Bai WW, et al. Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol 2015; 30:591–599. [DOI] [PubMed] [Google Scholar]
- 37.Nakahara T, Hyogo H, Yoneda M, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol 2014; 49:1477–1484. [DOI] [PubMed] [Google Scholar]
- 38.Dyal HK, Aguilar M, Bhuket T, et al. Concurrent obesity, diabetes, and steatosis increase risk of advanced fibrosis among HCV patients: a systematic review. Dig Dis Sci 2015; 60:2813–2824. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez FA1, Van den Eynde E, Perez-Hoyos S, et al. Liver stiffness and aspartate aminotransferase levels predict the risk for liver fibrosis progression in hepatitis C virus/HIV-coinfected patients. HIV Med 2015; 16:211–218. [DOI] [PubMed] [Google Scholar]


