Abstract
This study investigated the possible relationship between endocarditis and overall and individual cancer risk among study participants in Taiwan.
We used data from the National Health Insurance program of Taiwan to conduct a population-based, observational, and retrospective cohort study. The case group consisted of 14,534 patients who were diagnosed with endocarditis between January 1, 2000 and December 31, 2010. For the control group, 4 patients without endocarditis were frequency matched to each endocarditis patient according to age, sex, and index year. Competing risks regression analysis was conducted to determine the effect of endocarditis on cancer risk.
A large difference was noted in Charlson comorbidity index between endocarditis and nonendocarditis patients. In patients with endocarditis, the risk for developing overall cancer was significant and 119% higher than in patients without endocarditis (adjusted subhazard ratio = 2.19, 95% confidence interval = 1.98–2.42). Regarding individual cancers, in addition to head and neck, uterus, female breast and hematological malignancies, the risks of developing colorectal cancer, and some digestive tract cancers were significantly higher. Additional analyses determined that the association of cancer with endocarditis is stronger within the 1st 5 years after endocarditis diagnosis.
This population-based cohort study found that patients with endocarditis are at a higher risk for colorectal cancer and other cancers in Taiwan. The risk was even higher within the 1st 5 years after endocarditis diagnosis. It suggested that endocarditis is an early marker of colorectal cancer and other cancers. The underlying mechanisms must still be explored and may account for a shared risk factor of infection in both endocarditis and malignancy.
INTRODUCTION
Infectious endocarditis is an infection of the endocardium and typically involves 1 or more heart valves. If left untreated, endocarditis can cause other complications and be life threatening. It has an estimated annual incidence of 3 to 9 cases per 100,000 persons in industrialized countries.1–3 The mean age in the reported series varied between 36 and 69 years, and the incidence increased with age.1 The male to female ratio ranged from 1.2:1 to 2.5:1.4 Streptococci and staphylococci accounted for 80% of infective endocarditis cases.1,3 An earlier study in Taiwan revealed that the mean annual crude incidence was 7.6 per 100,000 inhabitants, and the incidence was significantly higher in men than in women (10.4 vs 4.6 per 100,000; P < 0.001). Staphylococcal (32%) and Streptococcal species (61%) were the most common causative pathogens.5
Infectious endocarditis was suggested to be related to colon cancer in as early as 1951 by McCoy and Mason.6 However, the association of Streptococcus gallolyticus with colorectal neoplasia was not recognized until the 1970s.7–9 A Danish nationwide study evaluated endocarditis and the risk of cancer and found that endocarditis is a strong marker for prevalent occult cancer and a predictor of modestly increased long-term cancer risk.10 In Taiwan, cancer has been ranked as the leading cause of mortality for more than 3 decades, and colorectal cancer has been the most common malignancy since 2006. The age-adjusted incidence rate for colorectal cancer incidence was 43.77 per 100,000 persons in Taiwan in 2011,11 an increase from 2007 to 2011 of 15.3% and 9.3% for Taiwanese men and women, respectively.12 We hypothesized that Taiwanese patients with endocarditis would have a higher colorectal cancer risk and conducted a population-based cohort study to verify it. In addition, we wanted to know if overall cancer or any individual cancer risk was related to endocarditis.
MATERIALS AND METHODS
Data Source
The National Health Insurance Research Database (NHIRD) was established using data from the single-payer National Health Insurance (NHI) program; the NHIRD is maintained by Taiwan's National Health Research Institutes. The NHI program, launched in 1995, covers approximately 99% of the 23.75 million residents in Taiwan.13 Every person included in the NHIRD is anonymous, with their individual privacy maintained. All NHI datasets can be interlinked with the scrambled personal identification number of each person. For this retrospective cohort study, we used an NHIRD subset comprising the Registry for Catastrophic Illness Patient Database (RCIPD) and the Registry of Beneficiaries, which contains healthcare data including files of inpatient claims. Each disease was identified on the basis of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This study was approved by the Institutional Review Board at China Medical University and Hospital in Taiwan (CMUHIO4-REC2-115).
Sampled Participants
From the inpatient claims, we selected patients aged 20 years who were diagnosed with endocarditis (ICD-9-CM codes: 391 and 421) from January 1, 2000 to December 31, 2010 as the endocarditis cohort. The index date was the date of the endocarditis diagnosis at admission. Those with a history of cancer (ICD-9-CM codes: 140–208) before the index date were excluded. The comparison cohort comprised patients who had no history of endocarditis or cancer; they were frequency matched with the endocarditis cohort at a ratio of 1:4, according to age (every 5 years), sex, and index year.
Outcome
Both cohorts were followed until a diagnosis of cancer (ICD-9-CM codes: 140–195, 200–208) or until loss to follow-up, death, termination of insurance, or the end of 2011. Cancer was identified from the RCIPD. Registration for a catastrophic illness requires a diagnosis made by a physician and pathological confirmation or other supporting medical information; these documents are formally reviewed by the Bureau of NHI.
Comorbidity
The Charlson comorbidity index score (CCI score) of each participant was counted using the claims data for hospitalizations at baseline. The CCI score is a scoring system that includes weighting factors for critical concomitant diseases; it has been validated for use with administrative databases that are coded using ICD-9-CM.14,15 We also incorporated an inpatient diagnosis file to ascertain comorbidities, including drug abuse (ICD-9-CM codes: 304 and 305), degenerative heart valves (ICD-9-CM codes: 390–392, 393–398, 424.00–424.09, 424.10–424.19, 424.90, 424.91–424.92, 746, 746.61,746.63, 746.60, and 746.6), and operations on valves and septa of the heart (ICD-9-OP code: 35).
Statistical Analysis
The proportionate distributions of demographic characteristics, CCI score, and comorbidity between the cohorts with and without endocarditis were compared using the Chi-square test for categorical variables and t-test for continuous variables. The incidence density rates of overall cancer and subdivision cancer per 1000 person-years of follow-up for each cohort were calculated. We used the Fine and Gray16 competing risks regression analysis to estimate the subhazard ratio (SHR) and 95% confidence interval (CI) for cancer in patients with endocarditis, as compared with the nonendocarditis cohort. The multivariable models were simultaneously adjusted for age, sex, CCI score, drug abuse, degenerative heart valves, and operations on valves and septa of the heart. All of the analyses were performed using the SAS statistical package (SAS System for Windows, Version 9.4; SAS Institute, Inc., Cary, NC). A P value of <0.05 was considered statistically significant.
RESULTS
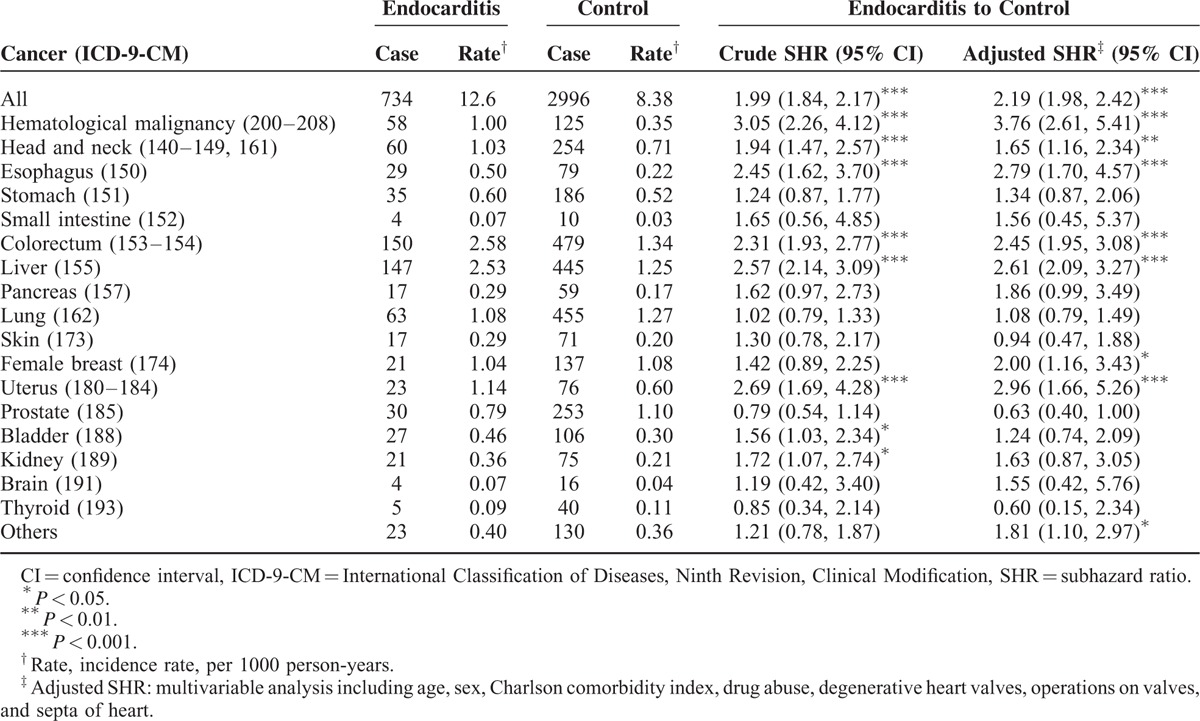
The study collected a total of 14,534 endocarditis patients and 4-fold matched control participants (n = 58,136) with a similar distribution of age and gender (Table 1). Compared with the comparison cohort, the endocarditis patients were more likely to have a CCI score ≥1 (58.3% vs 17.0%), drug abuse (6.13% vs 0.07%), degenerative heart valves (39.6% vs 1.66%), and operations on valves and septa of the heart (16.3% vs 0.09%). The mean follow-up time was 4.61 (SD = 3.83) and 6.15 (SD = 3.56) years for the endocarditis and comparison cohorts, respectively (data not shown). Overall, the incidence of cancer was 1.49-fold higher in the endocarditis cohort than in the comparison cohort (12.6 vs 8.38 per 1000 person-year), with the adjusted subhazard ratio (aSHR) being 2.19 (95% CI = 1.98–2.42). Patients with endocarditis had a significantly higher risk of developing hematological malignancy (aSHR = 3.76, 95% CI = 2.61–5.41) and cancer of the head and neck (aSHR = 1.65, 95% CI = 1.16–2.34), esophagus (aSHR = 2.79, 95% CI = 1.70–4.57), colorectum (aSHR = 2.45, 95% CI = 1.95–3.08), liver (aSHR = 2.61, 95% CI = 2.09–3.27), female breast (aSHR = 2.00, 95% CI = 1.16–3.43), and uterus (aSHR = 2.96, 95% CI = 1.66–5.26), than did the comparison cohort (Table 2).
TABLE 1.
Comparison of Demographics and Comorbidity Between Endocarditis Patients and Controls
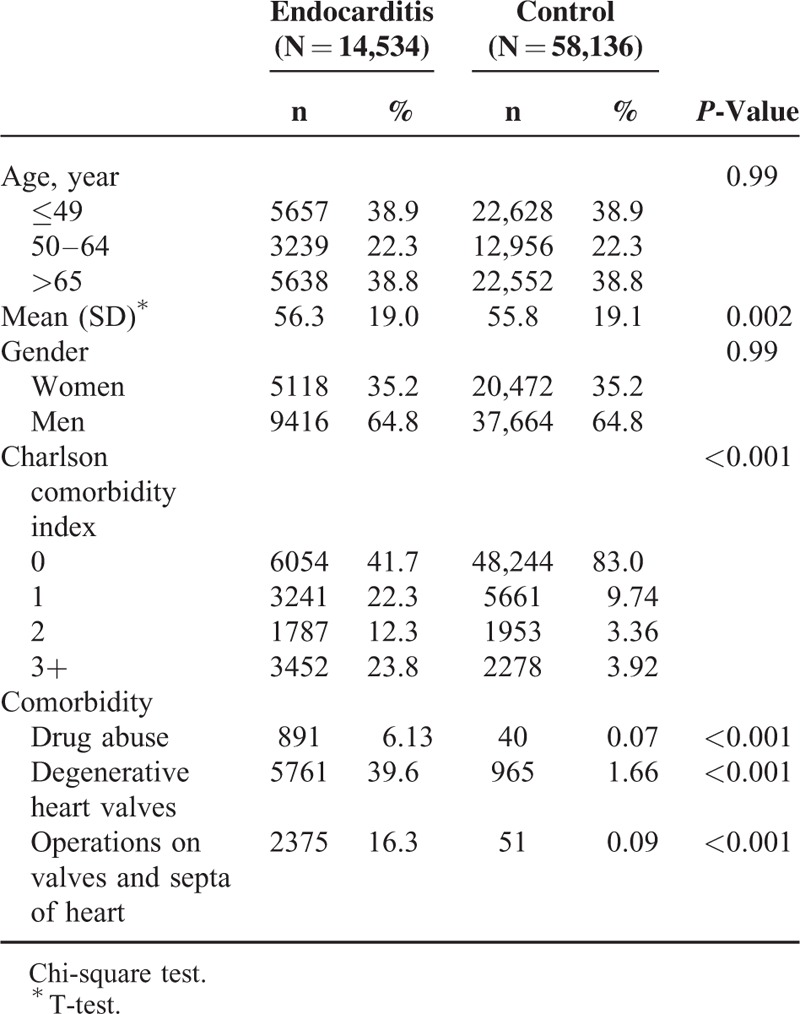
TABLE 2.
Comparison of Incidence and Competing Risks Regression Analysis Estimated Subhazard Ratio of Subdivision Cancer According to Endocarditis Status
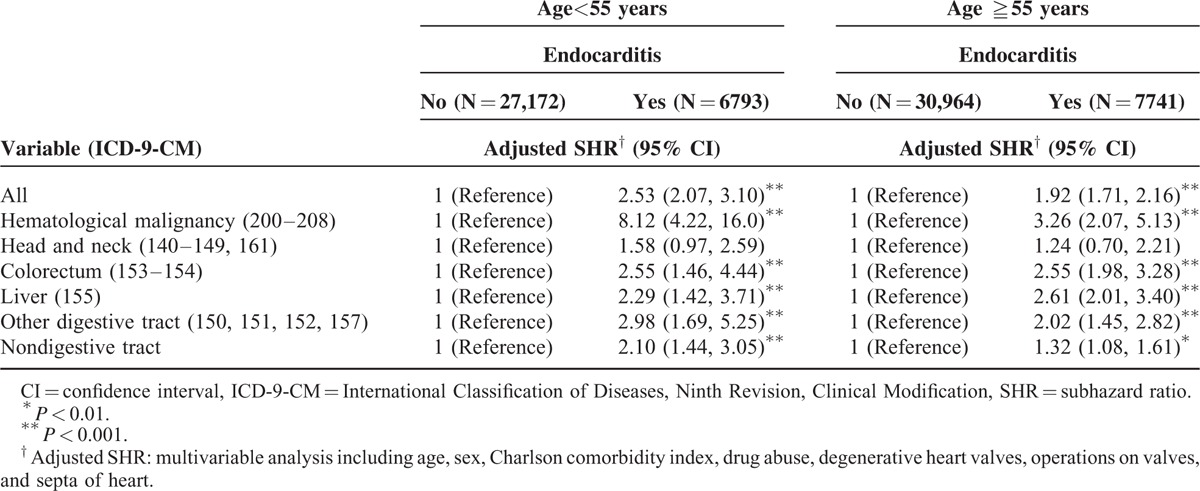
Among patients aged <55 and ≥55 years, patients with endocarditis had a higher risk of hematological malignancy and of colorectum, liver, other digestive tract, and nondigestive tract cancers than did those without endocarditis (Table 3).
TABLE 3.
Competing Risks Regression Analysis Estimated SHR and 95% CIs of Subdivision Cancer Associated With Endocarditis by Age
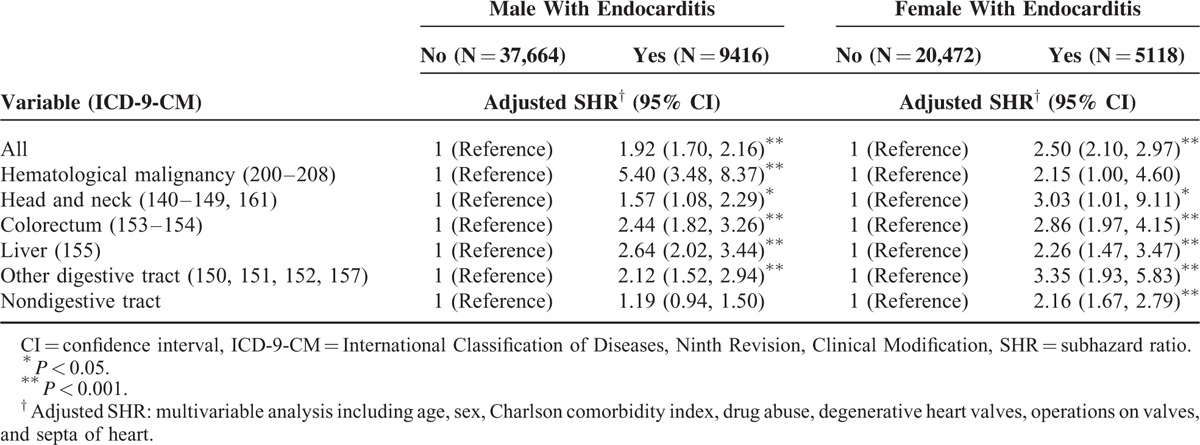
The men with endocarditis exhibited a significantly higher risk of hematological malignancy, cancer of the head and neck and of colorectum, liver, and other digestive tract cancers than did the men without endocarditis (Table 4). Women with endocarditis had a higher risk cancer of the head and neck, colorectum, liver, other digestive tract, and nondigestive tract cancers than did women without endocarditis.
TABLE 4.
Competing Risks Regression Analysis Estimated SHR and 95% CIs of Subdivision Cancer Associated With Endocarditis by Sex
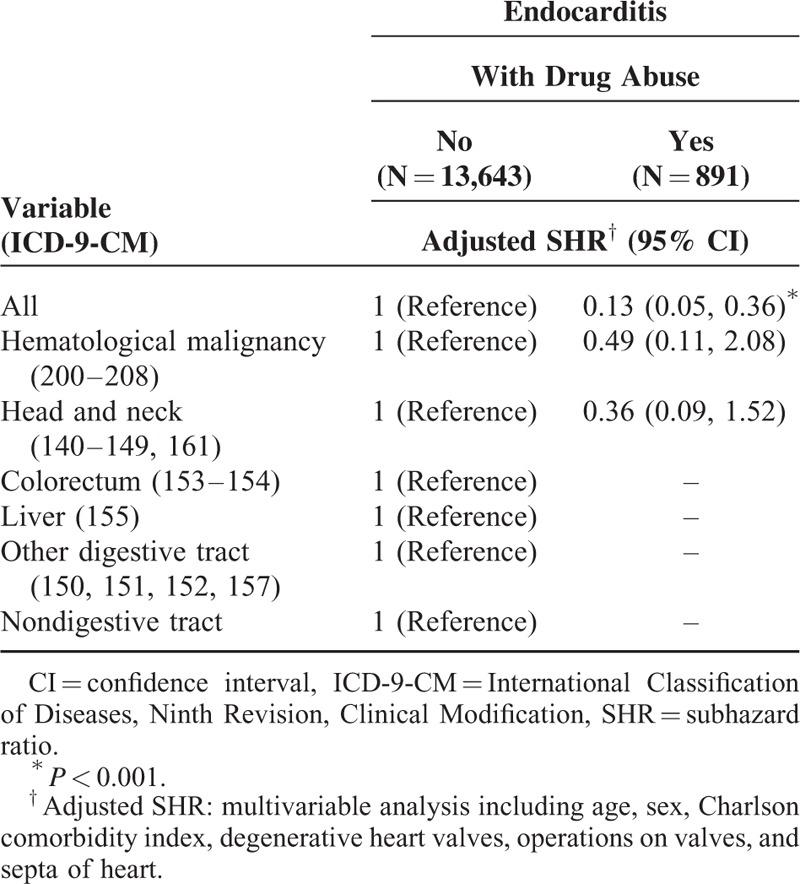
Patients within the 1st 5 years after endocarditis diagnosis exhibited a significantly higher risk of hematological malignancy, cancer of the head and neck and of colorectum, liver, other digestive tract cancers, and nondigestive tract cancers than did patients without endocarditis (Table 5). However, patients with longer than 5 years follow-up after endocarditis diagnosis had a higher risk of colorectal cancer than did those without endocarditis (aSHR = 1.86, 95% CI = 1.17–2.96). Endocarditis patients with drug abuse, however, had a significantly lower risk for overall cancer risk compared with endocarditis patients without drug abuse (aSHR = 0.13, 95% CI = 0.05–0.36) (Table 6).
TABLE 5.
Competing Risks Regression Analysis Estimated SHR and 95% CIs of Subdivision Cancer Associated With Endocarditis by Years After Endocarditis Diagnosis
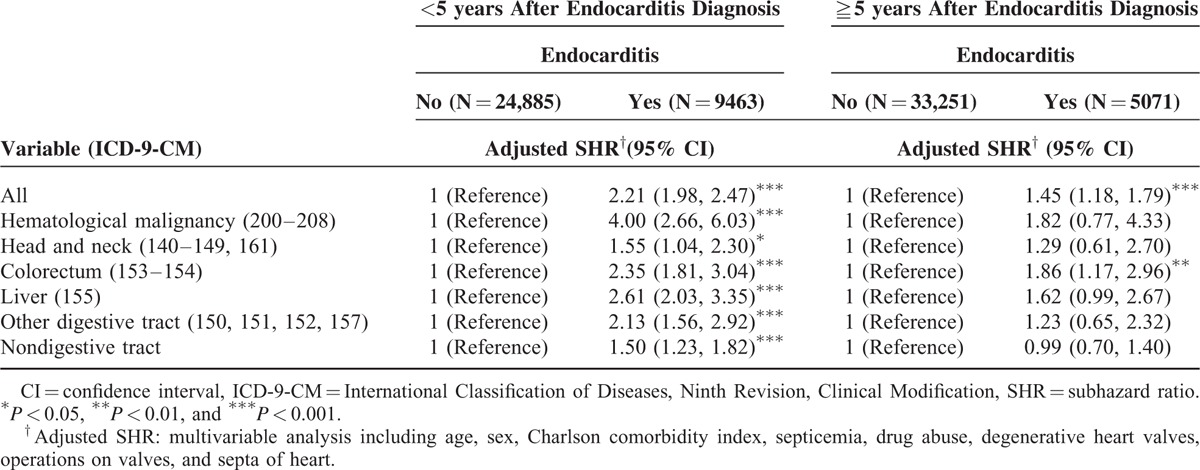
TABLE 6.
Competing Risks Regression Analysis Estimated SHR and 95% CIs of Subdivision Cancer Associated With Endocarditis by Drug Abuse
DISCUSSION
In this substantial, nationwide, population-based cohort study, we hypothesized that Taiwanese patients with endocarditis would have a higher risk of colorectal cancer. As expected, we observed that endocarditis has an over 2-fold increased risk of colorectal cancer. In addition, the risks of developing overall cancer and some digestive tract cancers, as well as head and neck, uterus, and female breast cancers and hematological malignancies in the endocarditis group were significantly higher than in the comparison group. Additional analyses by age, sex, and years after endocarditis diagnosis exhibited different patterns among various cancer sites, with endocarditis patients who had shorter times after endocarditis diagnosis tending to exhibit significantly higher risks.
The global cancer burden has been increasing rapidly over the past 30 years.17 It is also a major public health concern in Taiwan, with aging populations and lifestyle changes. Colorectal cancer is the 3rd most common newly diagnosed cancer among men and women in the United States and accounts for 10% to 15% of all cancers.18 Although the long-term trend of incidence has decreased in the United States,18 the burden of colorectal cancer exhibits a rising trend in the Asia-Pacific region and is now the 3rd most common malignant disease in both men and women in Asia.19 In Taiwan, colorectal cancer has been the most common malignancy since 2006.11 The NHI program in Taiwan has successfully provided quality health care, comprehensive benefits, and convenient access to treatment20 and is a suitable resource of data for population-based studies. We used the NHIRD for conducting a cohort study recently and found an increased risk of breast cancer in patients with multiple sclerosis.21 The positive findings encouraged us to explore other possible risk factors of cancer. This current study used a similar design and endeavored to determine whether endocarditis has any relationship with cancer risk.
As expected, our data revealed a significantly higher risk of colorectal cancer among endocarditis patients, which is consistent with earlier studies.6–10,22,23 As early as in 1951, McCoy and Mason6 reported a case and indicated a relationship between sigmoid carcinoma and the presence of infectious endocarditis. In 1974, Roses et al7 described 3 patients who presented with signs and symptoms of both bacterial endocarditis and carcinoma of the colon or rectum. Previous studies have identified that infectious endocarditis from S. gallolyticus is associated with colonic carcinoma.8,9,22 Recently, Takamura et al recognized that infective endocarditis caused by S. gallolyticus subspecies pasteurianus is also related to colon cancer.23 Although endocarditis is suggested to be a substantial clinical marker for the presence of occult colonic cancer,10,24 tumors have been reported years after an episode of endocarditis.25,26
In addition to colorectal cancer, previous studies have suggested that other cancers may also be related to endocarditis.10,24,27 Thomsen et al10 conducted a nationwide cohort study in Denmark and found that endocarditis is a critical clinical marker for the presence of occult cancer. Cancer risk was highly elevated, particularly for intraabdominal cancers and hematological malignancies, during the 1st 3 months of follow-up and remained substantially increased for several years. They concluded that endocarditis was a marker of modestly increased long-term cancer risk, probably related to shared risk factors including lifestyle andimmunosuppression.10 Partially consistent with their findings, our results showed that the risk of developing cancer in some digestive tract organs including the esophagus and liver in addition to the head and neck, uterus, and female breast and of developing hematological malignancies in patients of the endocarditis group was significantly higher. By contrast, a lack of association of S. gallolyticus with noncolonic gastrointestinal carcinoma was also reported.28
A large difference in CCI between endocarditis and nonendocarditis patients was acknowledged in this study. CCI is used to predict clinical outcome for a patient with a range of comorbid conditions. Although we adjusted it in the multivariable analyses, it still can reflect the relatively poorer outcome for endocarditis group compared with nonendocarditis group.
Because of the relatively low number of cases for each individual cancer site, the risk estimates were unstable and the explanatory power was inadequate to perform a stratified analysis. Therefore, we pooled our patients and regrouped them for further stratified analyses by sex, age, and years after endocarditis diagnosis. The diverse patterns among the different cancer sites stratified by age and sex were difficult to interpret. However, our data indicated that significantly higher risks were more likely to be observed among the patients within the 1st 5 years after endocarditis diagnosis (Table 5), which is consistent with Thomsen et al.10
We unexpected found that endocarditis patients with drug abuse had a significantly decreased risk for overall cancer risk compared with endocarditis patients without drug abuse. We did not have the clue to explain it, but assumed that some drug abuser are in prison and not convenient to have regular health examinations as the general population, which may lower the opportunity to detect malignancy. We also analyzed the age of drug abusers compared to the nonabusers and found that the drug abusers were significantly younger than the nonabusers (mean age was 33.8 and 56 years for drug abusers and nonabusers, respectively, P < 0.001). Cancer in young people is not so frequent. Although we have adjusted age in the analysis, residual confounding effect may still partially distort the result.
The plausible mechanisms of the association between endocarditis and cancers are still lacking. Cancer is more common after endocarditis in subjects who have had an endocarditis probably because cancer was already present at the time of endocarditis onset, but not diagnosed. The best model is again S. gallolyticus endocarditis. In these patients, more than colorectal cancer, colonic polyps are common.29 These are the portal of entry of bacteria into the blood stream. If, after hospitalization for endocarditis is ended, the patient does not perform a colonoscopy, the polyp is like to progress to colorectal cancer. The same applies for other gastro-intestinal cancers. Moreover, our data indicated that cancers are more likely to be observed among endocarditis patients within the 1st 5 years after diagnosis. This timing is compatible with the presence of cancer already at the time endocarditis occurred, although at a very early and often presymptomatic stage. Thus, endocarditis is likely an early marker of cancer. However, the role of cancer in endocarditis pathogenesis may not be confined to entry of mucosal bacteria into the bloodstream. Before bacteria localize to the endocardium, giving rise to the mature endocarditis vegetation, a nonbacterial thrombotic lesion can occur. It is well known that cancer is a major cause of acquired thrombophilia.30 Thus, we can hypothesize that an underlying, yet undiscovered cancer, can favor nonbacterial thrombotic endocarditis and associate with endocarditis.
There is increasing evidence that bacterial or viral infections play an important role in cancer development.31,32 Approximately 18% of the global cancer burden has been attributed to infectious agents.33 Well-known bacterial or viral carcinogens include Helicobacter pylori and gastric cancer;34 hepatitis B and C viruses and hepatocellular carcinoma;35 human papilloma viruses and cervical, anal, and oropharyngeal cancers;36,37 and Epstein–Barr virus and Hodgkin lymphoma, Burkitt lymphoma, and nasopharyngeal carcinoma.38,39 Furthermore, S. gallolyticus is related to both infectious endocarditis and colonic carcinoma.8,9,22 Another possible linkage between endocarditis and malignancy occurs through immunosuppression. Immunocompromised patients are vulnerable to hematological malignancies, other cancers, and infection.40,41 In addition to colonic involvement, S. gallolyticus endocarditis was also suggested to be related to chronic liver disease,42 and liver cirrhosis may progress to hepatocellular carcinoma,43 which may be the relationship between endocarditis and liver cancer. Nonetheless, the causality of endocarditis and malignancy is less likely, and shared risk factors (e.g., infection and immunosuppression) are more plausible. Abdulamir et al44 reviewed the literature and discovered that substantial evidence supported the etiological role of S. gallolyticus in the development of colorectal tumors.
LIMITATIONS
This study has the following strengths: a population-based design, the generalizability of its findings, and a low loss to follow-up rate because of its longitudinal design. Furthermore, the NHIRD covers a highly representative sample of Taiwan's general population because the reimbursement policy is universal and operated by a single buyer, the government. All of the NIH insurance claims are scrutinized by medical reimbursement specialists and peer reviewed. However, several intrinsic limitations of the database should be addressed before interpreting the data. First, we could not identify the infection organisms of endocarditis patients, which prevented us from performing more sophisticated analyses in specifying the role of S. gallolyticus in the association of endocarditis and cancer. Second, information regarding the life style or behavior of patients in the NHI database is not available, making it impossible to adjust for health behavior-related factors such as alcohol consumption and smoking. Alcohol consumption is suggested to be a risk factor for both colon cancer45 and endocarditis,46 as well as for other cancers.47,48 Although smoking is a well-documented risk factor for many cancers,48,49 its relationship to endocarditis remains unclear. Third, the reliability of the evidence derived from a retrospective cohort study is generally lower than that of randomized trials because a cohort study design is subject to many biases related to residual confounding, which can affect mainly low risk estimates in many observational studies. Despite the limitations of the administrative data, the data regarding endocarditis and cancer diagnoses and follow-up status in this study are highly valid.
CONCLUSION
This population-based cohort study showed a higher risk of colorectal cancer among endocarditis patients in Taiwan. In addition, the risks of overall cancer and some other individual cancers were also elevated, with this phenomenon being more evident in patients within the 1st 5 years after a diagnosis of endocarditis. It implied that endocarditis represents a strong marker of a higher probability to develop a cancer in the following years. Shared risk factors (e.g., infection and immunosuppression), which linked endocarditis and malignancy, were suggested.
Acknowledgments
The authors thank Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan) for the support.
Footnotes
Abbreviations: aSHR = adjusted subhazard ratio, CI = confidence interval, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database.
J-AL and L-MS contributed equally to this work.
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: Conception/Design: L-MS, J-AL, C-HK, L-RL; Provision of study materials: J-AL, C-HK; Collection and/or assembly of data: L-MS, C-HK, L-RL; Data analysis and interpretation; Manuscript writing; and Final approval of manuscript: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
All authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hill EE, Herijgers P, Herregods MC, et al. Evolving trends in infective endocarditis. Clin Microbiol Infect 2006; 12:5–12. [DOI] [PubMed] [Google Scholar]
- 2.Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol 2012; 59:1968–1976. [DOI] [PubMed] [Google Scholar]
- 3.Hoen B, Duval X. Clinical practice. Infective endocarditis. N Engl J Med 2013; 368:1425–1433. [DOI] [PubMed] [Google Scholar]
- 4.Fedeli U, Schievano E, Buonfrate D, et al. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC infect Dis 2011; 11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Tsai WC, Liu PY, et al. Epidemiologic features of infective endocarditis in Taiwanese adults involving native valves. Am J Cardiol 2007; 100:1282–1285. [DOI] [PubMed] [Google Scholar]
- 6.McCoy WC, Mason JM. Enterococcal endocarditis associated with carcinoma of the sigmoid: report of a case. J Med Assoc Stat Alab 1951; 21:162–166. [PubMed] [Google Scholar]
- 7.Roses DF, Richman H, Localio SA. Bacterial endocarditis associated with colorectal carcinoma. Ann Surg 1974; 179:190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein RS, Recco RA, Catalano MT, et al. Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med 1997; 297:800–802. [DOI] [PubMed] [Google Scholar]
- 9.Waisberg J, Matheus Cde O, Pimenta J. Infectious endocarditis from Streptococcus bovis associated with colonic carcinoma: case report and literature review. Arq Gastroenterol 2002; 39:177–180. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen RW, Farkas DK, Friis S, et al. Endocarditis and risk of cancer: a Danish nationwide cohort study. Am J Med 2013; 126:58–67. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Statistics Annual Report: Taiwan Cancer Registry. Available from http://tcr.cph.ntu.edu.tw/main.php?Page = N2 [Accessed June 19, 2015]. [Google Scholar]
- 12.Cancer Statistics: Cancer Incidence Trends. Taiwan Cancer Registry. Available from http://tcr.cph.ntu.edu.tw/main.php?Page = A5B2 [Accessed June 19, 2015]. [Google Scholar]
- 13.Database NHIR. Taiwan, http://nhird.nhri.org.tw/en/Background.html [Accessed June 19, 2015]. [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting aclinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 17.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277–300. [DOI] [PubMed] [Google Scholar]
- 19.Sung JJ, Lau JY, Goh KL, et al. Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005; 6:871–876. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CJ, Chen YC, Chen CJ, et al. Taiwan Cancer Registry Task Force. Cancer trends in Taiwan. Jpn J Clin Oncol 2010; 40:897–904. [DOI] [PubMed] [Google Scholar]
- 21.Sun LM, Lin CL, Chung CJ, et al. Increased breast cancer risk for patients with multiple sclerosis: a nationwide population-based cohort study. Eur J Neurol 2014; 21:238–244. [DOI] [PubMed] [Google Scholar]
- 22.Vaska VL, Faoagali JL. Streptococcus bovis bacteraemia: identification within organism complex and association with endocarditis and colonic malignancy. Pathology 2009; 41:183–186. [DOI] [PubMed] [Google Scholar]
- 23.Takamura N, Kenzaka T, Minami K, et al. Infective endocarditis caused by Streptococcus gallolyticus subspecies pasteurianus and colon cancer. BMJ Case Rep 2014; pii: bcr2013203476. doi: 10.1136/bcr-2013-203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Madani R, Mukhtar H. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis 2010; 12:164–171. [DOI] [PubMed] [Google Scholar]
- 25.Robbins N, Klein RS. Carcinoma of the colon 2 years after endocarditis due to Streptococcus bovis. Am J Gastroenterol 1983; 78:162–163. [PubMed] [Google Scholar]
- 26.Muhlemann K, Graf S, Tauber MG. Streptococcus bovis clone causing two episodes of endocarditis 8 years apart. J Clin Microbiol 1999; 37:862–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corredoira J, Alonso MP, Coira A, et al. Association between Streptococcus infantarius (formerly S. bovis II/1) bacteremia and noncolonic cancer. J Clin Microbiol 2008; 46:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein RS, Warman SW, Knackmuhs GG, et al. Lack of association of Streptococcus bovis with noncolonic gastrointestinal carcinoma. Am J Gastroenterol 1987; 82:540–543. [PubMed] [Google Scholar]
- 29.Grinberg M, Mansur AJ, Ferreira DO, et al. Endocarditis caused by Streptococcus bovis and colorectal neoplasms. Arq Bras Cardiol 1990; 54:265–269. [PubMed] [Google Scholar]
- 30.Decousus H, Moulin N, Quenet S, et al. Thrombophilia and risk of venous thrombosis in patients with cancer. Thromb Res 2007; 120:S51–S61. [DOI] [PubMed] [Google Scholar]
- 31.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med 2000; 248:171–183. [DOI] [PubMed] [Google Scholar]
- 32.Vogelmann R, Amieva MR. The role of bacterial pathogens in cancer. Curr Opin Microbiol 2007; 10:76–81. [DOI] [PubMed] [Google Scholar]
- 33.Vedham V, Divi RL, Starks VL, et al. Multiple infections and cancer: implications in epidemiology. Technol Cancer Res Treat 2014; 13:177–194. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira RM, Machado JC, Figueiredo C. Clinical relevance of Helicobacter pylori vacA and cagA genotypes in gastric carcinoma. Best Pract Res Clin Gastroenterol 2014; 28:1003–1015. [DOI] [PubMed] [Google Scholar]
- 35.Momin B, Richardson L. An analysis of content in comprehensive cancer control plans that address chronic hepatitis B and C virus infections as major risk factors for liver cancer. J Community Health 2012; 37:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walboomers JM, Jacobs MV, Manos MM, et al. Human papilloma virus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19. [DOI] [PubMed] [Google Scholar]
- 37.Monk BJ, Tewari KS. The spectrum and clinical sequelae of human papilloma virus infection. Gynecol Oncol 2007; 107:S6–S13. [DOI] [PubMed] [Google Scholar]
- 38.Maeda E, Akahane M, Kiryu S, et al. Spectrum of Epstein-Barr virus-related diseases: a pictorial review. Jpn J Radiol 2009; 27:4–19. [DOI] [PubMed] [Google Scholar]
- 39.Chen HX, Lai CH, Hsu HY, et al. The bacterial interactions in the nasopharynx of children receiving adenoidectomy. Biomedicine (Taipei) 2015; 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotton SJ, Belcher J, Rose PK, et al. The risk of a subsequent cancer diagnosis after herpes zoster infection: primary care database study. Br J Cancer 2013; 108:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norgaard M, Larsson H, Pedersen G, et al. Risk of bacteraemia and mortality in patients with haematological malignancies. Clin Microbiol Infect 2006; 12:217–223. [DOI] [PubMed] [Google Scholar]
- 42.Tripodi MF, Adinolfi LE, Ragone E, et al. Streptococcus bovis endocarditis and its association with chronic liver disease: an underestimated risk factor. Clin Infect Dis 2004; 38:1394–1400. [DOI] [PubMed] [Google Scholar]
- 43.Bartolomeo N, Trerotoli P, Serio G. Progression of liver cirrhosis to HCC: an application of hidden Markov model. BMC Med Res Methodol 2011; 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulamir AS, Hafidh RR, Abu Bakar F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res 2011; 30:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su LJ, Arab L. Alcohol consumption and risk of colon cancer: evidence from the national health and nutrition examination survey I epidemiologic follow-up study. Nutr Cancer 2004; 50:111–119. [DOI] [PubMed] [Google Scholar]
- 46.Davidson DM. Cardiovascular effects of alcohol. West J Med 1989; 151:430–439. [PMC free article] [PubMed] [Google Scholar]
- 47.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health 2007; 30:38–41.44-47. [PMC free article] [PubMed] [Google Scholar]
- 48.Secretan B, Straif K, Baan R, et al. A review of human carcinogens – Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009; 10:1033–1034. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz S, Messerschmidt H, Doren M. Psychosocial risk factors for cancer development. Med Klin (Munich) 2007; 102:967–979. [DOI] [PubMed] [Google Scholar]


