Abstract
The aims of the present study were to examine the relationship between serum B-cell activating factor belonging to the tumor necrosis factor family (BAFF) levels and serum interferon-γ-inducible protein-10 (IP-10) levels in patients with autoimmune hepatitis (AIH).
A total of 80 corticosteroid therapy naive AIH patients were analyzed in this analysis. First, we examined the relationship between pretreatment serum BAFF and IP-10 levels and liver histological findings. Next, we investigated the relationship of pretreatment serum BAFF and IP-10 levels and aspartate aminotransferase value (AST), alanine aminotransferase value, and serum Immunoglobulin G (IgG) level as serum liver inflammation markers.
Our study included 14 men and 66 women with the median (range) age of 64 (21–83) years. The serum BAFF levels ranged from 122.5 to 7696.0 pg/mL (median value, 1417.8 pg/mL), whereas the serum IP-10 levels ranged from 142.0 to 4198.7 pg/mL (median value, 640.1 pg/mL). The serum BAFF levels were significantly stratified in each 2 liver inflammation stage. Similarly, the serum IP-10 levels were significantly stratified in each 2 liver inflammation stage. Among 3 serum inflammation markers, AST value had the highest rs value in terms of the relationship with BAFF level (rs = 0.511, P < 0.001) and IP-10 level (rs = 0.626, P < 0.001). In addition, the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.561, P < 0.001). In patients without advanced fibrosis (F3 or more), the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.658, P < 0.001), whereas in patients with advanced fibrosis, the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.542, P < 0.001).
In conclusion, both BAFF and IP-10 are useful for predicting the degree of liver inflammation activity in AIH. BAFF and IP-10 may have the common clinical implication for liver inflammation activity for AIH patients.
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic inflammatory immunodependent disease of the liver mainly occurring in women.1–4 Corticosteroids alone or in combination with immunosuppressant drugs are the mainstay therapies for patients with AIH.3–6 These therapies mostly respond well, leading to biochemical remission, which is widely considered a satisfactory treatment end point for AIH patients, although there exist nonresponders for treatments.3–6
Serum cytokines are thought to be linked to the pathogenesis in AIH via immune dysregulation. The B-cell activating factor belonging to the tumor necrosis factor family (BAFF), which is a cytokine critical for development and proper selection of B-cells, is expressed by T-cells and dendritic cells.7 Particularly, the BAFF/BAFF-receptor pathway is crucial to the survival and growth of mature B-cells.8 In patients with systemic lupus erythematosus or systemic sclerosis, it has been demonstrated that serum BAFF levels were associated with disease severity.9,10 In chronic liver diseases, serum BAFF levels are reported to increase in patients with autoimmune liver diseases and hepatitis virus-related chronic liver disease.11–16 In addition, a previous study reported that corticosteroid therapy resulted in marked reduction in serum BAFF levels in AIH patients and BAFF contributes to liver injury and disease development for AIH patients.12 BAFF has been also implicated as a key player in the pathophysiology of hematologic malignancies.8,17–19
Inflammation in the liver is also regulated by chemokines, which regulate the activities and migration of Kupffer cells, hepatocytes, endothelial cells, hepatic stellate cells, and circulating immune cells.20 Interferon-γ-inducible protein-10 (IP-10) is a T-cell-specific CXC chemokine of 77 amino acids in its mature form and it targets the CXCR3 receptor and attracts monocytes, natural killer cells, and T lymphocytes.21,22 Intrahepatic and serum IP-10 levels have been reproducibly linked to the extent of hepatitis C virus (HCV)-related liver inflammation and fibrosis.23,24 On the other hand, a previous study demonstrated that serum IP-10 may help to recruit T-cells to the hepatic lesions in autoimmune liver diseases as well as in chronic viral hepatitis.25 Chemokines are considered to be requisites for directing, mobilizing, and positioning the effector cells in immune-mediated liver injury and feasible therapeutic targets in autoimmune liver diseases.26
However, to the best of our knowledge, there have been no reports investigating the relationship between serum BAFF levels and serum IP-10 levels for patients with AIH. The synergic effect or interaction of BAFF and IP-10 in AIH patients is unclear. The aims of this study were to examine the relationship between BAFF levels and serum IP-10 levels in patients with AIH.
PATIENTS AND METHODS
Subjects and AIH Diagnosis
Between April 2005 and April 2015, a total of 84 corticosteroid therapy naïve subjects with AIH were admitted at the Division of Hepatobiliary and Pancreatic disease, Department of Internal Medicine, Hyogo College of Medicine, Hyogo, Japan. Of these, data for both serum BAFF level and serum IP-10 level were available in 80 patients, who were analyzed in the present study. The BAFF level and IP-10 level were tested using stored sera. For all analyzed subjects, percutaneous liver needle biopsy under ultrasonographic guidance was performed. A diagnosis of AIH was made according to the revised scoring system for the diagnosis of autoimmune hepatitis reported by International Autoimmune Hepatitis Group.27 Our present study included patients with pretreatment AIH diagnostic score of 10 or more (probable AIH or definite AIH).27 For all analyzed subjects, there was no clear evidence of alcoholic or drug-induced liver disease and no evidence of concurrent hepatitis B virus (HBV) or HCV infection. First, we examined the relationship of pretreatment serum BAFF and IP-10 levels and liver histological findings. Next, we investigated the relationship of pretreatment serum BAFF and IP-10 levels and aspartate aminotransferase (AST) value, alanine aminotransferase (ALT) value, and serum IgG level as liver inflammation markers. Antinuclear antibody was tested by the indirect fluorescent antibody technique.28
The ethics committee of Hyogo college of medicine approved the present study protocol and this protocol complied with all of the provisions of the Declaration of Helsinki.
Liver Histological Findings
As for evaluation of liver histological findings, well-experienced pathologists in our hospital assessed liver biopsy samples. Liver inflammation activity stages were graded as A1 (mild degree of liver inflammation), A2 (moderate degree of liver inflammation), and A3 (severe degree of liver inflammation). Liver fibrosis stages were graded on a degree of F0–F4 (F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; F4, liver cirrhosis). In the present study, advanced fibrosis was defined as F3 or more.
Quantification of Serum BAFF Levels and Serum IP-10 Levels
The BAFF level was quantified using a human BAFF Quantikine ELISA (R&D Systems). The Serum IP-10 level was measured using commercially available Quantikine human CXCL10/IP-10 immunoassay kits (R and D systems, Minneapolis, MN).
Statistical Analysis
In continuous variables, the statistical analysis among groups was performed using the Kruskal–Wallis test, Mann–Whitney U test, or Spearman's rank correlation coefficient rs as appropriate. Data are expressed as the median value (range). Values of P < 0.05 were considered to be statistically significant. Statistical analysis was performed with the JMP 11 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline Characteristics
The baseline characteristics of the analyzed subjects (n = 80) are shown in Table 1. There were 14 men and 66 women with the median (range) age of 64 (21–83) years. Using the revised scoring system for AIH (pretreatment), 53 patients had >15 points (definite AIH) and the remaining 27 patients had 10 points or more and 15 points or less (probable AIH).27 As for histological findings, in terms of degree of inflammation activity, A3 was observed in 28 patients, A2 in 38 and A1 in 14 and in terms of degree of liver fibrosis, F4 was observed in 17 patients, F3 in 22, F2 in 23, and F1 in 18. The serum BAFF levels ranged from 122.5 to 7696.0 pg/mL (median value, 1417.8 pg/mL), whereas the serum IP-10 levels ranged from 142.0 to 4198.7 pg/mL (median value, 640.1 pg/mL).
TABLE 1.
Baseline Characteristics (n = 80)
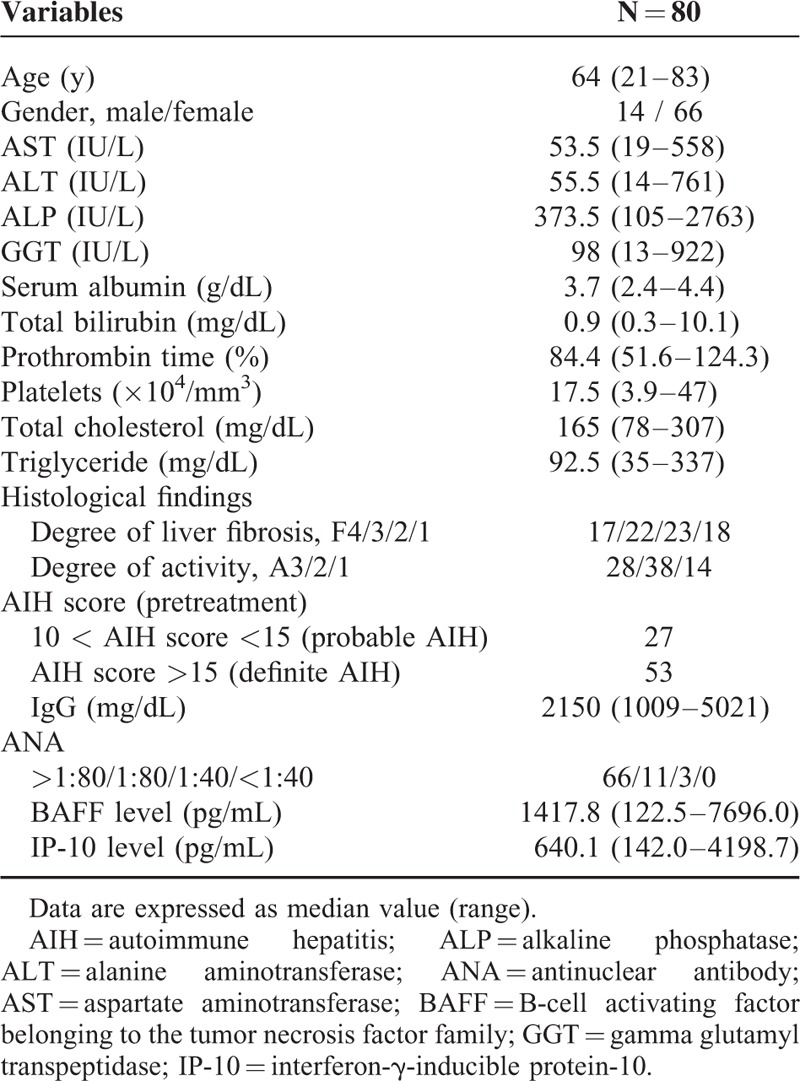
Serum BAFF and IP-10 Levels According to the Degree of Liver Inflammation Activity
The median BAFF levels (range) in each liver inflammation stage (A1–A3) are: 892.3 pg/mL (435.0–2760.0 pg/mL) in A1, 1376.8 pg/mL (339.0–4864.5 pg/mL) in A2, and 1985.0 pg/mL (122.5–7696.0 pg/mL) in A3 (A1 vs A2, P = 0.01; A2 vs A3, P = 0.01; A1 vs A3, P < 0.01; overall significance, P < 0.001) (Figure 1).
FIGURE 1.
Serum BAFF and IP-10 levels according to the degree of liver inflammation activity. The median BAFF levels (range) in each liver inflammation stage (A1–A3) are: 892.3 pg/mL (435.0–2760.0 pg/mL) in A1, 1376.8 pg/mL (339.0–4864.5 pg/mL) in A2, and 1985.0 pg/mL (122.5–7696.0 pg/mL) in A3 (A1 vs A2, P = 0.01; A2 vs A3, P = 0.01; A1 vs A3, P < 0.01; overall significance, P < 0.001). BAFF = B-cell activating factor belonging to the tumor necrosis factor family, IP-10 = interferon-γ-inducible protein-10.
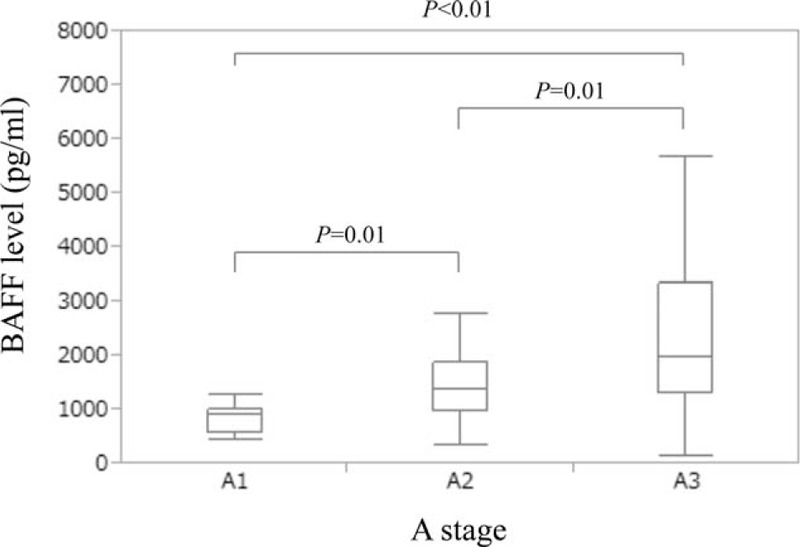
The median IP-10 levels (range) in each liver inflammation stage (A1–A3) are: 374.4 g/mL (142.0–884.0 pg/mL) in A1, 603.9 pg/mL (195.1–1835.9 pg/mL) in A2 and 1466.6 pg/mL (312.6–4198.7 pg/mL) in A3 (A1 vs A2, P = 0.02; A2 vs A3, P < 0.01; A1 vs A3, P < 0.01; overall significance, P < 0.001) (Figure 2).
FIGURE 2.
Serum IP-10 levels according to the degree of liver inflammation activity. The median IP-10 levels (range) in each liver inflammation stage (A1–A3) are: 374.4 g/mL (142.0–884.0 pg/mL) in A1, 603.9 pg/mL (195.1–1835.9 pg/mL) in A2, and 1466.6 pg/mL (312.6–4198.7 pg/mL) in A3 (A1 vs A2, P = 0.02; A2 vs A3, P < 0.01; A1 vs A3, P < 0.01; overall significance, P < 0.001). IP-10 = interferon-γ-inducible protein-10.
Serum BAFF and IP-10 Levels in Patients With or Without Advanced Fibrosis (F3 or More)
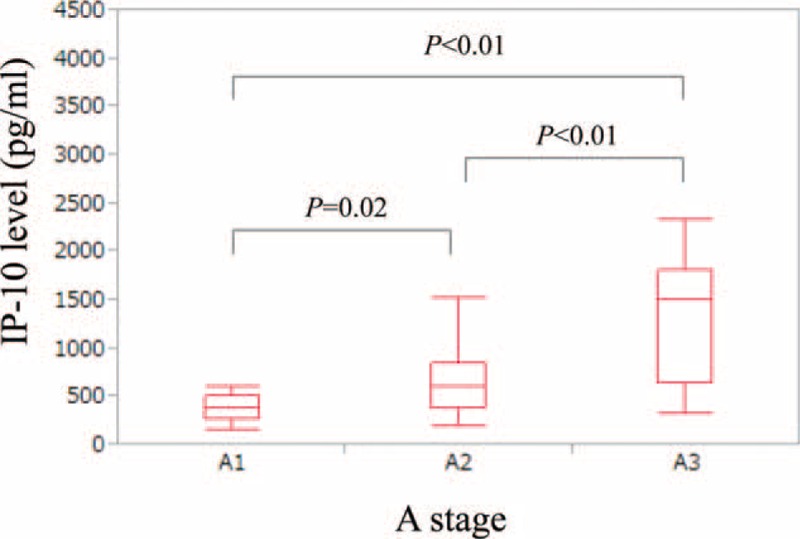
The median BAFF levels (range) in patients with or without advanced fibrosis (F3 or more) are: 1657.5 pg/mL (359.0–7696.0 pg/mL) in patients with advanced fibrosis and 1276.0 pg/mL (122.5–5681.5 pg/mL) in patients without advanced fibrosis (P = 0.039) (Figure 3A).
FIGURE 3.
Serum BAFF and IP-10 levels in patients with or without advanced fibrosis (F3 or more). (A) The median BAFF levels (range) in patients with or without advanced fibrosis (F3 or more) are: 1657.5 pg/mL (359.0–7696.0 pg/mL) in patients with advanced fibrosis and 1276.0 pg/mL (122.5–5681.5 pg/mL) in patients without advanced fibrosis (P = 0.039). (B) The median IP-10 levels (range) in patients with or without advanced fibrosis (F3 or more) are: 722.3 pg/mL (172.7–2317.4 pg/mL) in patients with advanced fibrosis and 614.3 pg/mL (142.0–4198.7 pg/mL) in patients without advanced fibrosis (P = 0.551). BAFF = B-cell activating factor belonging to the tumor necrosis factor family, IP-10 = interferon-γ-inducible protein-10.
The median IP-10 levels (range) in patients with or without advanced fibrosis (F3 or more) are: 722.3 pg/mL (172.7–2317.4 pg/mL) in patients with advanced fibrosis and 614.3 pg/mL (142.0–4198.7 pg/mL) in patients without advanced fibrosis (P = 0.551) (Figure 3B).
Relationship Between BAFF Level and Liver Inflammation Markers
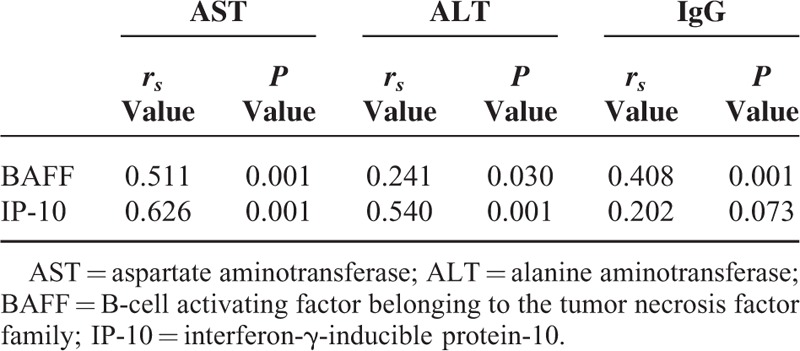
Among 3 variables of AST value, ALT value, and IgG level, AST value had the highest rs value in terms of the relationship with BAFF level (rs = 0.511, P < 0.001) (Table 2).
TABLE 2.
Correlation Between Liver Inflammation Markers and Serum BAFF Level and Serum IP-10 Level
Relationship Between IP-10 Level and Liver Inflammation Markers
Among 3 variables of AST value, ALT value, and IgG level, the AST value had the highest rs value in terms of the relationship with serum IP-10 level (rs = 0.626, P < 0.001) (Table 2).
Relationship Between BAFF level and IP-10 Level
The serum BAFF level significantly correlated with serum IP-10 level (rs = 0.561, P < 0.001) (Figure 4).
FIGURE 4.
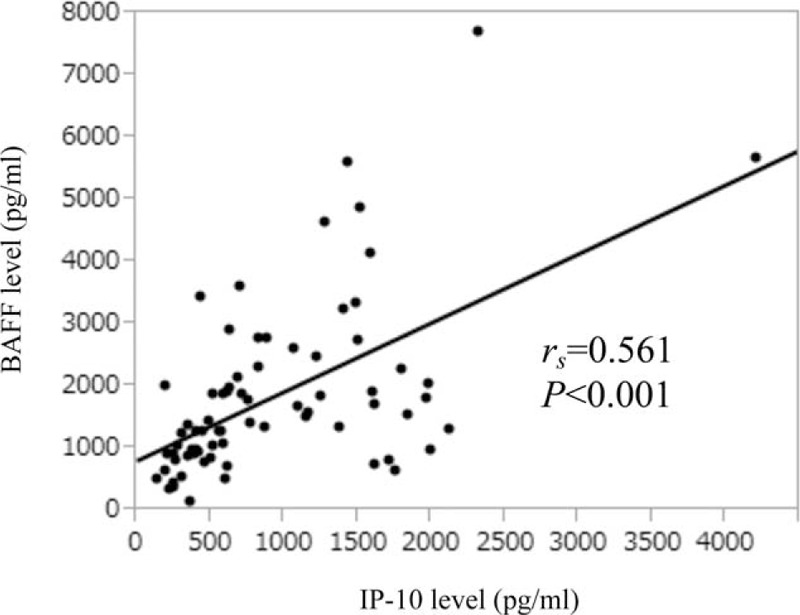
Relationship between BAFF level and IP-10 level. The Serum BAFF level significantly correlated with serum IP-10 level (rs = 0.561, P < 0.001). BAFF = B-cell activating factor belonging to the tumor necrosis factor family, IP-10 = interferon-γ-inducible protein-10.
Relationship Between BAFF Level and IP-10 Level in Patients With or Without Advanced Fibrosis (F3 or More)
We also performed subgroup analysis in patients with or without advanced fibrosis. In patients without advanced fibrosis, the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.658, P < 0.001) (Figure 5A), whereas in patients with advanced fibrosis, the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.542, P < 0.001) (Figure 5B).
FIGURE 5.
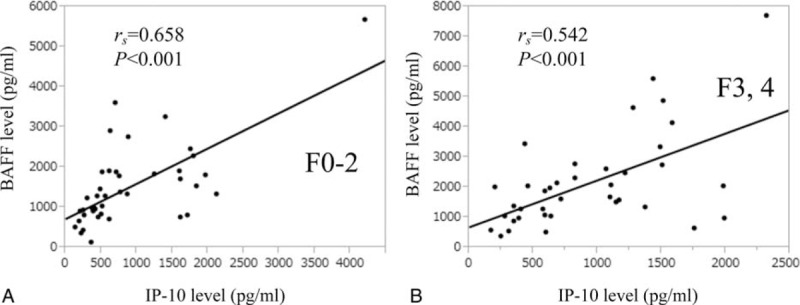
Relationship between BAFF level and IP-10 level in patients with or without advanced fibrosis (F3 or more). (A) In patients without advanced fibrosis, the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.658, P < 0.001). (B) In patients with advanced fibrosis, the serum BAFF level significantly correlated with serum IP-10 level (rs = 0.542, P < 0.001). BAFF = B-cell activating factor belonging to the tumor necrosis factor family, IP-10 = interferon-γ-inducible protein-10.
DISCUSSION
To the best of our knowledge, this is the first study for examining the relationship between serum BAFF levels and serum IP-10 levels in patients with AIH. These investigations can lead to the better understanding for the development of AIH. Furthermore, both BAFF and IP-10 have attracted much attention as liver inflammation markers among hepatologists.11–15,23–25,29 However, the synergic effect or interaction of BAFF and IP-10 in AIH patients remains unclear and thus there is urgent need for clarifying these issues. Thus, we conducted the current analysis.
In our results, BAFF levels and IP-10 levels were significantly stratified based on disease severity of inflammation, which are in line with previous studies.12,25 On the other hand, the mean value of BAFF level in our data was 1795.4 pg/mL, which is higher than that in patients with primary biliary cirrhosis (PBC) (722.8 pg/mL) and chronic hepatitis C (CHC) patients (871.0 pg/mL) in data of a previous study, whereas the median value of IP-10 in our data was 604.1 pg/mL, which is higher than that in PBC patients (571.5 pg/mL) and that in CHC patients (461.83 pg/mL) in our previous studies.12,29,30 The reason for the differences of BAFF level and IP-10 level in each liver disease etiology is unknown; however, individual cutoff values for each stage of liver inflammation activity should be determined for all chronic liver diseases.
It is of note that serum BAFF levels significantly correlated with serum IP-10 levels in the current analysis for all cases (rs = 0.561, P < 0.001) and for patients with (rs = 0.542, P < 0.001) and without (rs = 0.658, P < 0.001) advanced fibrosis. These results may shed some lights on the understanding for the development of AIH. BAFF is expressed by T-cells and dendritic cells and is related to the survival and maturation of B-cells and is also indicated to augment both T-cell and B-cell responses, particularly Th1-type responses, whereas serum IP-10 can recruit T-cells to the hepatic lesions in autoimmune liver diseases.7,25,31 The synergic effect or the interaction of BAFF and IP-10 may be associated with the development of AIH. However, further examinations will be needed to confirm these results as other cytokines or chemokines than BAFF or IP-10 can be correlated with liver inflammation.26,32–34
BAFF levels in patients with advanced fibrosis were significantly higher than those without advanced fibrosis in our analysis (P = 0.039). Yang et al reported that in patients with HBV-related liver disease, serum BAFF levels in liver cirrhosis were significantly higher than those in chronic hepatitis, which are consistent with our current results.16 One possible for these results is that in our analysis, the proportion of patients with A2 or A3 in patients with advanced fibrosis (87.2%, 34/39) was higher than that in patients without advanced fibrosis (78.0%, 32/41), whereas IP-10 levels in patients with advanced fibrosis were similar to those without advanced fibrosis (P = 0.551). In our previous study in CHC patients, we demonstrated that serum IP-10 levels significantly correlated with the degree of liver fibrosis.29 Differences of etiologies of liver diseases may be associated with these results.
Although our study had the limitation of small sample size, our current results indicate that both BAFF and IP-10 are useful for predicting the degree of liver inflammation activity in AIH and these markers have the common clinical implication for liver inflammation activity for AIH patients.
Acknowledgments
The authors would like to thank Nozomi Kanazawa, Yoko Matsushita, and Sayaka Fujii for data collection.
Footnotes
Abbreviations: AIH = autoimmune hepatitis, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BAFF = B-cell activating factor belonging to the tumor necrosis factor family, CHC = chronic hepatitis C, HBV = hepatitis B virus, HCV = hepatitis C virus, IP-10 = interferon-γ-inducible protein-10, PBC = primary biliary cirrhosis.
Funding: in the past year, SN received financial support from Chugai Pharmaceutical, MSD, Dainippon Sumitomo Pharma, Ajinomoto Pharma, and Otsuka Pharmaceutical.
The remaining authors declare that they have no conflicts of interest.
REFERENCES
- 1.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis—Update 2015. J Hepatol 2015; 62:S100–S111. [DOI] [PubMed] [Google Scholar]
- 2.Abe M, Mashiba T, Zeniya M, et al. Autoimmune Hepatitis Study Group–Subgroup of the Intractable Hepato-Biliary Disease Study Group in Japan. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol 2011; 46:1136–1141. [DOI] [PubMed] [Google Scholar]
- 3.Krawitt EL. Autoimmune hepatitis. N Engl J Med 2006; 354:54–66. [DOI] [PubMed] [Google Scholar]
- 4.Yang F, Wang Q, Jia J, et al. Autoimmune hepatitis: East meets West. J Gastroenterol Hepatol 2015; 30:1230–1236. [DOI] [PubMed] [Google Scholar]
- 5.Onji M, Zeniya M, Yamamoto K, et al. Autoimmune hepatitis: diagnosis and treatment guide in Japan, 2013. Hepatol Res 2014; 44:368–370. [DOI] [PubMed] [Google Scholar]
- 6.Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol 2014; 60:210–223. [DOI] [PubMed] [Google Scholar]
- 7.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999; 189:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Li JY, Xu W. Role of BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit Rev Oncol Hematol 2014; 91:113–122. [DOI] [PubMed] [Google Scholar]
- 9.Zhao LD, Li Y, Smith MF, Jr, et al. Expressions of BAFF/BAFF receptors and their correlation with disease activity in Chinese SLE patients. Lupus 2010; 19:1534–1549. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita T, Hasegawa M, Yanaba K, et al. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum 2006; 54:192–201. [DOI] [PubMed] [Google Scholar]
- 11.Migita K, Ilyassova B, Kovzel EF, et al. Serum BAFF and APRIL levels in patients with PBC. Clin Immunol 2010; 134:217–225. [DOI] [PubMed] [Google Scholar]
- 12.Migita K, Abiru S, Maeda Y, et al. Elevated serum BAFF levels in patients with autoimmune hepatitis. Hum Immunol 2007; 68:586–591. [DOI] [PubMed] [Google Scholar]
- 13.Toubi E, Gordon S, Kessel A, et al. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun 2006; 27:134–139. [DOI] [PubMed] [Google Scholar]
- 14.Sène D, Limal N, Ghillani-Dalbin P, et al. Hepatitis C virus-associated B-cell proliferation—the role of serum B lymphocyte stimulator (BLyS/BAFF). Rheumatology (Oxford) 2007; 46:65–69. [DOI] [PubMed] [Google Scholar]
- 15.Mackay IR, Groom J, Mackay CR. Levels of BAFF in serum in primary biliary cirrhosis and autoimmune diabetes. Autoimmunity 2002; 35:551–553. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Li N, Wang Y, et al. Serum levels of B-cell activating factor in chronic hepatitis B virus infection: association with clinical diseases. J Interferon Cytokine Res 2014; 34:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengeveld PJ, Kersten MJ. B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J 2015; 5:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer G, Bosch R, Hodgson K, et al. B cell activation through CD40 and IL4R ligation modulates the response of chronic lymphocytic leukaemia cells to BAFF and APRIL. Br J Haematol 2014; 164:570–578. [DOI] [PubMed] [Google Scholar]
- 19.Rihacek M, Bienertova-Vasku J, Valik D, et al. B-Cell activating factor as a cancer biomarker and its implications in cancer-related cachexia. Biomed Res Int 2015; 792187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014; 147:577–594 e1. [DOI] [PubMed] [Google Scholar]
- 21.Grebely J, Feld JJ, Applegate T, et al. Plasma interferon-gamma-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology 2013; 57:2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diago M, Castellano G, García-Samaniego J, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut 2006; 55:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey CE, Post JJ, Palladinetti P, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol 2003; 74:360–369. [DOI] [PubMed] [Google Scholar]
- 24.Zeremski M, Petrovic LM, Chiriboga L, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 2008; 48:1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishioji K, Okanoue T, Itoh Y, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol 2001; 123:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czaja AJ. Review article: chemokines as orchestrators of autoimmune hepatitis and potential therapeutic targets. Aliment Pharmacol Ther 2014; 40:261–279. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999; 31:929–938. [DOI] [PubMed] [Google Scholar]
- 28.Mahler M, Meroni PL, Bossuyt X, et al. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J Immunol Res 2014; 315179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H, Enomoto H, Nasu A, et al. Clinical significance of pretreatment serum interferon-gamma-inducible protein 10 concentrations in chronic hepatitis C patients treated with telaprevir-based triple therapy. Hepatol Res 2014; 44:E397–E407. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa H, Enomoto H, Iwata Y, et al. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-γ-inducible protein-10 in primary biliary cirrhosis. Hepatol Res 2015; doi:10.1111/hepr.12595. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland AP, Ng LG, Fletcher CA, et al. BAFF augments certain Th1-associated inflammatory responses. J Immunol 2005; 174:5537–5544. [DOI] [PubMed] [Google Scholar]
- 32.Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol 2014; 61 (1 Suppl):S14–25. [DOI] [PubMed] [Google Scholar]
- 33.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 2015; 61:1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology 2013; 57:1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]


