Abstract
The combination of low rather than high dose of dextromethorphan (DXM) with amlodipine (AM) could improve blood pressure (BP) reduction in hypertensive animals. The study aimed to evaluate the feasibility of different doses of DXM combined with standard AM treatment in clinical hypertension.
This was a prospective, 14-week, dose-escalation, multicenter study. After 2-week run-in period with AM 5 mg/day, hypertensive patients who got the BP goal of 140/90 mmHg kept receiving AM monotherapy for another 12 weeks. The nonresponders, while kept on AM 5 mg/day, received additional DXM treatment for 3 sequential dose-titrated periods with initially 2.5 mg/day, followed by 7.5 mg/day, and finally 30 mg/day. Each period was for 4 weeks. The patients at BP goal after each treatment period were defined as the responders and kept on the same combination till the end of the study. The responder rate of each treatment period was recorded. The changes of BP and serum antioxidant/endothelial markers between week 14 and week 2 were evaluated.
Of the 103 patients initially enrolled, 89 entered the treatment period. In the 78 patients completing the study, 31 (40%) at BP goal after 2-week AM run-in kept on AM monotherapy (DXM0). The addition of 2.5 (DXM2.5) and 7.5 mg/day (DXM7.5) of DXM enabled BP goal achievement in 22 (47%) nonresponders to AM monotherapy including 16 (29%) with DXM2.5 and 6 (18%) with DXM7.5. Only 4 patients (16%) reached BP goal with the combination of DXM 30 mg/day (DXM30). Overall, 73% of the 78 patients reached BP goal at the end of the 14-week study. Mean systolic BP was reduced by 7.9% ± 7.0% with DXM2.5 (P < 0.001) and by 5.4% ± 2.4% with DXM7.5 (P = 0.003) respectively at week 14 from that at week 2, which was unchanged in either DXM0 or DXM30 group. Besides, the effects of combination treatment were particularly significant in the patients with impaired endothelial function suggested by reduced serum NOx level at baseline.
Accordingly, the combination with low dose of DXM was feasible to improve BP control in patients who failed to achieve the BP goal by standard AM monotherapy. The benefit effects might be significant especially in patients with impaired endothelial function.
INTRODUCTION
Hypertension is the most important risk factor for cardiovascular disease and stroke, affects nearly 1 billion people (about 26% of the adult population) worldwide in 2000, and this is predicted to increase to 1.56 billion by 2025.1 Randomized controlled trials confirmed that lowering blood pressure (BP) significantly reduces the cardiovascular morbidity and mortality at all ages.2–5 Although safe and effective strategies for the prevention and control of high BP have been widely available, overall control rates of hypertension remains unsatisfactory in most countries.6–11 Previous data in Taiwan suggested that hypertension control rate was around one-third in both men and women,12 which were similar to those in the United States13 and European countries.6,9 It was estimated that if all hypertensive patients were treated sufficiently to reach the goal specified in current clinical guidelines, 46,000 deaths might be averted each year in the United States.14
Calcium channel blockers (CCBs), with remarkable efficacy and favorable safety profiles, are one of the most popular first-line antihypertensive agents.15 Amlodipine (AM), a long-acting calcium channel blocker, is commonly prescribed for the treatment of mild to moderate hypertension.16 However, only half of the patients could reach the BP goal with the standard AM treatment.16 It was also known that increasing the dosage of AM might significantly increase the incidence of peripheral edema because of potent arterial vasodilatory effects of CCBs.17 Thus, additional combined treatment is usually required.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases have been shown to contribute to the pathogenesis of hypertension.18 The experimental studies showed that the increased production of superoxide anion (O2−) derived from NAD (P)H oxidase counteracts the enhanced production of NO derived from nitric oxide synthases (iNOS), occurring in hypertension.19–21 It suggested that the inhibition of NADPH oxidase might lower BP in hypertensive patients. Dextromethorphan (DXM) is a dextrorotatory morphinan and is widely used as a nonopioid cough suppressant in a variety of over-the-counter remedies. DXM possesses neuroprotective activity and also effectively inhibited the production of reactive oxygen species (ROS) depending on the inhibition of NADPH oxidase activity.22–25 Some evidence suggested that inflammation can lead to the development of hypertension, and the oxidative stress and endothelial dysfunction are involved in the inflammatory cascade.26 A previous clinical study demonstrated that daily oral administration of DXM for 6 months significantly improved endothelial function and decreased vascular inflammation in heavy smokers.27
Our previous preclinical study also demonstrated that the combination therapy of low rather than high dose of AM with DXM in spontaneous hypertensive rats (SHRs) has a significant greater BP reduction than that of AM monotherapy.28 In addition to the antihypertensive effect, DXM in low dose also showed the effect on the improvement of endothelial function and the prevention of aortic hypertrophy that was considered might be because of the antioxidant effects on NADPH oxidase. DXM could enhance the antihypertensive effects of AM in the combination therapy. The combination therapy of AM and DXM can improve the vasomotor function and reduce the thickness of media layer of aorta in SHRs.28 Accordingly, the purpose of this study was to evaluate the clinical feasibility of different doses especially the low dose of DXM in combination with standard AM treatment for mild-to-moderate hypertensives. Further evaluation of the changes of serum biomarkers was also done to elucidate the potential interaction of oxidative stress, vascular inflammation, and endothelial dysfunction with the effects of DXM in hypertensive patients.
METHODS
Study Design
This was a single blind, prospective, dose-escalation design, multicenter, phase II study conducted in 9 medical centers and regional hospital in Taiwan between 2012 and 2014. Hypertensive patients were screened by cardiologists or endocrinologists at out-patient clinic in each hospital. They were then enrolled if the following criteria were fulfilled, which included age 55 to 75 years, history of mild-to-moderate essential hypertension with or without regular treatment, and elevated sitting SBP or DBP or both (90 mmHg ≤ DBP <110 mmHg and/or 140 mmHg ≤SBP <180 mmHg) at enrollment. Patients with secondary hypertension, severe hypertension (mean sitting DBP ≥ 110 mmHg and/or mean sitting SBP ≥ 180 mmHg), endocrine disease or type 1 diabetes mellitus, type 2 diabetes mellitus with poor glucose control, known cardiovascular disease (such as angina pectoris, myocardial infarction, coronary artery bypass graft, stroke) within 3 months before enrollment, significant hepatic or renal dysfunction, or any serious disease that might interfere with treatment were excluded. The BP goal of this study was sitting SBP <140�mmHg and DBP <90 mmHg after each period of treatment.
Study Protocol
Patients who fulfilled the enrollment criteria were asked to stop all the cardiovascular medications that might alter BP if there were. In each patient, the sitting BP was re-checked as the standard method to confirm the presence of hypertension just before the enrollment in the morning hours. Initially, they were administered with AM 5 mg (in a capsule) daily for 2 weeks as the run-in period of the study. Two weeks later, the hypertensive patients who met the treatment BP goal (sitting BP <140/90 mmHg in the morning hours) were kept with AM monotherapy (in a same capsule) for another 12 weeks till the end of the study (DXM0). The others were then given AM 5 mg (in a same capsule) combined with DXM 2.5 mg (in another capsule) daily for 4 weeks as the first combination treatment period. After the 4-week treatment, the patients who met the BP goal were kept with the combination of AM 5 mg and DXM 2.5 mg daily for another 8 weeks till the end of the study (DXM2.5). The others who did not meet the BP goal were then given AM 5 mg (in a same capsule) combined with DXM 7.5 mg (in another capsule similar to that of DXM 2.5 mg) daily for another 4 weeks as the second combination treatment period. After the 4-week treatment, the patients who met the BP goal were kept with the combination of AM 5 mg and DXM 7.5 mg daily for another 4 weeks till the end of the study (DXM7.5). The patients who did not meet the BP goal were given AM 5 mg (in a capsule) with the highest combination dose of DXM 30 mg (in another capsule similar to that of DXM 2.5 mg) daily for the final 4 weeks as the third combination treatment period. Therefore, the patients enrolled in this study were allocated to 4 different regimens (DXM0 group, DXM2.5 group, DXM7.5 group, and DXM30 group) according to their response of BP to different doses of DXM. The subject enrollment and diagram of study design were shown in Figure 1.
FIGURE 1.
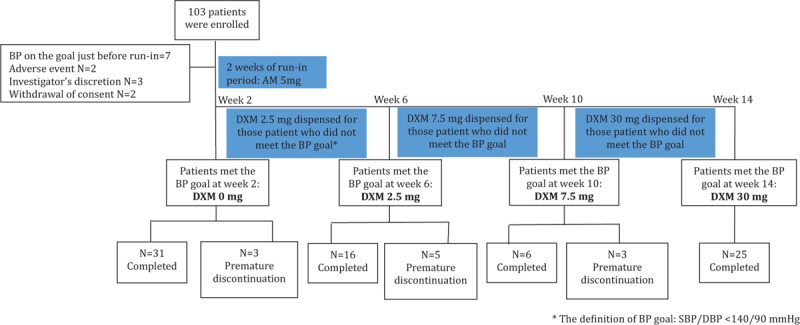
Enrollment and disposition of subjects.
The study patients were evaluated by the same physicians and/or research assistants for potential physiological and BP changes both before and after each period of treatment. In each visit, those patients presented with severe hypertension (mean sitting DBP ≥110 mmHg and/or mean sitting SBP ≥180 mmHg) were immediately dropped out from the study for safety reason.
The study protocol was approved by both the joint institutional review board and each corresponding institutional review board (ClinicalTrial.gov Identifier: NCT01614366). All the study patients gave written inform consents before entering the study.
Blood Pressure Measurement
The office BP was measured in the morning hours at each visit with an electronic sphygmomanometer for each study patient. After the patients have rested quietly in the seated position for 5 to 10 minutes, 2 measurements were taken at 1-minute intervals. All measurements were made on the same arm and the same equipment by the same assistant. In each treatment group, both the achieve rate of BP goal and the BP changes from week 2 to the end of 14-week treatment were recorded for the evaluation of efficacy.
Plasma Antioxidant, Anti-inflammatory and Endothelial Profile Measurement
To evaluate the potential effects of DXM and AM on plasma antioxidant as well as endothelial profiles, all the patients were requested to stop the intake of antioxidant vitamins and specific foods or nutritional supplements with antioxidant activity before entering the study. In each visit, a total of 15-cc blood was drawn from the peripheral veins of each study patient following a 20-minute rest in sitting position after an overnight fasting.
In each study patient, the plasma endothelial profiles including plasma NOx, and asymmetric dimethylarginine (ADMA), anti-inflammatory profile including high-sensitive C-reactive protein (hs-CRP), and antioxidant profiles including serum peroxynitrate (3-NT), oxidized low-density lipoprotein (OX-LDL), glutathione peroxide (GPx) activity (IU/g Hb), and total antioxidant status (TAS, mmol/L) were examined at each visit to explore whether addition of DXM attenuates the oxidation stress in addition to BP reduction.
Safety Profile Assessment
Safety assessments on all enrolled subjects included adverse events, vital signs, and laboratory tests (hematology, biochemistry, and urinary). Any adverse events and concomitant medications/therapies were recorded on the Case Report Forms (CRFs) throughout the study.
Statistical Analysis
The primary analysis was performed using the population of the patients on treatment by protocol defined as the study patients who had the BP measurement after each treatment regimen and with compliance of ≥70%. Descriptive statistics (means, standard deviations, frequencies) were presented for each of outcome measures at enrollment and at each follow-up visit. The primary efficacy variable represented as SBP reduction from baseline was analyzed with paired t test by each treatment group. For other efficacy parameters, such as DBP and antioxidant markers, the same analysis was performed as that of SBP. To compare the mean of reduction in BP among 4 different regimens, one-way analysis of variance was performed to find the significant differences. For those factors (ie, obesity, cigarette smoking, etc) that might influence BP value, the difference in antihypertensive response by different medications between the patient with and those without the risk factors was analyzed by χ2 test (see supplementary material).
RESULTS
The disposition of study patients is shown in Figure 1. A total of 103 patients fulfilled the entry criteria were enrolled and entered the run-in treatment period (AM 5 mg daily for 2 weeks). Of note, 14% of subjects withdrew prematurely during the run-in period (please see Figure 1 for the reasons of withdrawal). Thus, the intention-to-treat population was constituted of 89 patients. The primary analysis was performed using the population of the patients on treatment by protocol defined as the study patients who had the BP measurement after each treatment regimen and with compliance of ≥70%. Accordingly, the 11 patients entering the treatment period (3 with AM 5 mg/day, 5 with AM 5 mg/day and DXM 2.5 mg/day, and 3 with AM 5 mg/day and DXM 7.5 mg/day) were excluded from analysis. Finally, a total of 78 patients were analyzed.
Among a total of 78 patients on treatment by protocol, 31 patients (40%) reached the BP goal after AM 5 mg/day treatment for 2 weeks (DXM0 group). Of the nonresponders, 16 patients (34%) achieved the BP goal with the combination of AM 5 mg/day with DXM 2.5 mg/day (DXM2.5 group). The uptitration of DXM to 7.5 mg/day enabled 6 more patients (13%) meet BP goal (DXM7.5 group). Finally, only 4 patients (8%) achieved the BP goal in the 25 patients with DXM 30 mg/day (DXM30 group).
Baseline Characteristics of the Study Patients
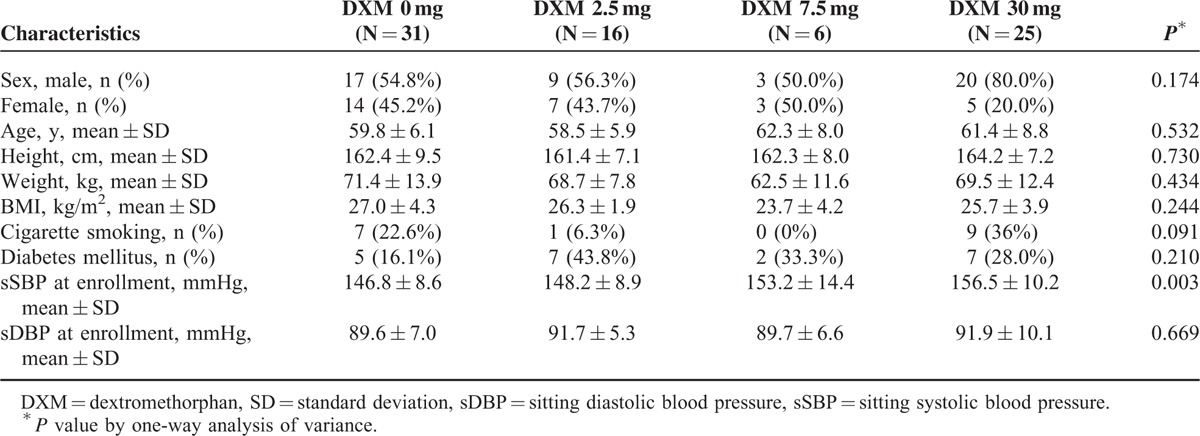
The demographic characteristics of these patients at baseline (week 2) and BP values measured at enrollment (week 0) are shown in Table 1. None of any characteristics demonstrated statistical difference across different dosing groups except SBP value at enrollment. However, those patients who did not respond to AM monotherapy in run-in period had higher baseline SBP level at enrollment.
TABLE 1.
Demographics Distribution at Baseline of the Patients on Treatment by Protocol
Blood Pressure Changes
In the DXM0 group (n = 31), the mean SBP was 127.2 ± 6.9 mmHg at week 2, which remained stable with AM 5 mg daily through the end of the study. In the DXM2.5 group (n = 16), SBP reduced significantly and constantly from baseline. The reductions were 14.0 ± 8.1 mmHg (P < 0.001), 9.4 ± 12.1 mmHg (P = 0.007), and 11.9 ± 10.8 mmHg (P = 0.003) at week 6, 10, and 14, respectively. In the DXM7.5 group (n = 6), SBP was constantly reduced from baseline by 10.8 ± 10.2 mmHg (P = 0.049) and 7.7 ± 3.4 mmHg (P = 0.003) at week 10 and week 14, respectively. In DXM30 group (n = 25), there was no change in mean SBP from baseline to the end of the study. Among them, only 4 patients achieved the BP goal by a significant reduction of 15.3 ± 5.5 mmHg in SBP (P = 0.011). The numeric SBP changes (mmHg) and the percentage of change at the end of study (week 14) from baseline (week 2) with different treatment regimens were shown in Figure 2. There were significant reductions in percentage of SBP changes at the end of the study from baseline in DXM2.5 (−7.9% ± 7.0%, P < 0.001) and in DXM7.5 group (−5.4% ± 2.4%, P = 0.003) respectively. The similar trend of DBP reduction was also found in DXM2.5 group and DXM7.5 group (Figure 2).
FIGURE 2.
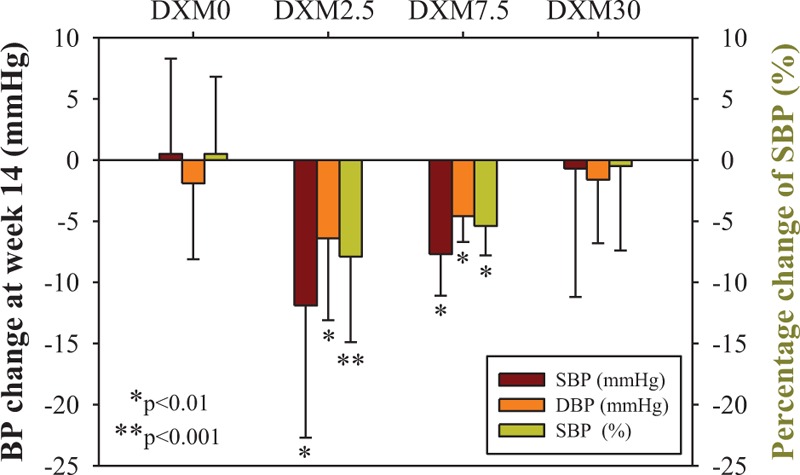
The change of systolic blood pressure (SBP) and diastolic blood pressure (DBP) from baseline (week 2) to end of study (week 14) for each treatment regimen. Four treatment regimens, DXM0, DXM2.5, DXM7.5, and DXM30, were consisted of 31, 16, 6, and 25 patients respectively. P value was examined by paired t test for the comparison of BP value (mmHg) or percentage of change (%) between week 2 and week 14.
The Rate of BP Goal Achieved
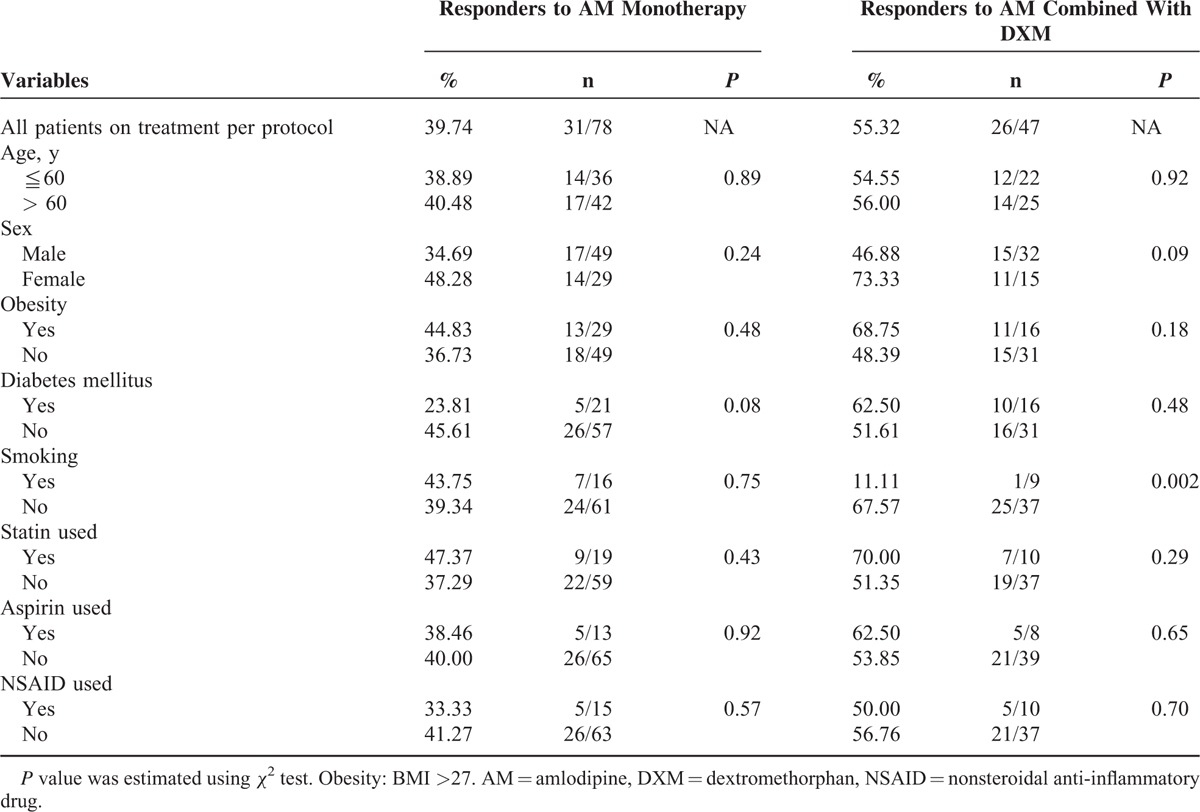
For the responder rate to achieving BP goal with individual treatment by protocol, 40% of the 78 patients reached BP goal with AM monotherapy. There were further improvements of BP control in 55% (26/47) of the nonresponders to AM alone. Overall, 73% of the 78 patients reached the BP goal with either monotherapy or different combinations at the end of the 14-week study. The addition of low-dose (2.5 or 7.5 mg/day) DXM improved BP goal achievement in 47% (22/47) of the nonresponders to AM alone.
The BP goal achievement was compared according to the presence or absence of risk factors (age, sex, obesity, cigarette smoking and so on) and the medication used (Table 2). Compared to that in the smokers, the responder rate to any combination of DXM with AM was significantly higher in patients without cigarette smoking. The above phenomenon was not seen in the responders to AM monotherapy.
TABLE 2.
BP Goal Achievement in Both AM Monotherapy and the Combination of AM and DXM Respectively for the Patients Stratified by Cardiovascular Risk Factors
The Effects of Amlodipine Monotherapy According to Baseline Antioxidant and Endothelial Profiles
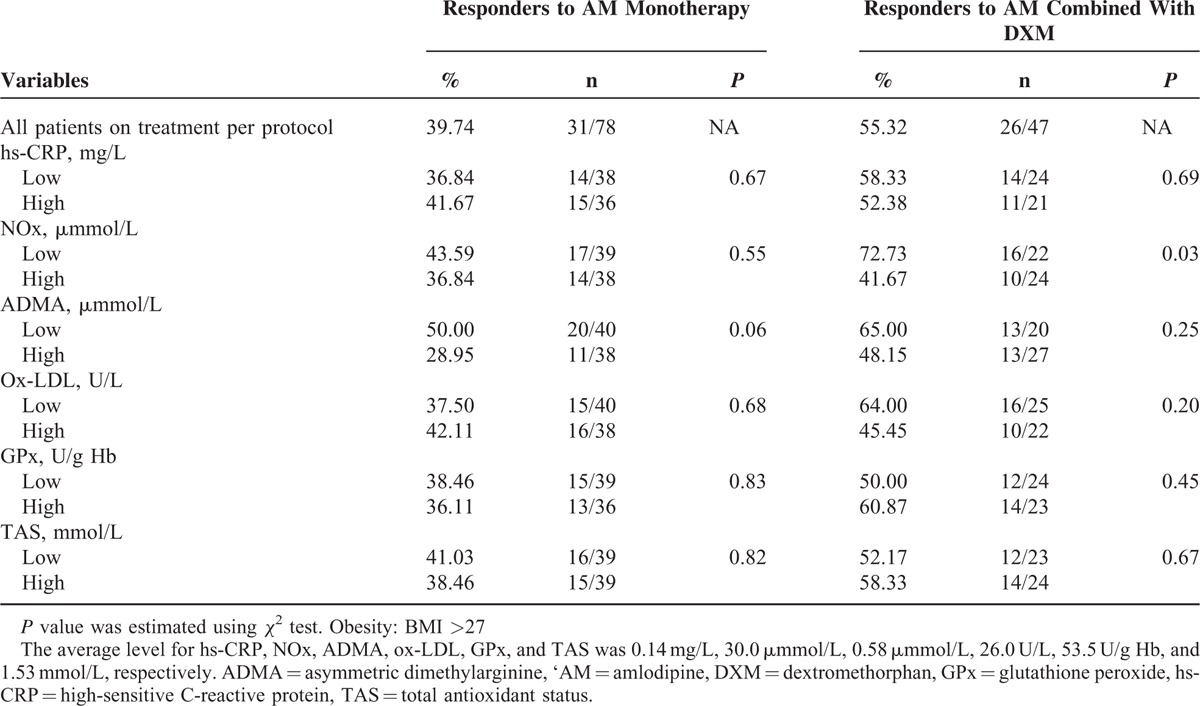
To examine whether the BP effects of amlodipine monotherapy or combined therapy might be modified by the baseline antioxidant condition and endothelial function, the subgroup analyses were conducted according to the patients’ baseline vale of antioxidant and endothelial markers. The subgroup analysis showed that among the responders to amlodipine monotherapy, the patients with higher baseline serum ADMA, an endogenous inhibitor of endothelial NO synthase tended to respond worse than those patients with lower baseline ADMA level (P = 0.06) (Table 3). Besides, the BP-lowering effects of amlodipine monotherapy were not modified by other antioxidant and endothelial markers at baseline. It seems that the BP-lowering effects of amlodipine monotherapy might be attenuated while the patients’ baseline endothelial function is impaired.
TABLE 3.
BP Goal Achievement Rate in AM Monotherapy and the Combination of AM and DXM Respectively for the Patients Stratified by the Baseline Value of Anti-oxidant/Anti-inflammatory markers (“low” Indicates the Baseline Level Below Average, “High” Indicated the Baseline Level Above Average)
The Effects of Combination of Dextromethorphan With Amlodipine According to Baseline Antioxidant and Endothelial Profiles
In all patients with combination treatment, serial subgroup analyses were done according to their baseline antioxidant condition and endothelial function. It was shown that compared with those patients with higher baseline serum NOx level, the patients with lower baseline serum NOx level responded significantly better to the combination treatment (P = 0.03) (Table 3). Besides, in patients with combination treatment, the baseline ratio of ADMA/NOx, an index of endothelial cell dysfunction, tended to reduce at the end of study. The above were not seen in patients with AM monotherapy (Figure 3). Taken together, combined treatment gave more significant BP reduction especially in patients with impaired baseline endothelial function. Furthermore, the addition of DXM to AM might improve endothelial function in hypertension patients responding poor to AM.
FIGURE 3.
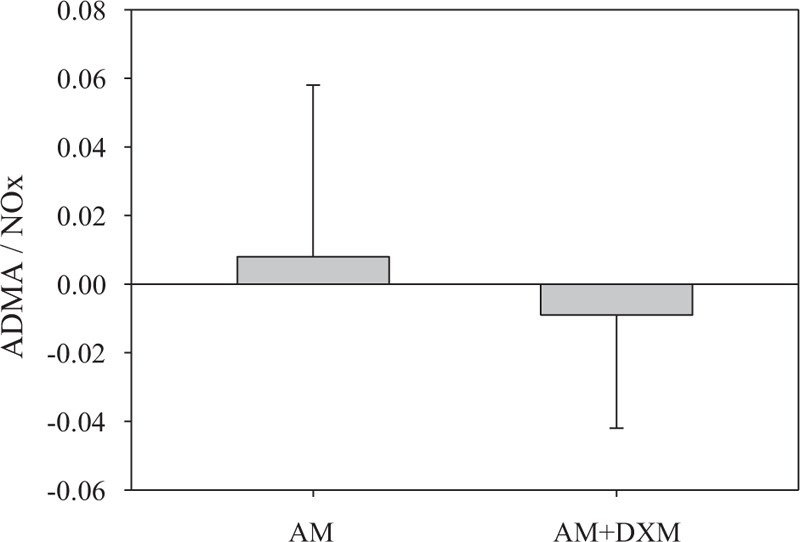
The change of ADMA/NOx ratio from baseline to end of study for responders to AM monotherapy and responders to AM combined with DXM respectively. “AM” represented as the responders to amlodipine monotherapy (N = 29). “AM + DXM” represented as the responders to amlodipine combined with dextromethorphan (N = 25).
Adverse Events
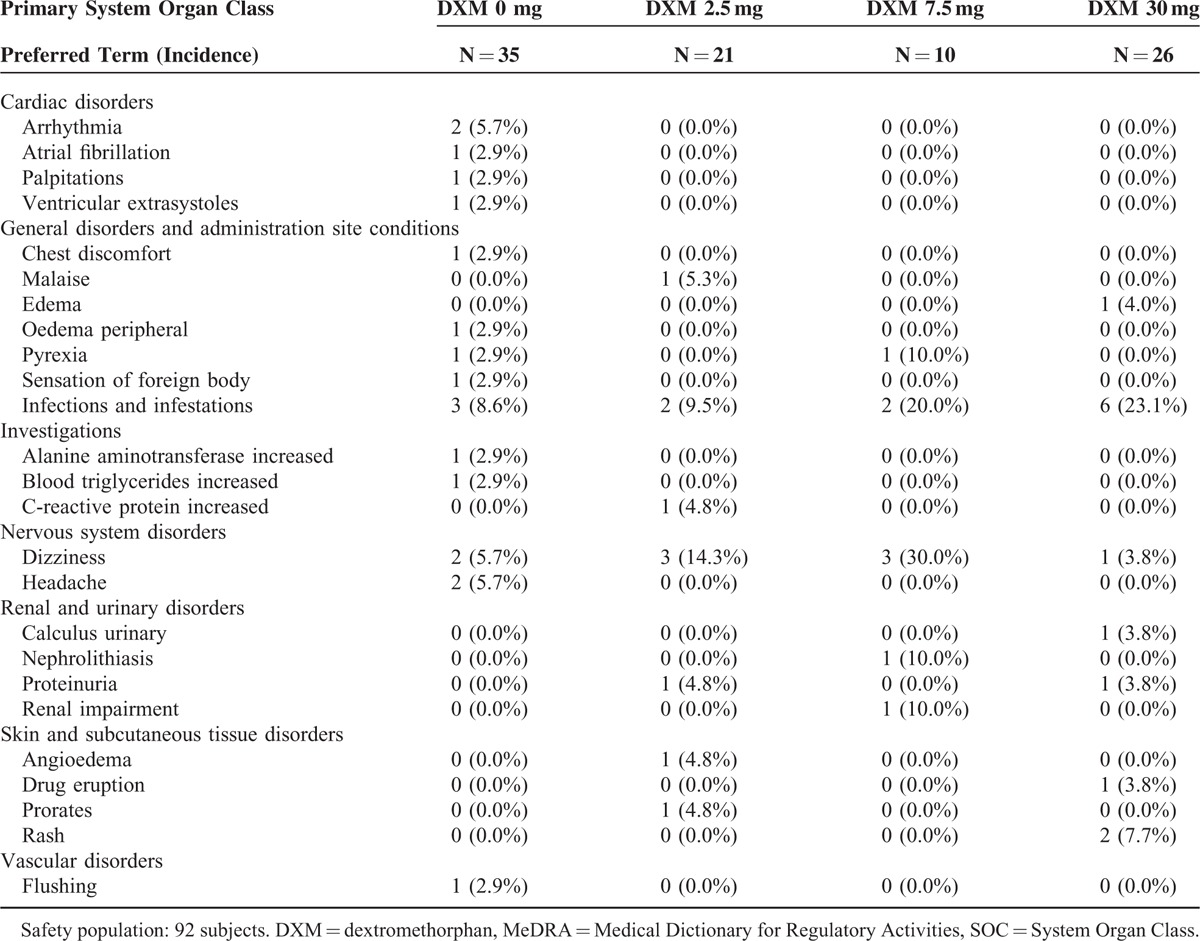
In the whole study period, a total of 101 adverse events (AEs) were reported by 50 patients (54.3%, 50/92). Most of them (81.2%. 82/101) were mild. Only 8 AEs were identified as severe in 5 patients, which included 2 patients in DXM30 group and 1 patient each in other 3 dosing groups. None of the above SAEs were determined as drug-related. However, another 7 AEs including angioedema, dizziness, and so on were related to study drugs in 7 different patients. There were no differences in AEs between the patients with AM monotherapy and those with the combinations of DXM. The summary of AEs classified according to MedDRA (Medical Dictionary for Regulatory Activities) version 17.0 was listed in Table 4.
TABLE 4.
Adverse Events Classified by MedDRA SOC for the Safety Population
DISCUSSION
This was the first study to assess the antihypertensive efficacy and safety profiles in the combination therapy of DXM and AM in clinical hypertension. As the first-in-human, concept-proven, prospective, dose-escalation, multicenter study, the present findings suggested the feasibility of the combination with low-dose DXM to improve BP control rate in patients who failed to achieve the BP goal by standard AM treatment. More interestingly, the patients with impaired endothelial function indicated by low NOx level at baseline may respond even better to this combination.
The major findings of this study included that after 2 weeks of AM monotherapy, only 31 (40%) of the 78 patients in the PP population reached the BP goal. The combination of DXM enabled 26 more patients achieve the treatment goal at the end of each 4-week treatment period, which included 16 (30%) of the 55 patients with DXM 2.5 mg, 6 (18%) of the 34 patients with DXM 7.5 mg, and 4 (16%) of the 25 patients with DXM 30 mg. In the responders of each treatment period, the SBP remained stable through the end of treatment, resulting in the significant reduction of mean SBP by 11.9 ± 10.8, 7.7 ± 3.4, and 15.3 ± 5.5 mmHg in the individual responders to DXM2.5, DXM7.5, and DXM30 treatment, respectively. At the end of the 14-week study, there were 21 patients (27%) not at BP goal even on DXM30. Thus, 73% of the 78 patients reached BP goal with either monotherapy or different combinations at the end of the 14-week study. It was significantly higher than the 40% responder rate to initial AM monotherapy (P < 0.05).
Interestingly, similar to the findings in our previous preclinical animal study,28 the effects of DXM on BP control are significantly presented in the addition with low dose of DXM (2.5 mg or 7.5 mg) rather than the higher dose of DXM (30 mg). In the present study, around 40% patients achieved the BP goal after 2 weeks of AM 5 mg treatment. However, it has been shown that up to 55% of hypertensive patients may achieve BP goal with AM 2.5 to 10 mg/day in 1 year.29 We cannot completely exclude the possibility that antihypertensive effects in DXM2.5 might be partially contributed by the delayed response from AM. However, in the current study, up-titration of DXM to 7.5 mg from week 6 to week 10 enabled additional 13% of the 47 non-responders to meet BP goal. It seems that the addition of DXM even after 8 weeks of AM treatment still have the beneficial effects on BP control. Taken together, the BP control effect by combination of DXM with AM may not be mainly due to the delayed response of AM. Alternatively, the combination effect could be raised even with the low dose of either 2.5 mg or 7.5 mg of DXM.
The NADH/NADPH oxidase is mainly expressed in endothelial cells and vascular smooth muscle cells (VSMCs).30,31 It was suggested that the development of endothelial dysfunction could be linked to an exaggerated production of O2− in aortas from SHRs.32 An enhanced production of O2− might result in inactivation of NO, generation of peroxynitrite,33 and impairing the NO-dependent relaxations.34,35 It was expected that the BP-lowering ability of DXM would corresponded to an elevation of NOx. Our data of subgroup analyses did show that the patients with lower serum NOx level at baseline responded better to the combination treatment than those with higher baseline NOx level did. The results suggest that the patients with endothelial dysfunction representing the consumption of NOx (possibly related to high oxidative stress) could be respondent to this combination treatment. It implicated that the patients with impaired endothelial function with high oxidative stress may be the potential target patients respondent to the combination treatment of addition of low-dose DXM to AM.
It has been known that endothelial dysfunction and increased oxidative stress could be found in smokers,27 and patients with metabolic syndrome36 and diabetes mellitus.37 DXM, an NADPH oxidase inhibitor, is believed have the anti-oxidation and anti-inflammatory effects. Therefore, one may speculate that DXM combined with AM could have the benefits on the hypertensive patients with the above-mentioned conditions. Our data showed that the patients without cigarette smoking at baseline responded better to the addition of DXM to AM than those with smoking did. One of the possibilities is that the patients with active cigarette smoking could have very high oxidative stress in addition to hypertension itself, which might overwhelm rapidly the potential effects of anti-oxidant treatment. Given that the patient number is limited in the present current study and the multiple, heterogeneous pathogenesis of hypertension, further investigations are indicated to examine the individual effects of combination treatment on different subgroups of hypertensive patients including the smokers and others.
It is not known whether AM and DXM may exert synergistic effects on BP control. It was shown in the present study that the BP-lowering effects of AM monotherapy tended less significant in patients with higher baseline ADMA level compared with those patients with lower baseline ADMA level. Since ADMA is an endogenous inhibitor of endothelial NO synthase, one may speculate that the effects of AM monotherapy might be attenuated by baseline endothelial dysfunction in hypertensive patients. However, although AM, a kind of calcium channel blockers, could reduce BP mainly by its direct vasodilatation effects on vascular smooth muscle cells, the combination of DXM seems to act on endothelial cells and might provide additional benefits on BP control by improving endothelial function. However, the effects of combined treatment were not modified by baseline hs-CRP level and other serum oxidative stress markers such as ox-LDL and TAS. It seems that the antioxidant effect of DXM might be mainly limited to the endothelial cellular level for the modification of intracellular rather than extracellular oxidative stress in vivo. Future in vitro and in vivo studies may be still required to clarify the detailed mechanisms for the BP-lowering effect of DXM, either alone or as the combined treatment.
According to the safety data, the frequent adverse events possibly related to treatment with DXM were mostly mild symptoms, such as dizziness and nausea, whereas peripheral edema might be associated with AM. Drug-related edema and dizziness only occurred in 2 patients each (a total of 7 patients experienced drug-related AEs). Most of the AEs were mild to moderate in severity. All the 8 SAEs reported by 5 subjects (5.4%, 5/92) were not considered related to study drug. Notably, the dose of DXM in the present study was relatively low (?30 mg), as compared with its clinical practice in suppressing cough. The side effects would be less likely to occur in this regimen. The combinational therapy in this study was well tolerated by patients with hypertension. These findings indicated that the addition of DXM displayed a favorable safety profile.
Although the current findings may be interesting, there are some limitations that should be mentioned. First, this study was designed to find out the optimal dose of the novel combination treatment with the short period of each treatment. Future long-term study is required to show the persistent effects of the combination treatment as the chronic treatment. Second, the present study design may be appropriate to find out the additional antihypertensive effects that could be contributed by add-on treatment of DXM. However, the effects of DXM monotherapy were not evaluated. Further study may be indicated to clarify this issue for potential clinical implication. Third, the number of the patients was limited in this study, which was even more limited in subgroup analyses. Thus, the current data are preliminary, which should be validated in future large-scaled study before final clinical implication could be indicated.
In conclusion, the results of this study proved the concept that the add-on of DXM provides additional efficacy over AM treatment alone in clinical hypertension. The combination therapy of AM 5 mg with DXM, especially on low dose of 2.5 mg or 7.5 mg, effectively decreased BP and exhibited a favorable safety profile in patients who had not sufficiently responded to AM 5 mg monotherapy. The patients with impaired endothelial function might respond better to this combination and therefore will be the potential target patients to the novel combination therapy. The findings of the present study may provide a rational to future prospective, multi-arms, double-blind, placebo or active-control clinical trials to confirm the potential clinical implication of DXM as the novel combination treatment with AM for mild-to-moderate hypertensive patients.
Supplementary Material
Acknowledgments
None.
Footnotes
Abbreviations: AM = Amlodipine, DBP = diastolic blood pressure, DXM = Dextromethorphan, SBP = systolic blood pressure.
This work was partially supported by National Yang-Ming University, Taipei, ROC and the funding of TSH Biopharm Corporation Ltd Taipei, Taiwan, ROC, and the UST-UCSD International Center of Excellence in Advanced Bio-engineering sponsored by the Taiwan National Science Council I-RiCE Program (NSC-99–2911-I-009–101).
The authors report no conflicts of interest.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Lancet 1990; 335:827–838. [DOI] [PubMed] [Google Scholar]
- 3.MacMahon S, Rodgers A, Neal B, et al. Blood pressure lowering for the secondary prevention of myocardial infarction and stroke. Hypertension 1997; 29:537–538. [DOI] [PubMed] [Google Scholar]
- 4.Wang JG, Staessen JA, Franklin SS, et al. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension 2005; 45:907–913. [DOI] [PubMed] [Google Scholar]
- 5.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 6.Falaschetti E, Chaudhury M, Mindell J, et al. Continued improvement in hypertension management in England: results from the health survey for England 2006. Hypertension 2009; 53:480–486. [DOI] [PubMed] [Google Scholar]
- 7.Wagner A, Sadoun A, Dallongeville J, et al. High blood pressure prevalence and control in a middle-aged French population and their associated factors: the MONALISA study. J Hypertens 2011; 29:43–50. [DOI] [PubMed] [Google Scholar]
- 8.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010; 303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 9.Tocci G, Rosei EA, Ambrosioni E, et al. Blood pressure control in Italy: analysis of clinical data from 2005-2011 surveys on hypertension. J Hypertens 2012; 30:1065–1074. [DOI] [PubMed] [Google Scholar]
- 10.Banegas JR, Graciani A, de la Cruz-Troca JJ, et al. Achievement of cardiometabolic goals in aware hypertensive patients in Spain: a nationwide population-based study. Hypertension 2012; 60:898–905. [DOI] [PubMed] [Google Scholar]
- 11.Prince MJ, Ebrahim S, Acosta D, et al. Hypertension prevalence, awareness, treatment and control among older people in Latin America, India and China: a 10/66 cross sectional population-based survey. J Hypertens 2012; 30:177–187. [DOI] [PubMed] [Google Scholar]
- 12.Su TC, Bai CH, Chang HY, et al. Evidence for improved control of hypertension in Taiwan: 1993-2002. J Hypertens 2008; 26:600–606. [DOI] [PubMed] [Google Scholar]
- 13.Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension 2007; 49:69–75. [DOI] [PubMed] [Google Scholar]
- 14.Farley TA, Dalal MA, Mostashari F, et al. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med 2010; 38:600–609. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization, I.S., o.H.W., G. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 16.Webster J, Robb OJ, Jeffers TA, et al. Once daily amlodipine in the treatment of mild to moderate hypertension. Br J Clin Pharmacol 1987; 24:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: issues and practical significance. J Clin Hypertens 2003; 5:330–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paravicini TM, Touyz RM. NADPH Oxidases, Reactive Oxygen Species, and Hypertension. Diabetes Care 2008; 31 (Supplement 2):S170–S180. [DOI] [PubMed] [Google Scholar]
- 19.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histo chem Cell Biol 2004; 122:339–352. [DOI] [PubMed] [Google Scholar]
- 20.Welch WJ. Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol 2006; 33:1002–1005. [DOI] [PubMed] [Google Scholar]
- 21.Redon J, Oliva MR, Tormos C, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 2003; 41:1096–1101. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Wang T, Qin L, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. FASEB J 2004; 18:589–591. [DOI] [PubMed] [Google Scholar]
- 23.Tortella FC, Pellicano M, Bowery NG. Dextromethorphan and neuromodulation: old drug coughs up new activities. Trends Pharmacol Sci 1989; 10:501–507. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther 2000; 293:607–617. [PubMed] [Google Scholar]
- 25.Liu Y, Qin L, LI G, et al. Dextromethorphan Protects Dopaminergic Neurons against Inflammation-Mediated Degeneration through Inhibition of Microglial Activation. J Pharmacol Exp Ther 2003; 305:212–218. [DOI] [PubMed] [Google Scholar]
- 26.Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu PY, Lin CC, Tsai WC, et al. Treatment with dextromethorphan improves endothelial function, inflammation and oxidative stress in male heavy smokers. J Thromb Haemost 2008; 6:1685–1692. [DOI] [PubMed] [Google Scholar]
- 28.Wu TC, Chao CY, Lin SJ, et al. Low-dose dextromethorphan, a NADPH oxidase inhibitor, reduce blood pressure and enhances vascular protection in experimental hypertension. PLoS ONE 2012; 7:e46067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ALLHAT Officers, Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic - the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 30.Zafari AM, Ushio-Fukai M, Akers M, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II–induced vascular hypertrophy. Hypertension 1998; 32:488–495. [DOI] [PubMed] [Google Scholar]
- 31.Ushio-Fukai M, Zafari AM, Fukui T, et al. p22 phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 1996; 271:23317–23321. [DOI] [PubMed] [Google Scholar]
- 32.Grunfeld S, Hamilton CA, Mesaros S, et al. Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension 1995; 377:854–857. [DOI] [PubMed] [Google Scholar]
- 33.Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 1990; 87:1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschudi M, Mesaros S, Lüscher TF, et al. Direct in situ measurement of nitric oxide in mesenteric arteries. Hypertension 1996; 27:32–35. [DOI] [PubMed] [Google Scholar]
- 35.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membranepermeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 1998; 32:59–64. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 2007; 112:375–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


