Supplemental Digital Content is available in the text
Keywords: critical care, mortality, prognosis, red cell distribution width, risk assessment, sepsis
Abstract
Sepsis is a common condition in the emergency department that is associated with high mortality. Red blood cell distribution width (RDW) has been used as a simple prognosis predictor for patients with community-acquired pneumonia, gram-negative bacteremia, and severe sepsis or septic shock. To evaluate the performance of RDW to predict in-hospital mortality among septic patients, we conducted a hospital-based retrospective cohort study in an emergency department of a tertiary teaching hospital. RDW was compared with other commonly used clinical prediction scores (Systemic Inflammatory Response Syndrome (SIRS), Mortality in Emergency Department Sepsis (MEDS) and the Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older (CURB65)). Of 6973 consecutive adult patients with a clinical diagnosis of sepsis and 2 sets of blood culture ordered by physicians, 477 (6.8%) died. The mortality group had higher RDW levels than the survival group (15.7% vs 13.8%). After dividing RDW into quartiles, the patients in the highest RDW quartile (RDW >15.6%; mortality, 16.7%) had more than twice the risk of in-hospital mortality compared with patients in the second highest quartile (RDW >14% and <15.6%; mortality, 7.3%), whereas the mortality rate in the lowest RDW quartile (<13.1%) was only 1.6%. The area under the receiver operating characteristic curve of RDW to predict mortality was 0.75 (95% confidence interval, 0.72–0.77), which is significantly higher than the areas under the curve of clinical prediction rules (SIRS, MEDS, and CURB65). After integrating RDW into these scores, all scores performed better in predicting mortality (0.73, 0.72, and 0.77, for SIRS, MEDS, and CURB65, respectively). RDW could be an independent predictor of mortality among septic patients. Clinicians could classify the septic patients into different risk groups according to RDW quartiles. For more accurate mortality prediction, RDW could be a potential parameter to be incorporated into clinical prediction rules.
1. Introduction
Sepsis is a common condition in the emergency department (ED) that is associated with high mortality. It is important to stratify patients at high risk of morbidity or mortality. Hence, several clinical prediction rules were developed for mortality prediction, which calculated multiple factors to formulate the risk scores. The well-known clinical prediction scores for sepsis include the Systemic Inflammatory Response Syndrome (SIRS) criteria,[1] the Mortality in Emergency Department Sepsis (MEDS) score,[2] and the Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older (CURB-65) score (supplementary Table 1–3).[3,4] However, utilizing these scores to predict mortality for septic patients in clinical settings requires complicated calculations and results in suboptimal performance.
Red blood cell distribution width (RDW) is routinely obtained from ED patients as part of the complete blood count report. It is calculated as the percentage of the standard deviation of red blood cell volume to the mean corpuscular volume, which reflects the variation of the red blood cell volume. A higher RDW level indicates higher variation of red blood cell size, which is also associated with anisocytosis in anemic patients. Recently, RDW was reported as an independent predictor of mortality among critically ill patients and patients with community-acquired pneumonia, gram-negative bacteremia, and severe sepsis or septic shock.[5–8] However, there is no direct performance comparison between common scoring systems or biomarkers among septic patients. In this retrospective cohort study, we attempted to evaluate the performance of this biomarker in comparison with other clinical prediction rules such as SIRS, MEDS, CURB-65 for predicting mortality among ED patients with sepsis.
2. Methods
2.1. Study design
The study was approved by the Chang Gung Memorial Hospital Institutional Research Board (102-2385C), and a requirement for informed consent was waived. This retrospective cohort study was conducted in the ED of a 3700-bed tertiary teaching hospital with approximately 180,000 ED visits annually. All consecutive adult (aged older than 18 years) septic patients who visited the ED during 2010 with 2 sets of blood cultures ordered by physicians and with a clinical diagnosis of sepsis as indicated by diagnosis codes were included in this study. Patients transferred from other wards, intensive care units (ICUs), or the EDs of local hospitals were excluded because the management (such as blood transfusions) in local hospitals could interfere with the RDW results.
2.2. Data collection
All the data were retrieved from electronic medical records. Variables were defined before data collection and entered in a standardized format during the data collection. Structured Query Language was utilized by trained research coordinators with predefined data collection forms to retrieve individual electronic medical records, which were stored in Microsoft Access (Microsoft, Redmond, WA) for subsequent chart review and data management. We double-checked the results of the electronic chart review by different program codes as well as by manual chart review. The basic demographic data, vital signs on ED arrival, symptoms and signs, underlying diseases, laboratory findings, microbiological results, and the final discharge status were collected. The data abstractors were blinded to the study objectives and hypothesis.
2.3. Laboratory measurements
RDW was an integral part of the automated complete blood count analysis and available on our Sysmex XE-2100 Automated Hematology System analyzer (Sysmex Corporation, Kobe, Japan). The normal laboratory range of RDW in our institution is 11.5% to 14.5%. C-reactive protein (CRP) levels were measured using a highly sensitive turbidimetric immunoassay with a monoclonal antibody to CRP coated on polystyrene beads with a lower limit of detection of 0.2 mg/L (Synchron CX Systems; Beckman Coulter, Brea, CA). The normal range of CRP is less than 5 mg/dL. Lactate was measured by an enzymatic method (lactate reagent; Beckman Coulter). The normal range of lactate is 4.5 to 19.8 mg/dL. Procalcitonin (PCT) was measured by the enzyme-linked immunosorbent assay (VIDAS B.R.A.H.M.S. PCT; BioMerieux, Durham, UK). A PCT value less than 0.5, between 0.5 and 2, between 2 and 10, and more 10 ng/mL indicates low, uncertain, and high risk of sepsis and probable sepsis/septic shock, respectively.
2.4. Outcome measurements
Our primary outcome was the performance of RDW in predicting in-hospital mortality among septic patients. We then compared performances between RDW, CRP, PCT, lactate, and clinical prediction scores such as SIRS, MEDS, and CURB-65 alone and incorporated with RDW.
2.5. Statistical analysis
Descriptive statistics of demographic and laboratory variables are reported as medians, interquartile ranges, numbers, and percentages. The χ2 test and 2-sample t test were used to test differences in mortality and survival between groups. The patients were further stratified a priori according to normal laboratory ranges and the quartile distribution of RDW results: low, <13.1%; moderate, >13.1% and <14%; high, >14% and <15.6%; and very high, >15.6%. The Cochran–Armitage test was used to test the significance of trend across RDW quartiles. Bivariate Logistic regression was utilized to evaluate the potential confounding between RDW, risk factors, and mortality. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate performance in predicting in-hospital mortality. Multivariate ROC curve regression analysis was performed to identify and adjust potential confounding between the performance of RDW, area under the ROC curve (AUC), and other risk factors of in-hospital mortality.[9] The correlated AUC was compared among RDW, biomarkers, and clinical prediction scores using partial paired ROC analysis. Statistical analyses were performed using the Stata (StataCorp, College Station, TX) package and ROCKIT (University of Chicago Medical Center, Chicago, IL). All P values <0.05 from 2-sided tests were considered statistically significant.
2.6. Subgroup analysis
Subgroup analysis was conducted a priori for patients with severe sepsis to evaluate the performance of RDW. We considered severe sepsis, according to its definition in the Surviving Sepsis Campaign as “sepsis-induced tissue hypoperfusion or organ dysfunction,” to include any of the following: sepsis-induced hypotension, lactate levels above normal laboratory upper limits, urine output <0.5 mL/kg/h for more than 2 hours despite adequate fluid resuscitation, acute lung injury with a ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) <250 in the absence of pneumonia as infection source, acute lung injury with a PaO2/FiO2 <200 in the presence of pneumonia as infection source, creatinine level >2.0 mg/dL (176.8 μmol/L), bilirubin level >2 mg/dL (34.2 μmol/L), platelet count <100,000/μL, and coagulopathy (international normalized ratio >1.5).[10] We also evaluate the performance of RDW in the subgroup of patients with malignancy.
3. Results
In total, 11,899 patients visited our ED with suspected sepsis as indicated by 2 sets of blood cultures ordered by emergency physicians during the study period. After excluding 2342 patients without probable or documented infection focus and 2546 patients transferred from other hospitals or with duplicated visits, 7011 patients were included. Because 38 patients lacked an initial RDW value (0.5%), we finally included 6973 patients in the analysis (Fig. 1).
Figure 1.
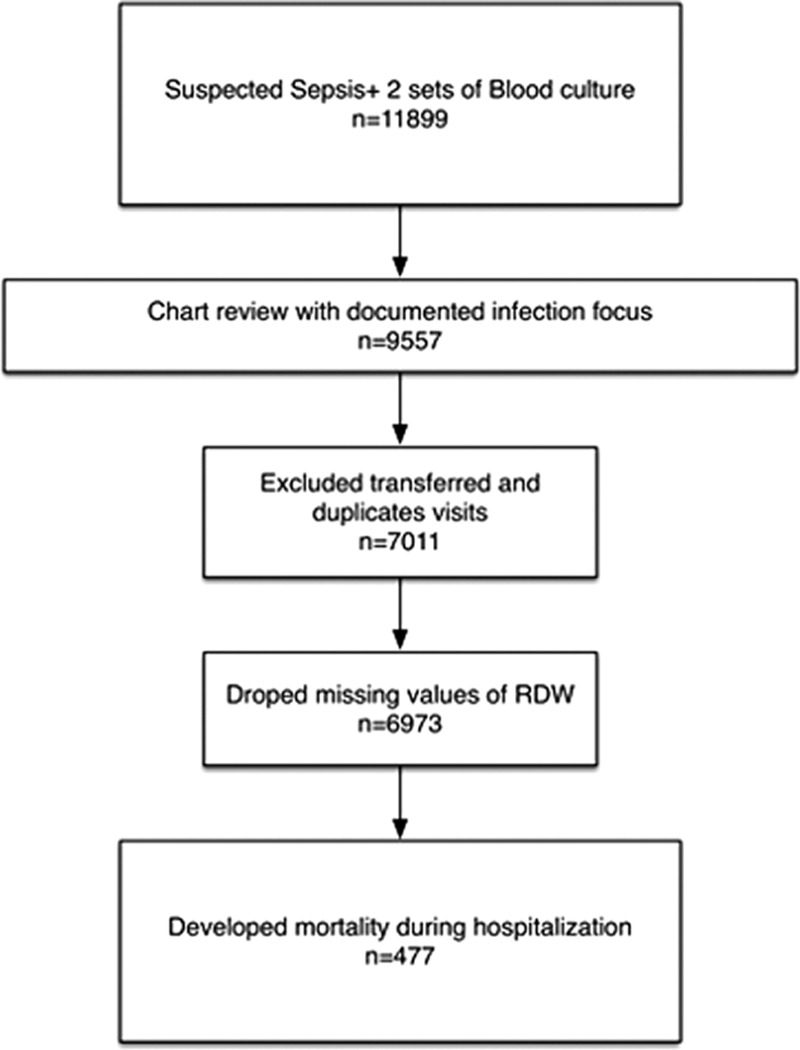
Flow chart of subject inclusion.
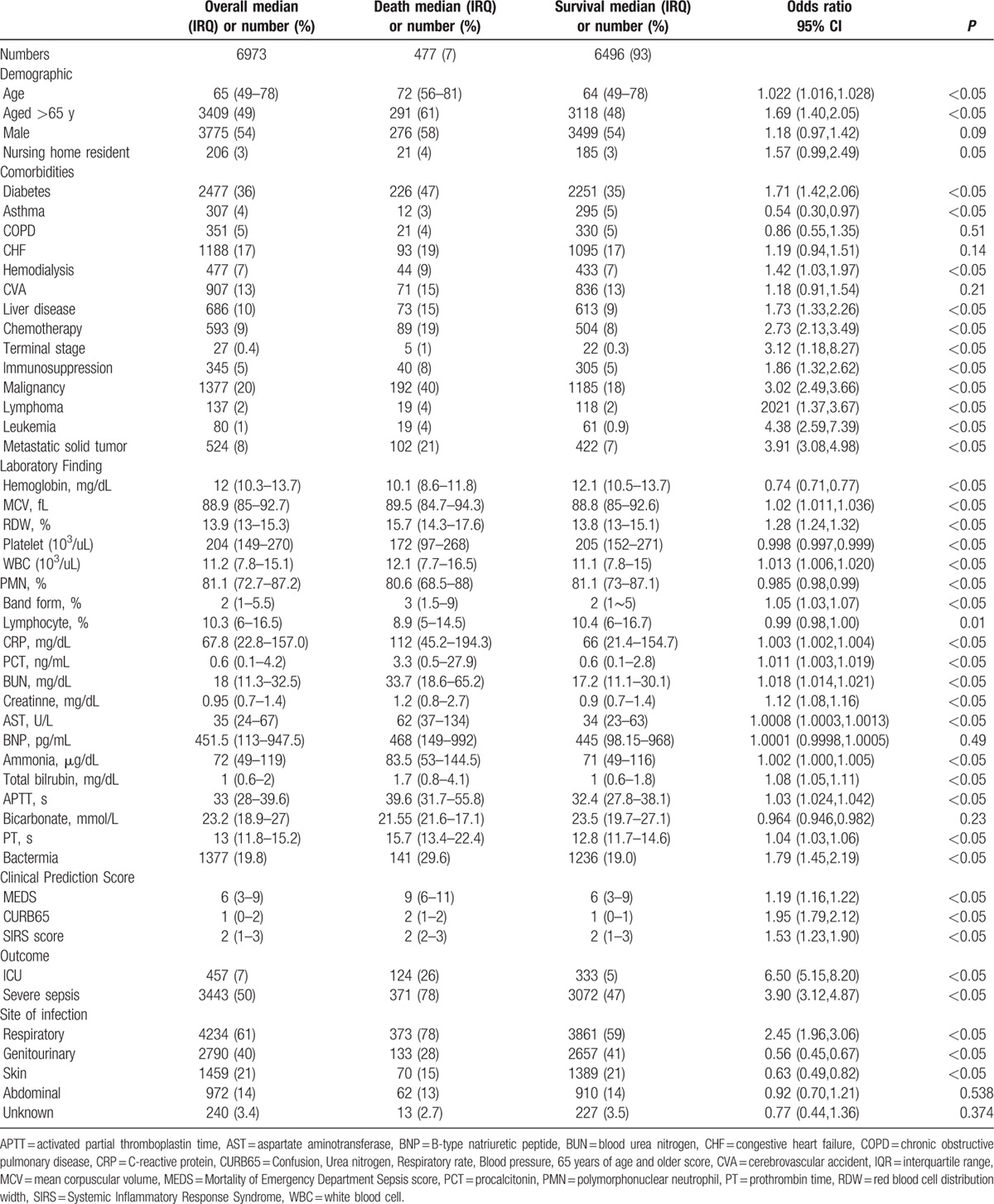
Among those included patients, 477 (6.8%) died during hospitalization (Table 1). Almost half of the patients were over 65 years of age (49%) and male (54%). The common underlying diseases were diabetes mellitus (36%), malignancy (20%), congestive heart failure (17%), cerebral vascular accident (13%), chronic liver disease (10%), and chronic kidney disease (7%). The patients who died were more likely to be elderly (age >65 years); to have diabetes mellitus, liver disease, malignancy, or immunosuppressive status; or to be under regular hemodialysis or chemotherapy (all P < 0.05). Regarding the laboratory data, a higher RDW percentage (15.7% vs 13.8%), higher white blood cell count, bandemia, increased prothrombin time and activated partial thromboplastin time, and higher levels of blood urea nitrogen (BUN), creatinine, aspartate aminotransferase, ammonia, total bilirubin, but lower hemoglobin levels and platelet counts, were associated with higher mortality (all P <0.05). Furthermore, patients who died also had higher CRP (112 vs 66 mg/dL) and procalcitonin (3.3 vs 0.6 ng/dL) levels. The most common site of infection which caused the episode of sepsis was respiratory tract infection, which was also associated with in-hospital mortality (OR 2.45, 95% CI 1.96–3.06). Not surprisingly, positive blood culture results were also associated with mortality (29.6% vs 19.0%, all P <0.05).
Table 1.
Demographic, laboratory finding, clinical prediction scores, outcome and site of infection.
To illustrate the capacity to discriminate the risk of mortality stratified by RDW quartile, we plotted mortality rates by RDW quartile (Fig. 2). For patients in the very high RDW quartile, the mortality rate was 16.7%, twice that of patients in the high quartile (7.3%, supplementary Table 4). On the contrary, the mortality rate of patients in the lowest RDW quartile was only 1.6% (diagnostic odds ratio: 5.69, 95% CI: 3.81–8.84, sensitivity = 93.7%, 95% CI: 91.2–95.6%). Using 12% as a cutoff of RDW, the sensitivity in predicting mortality would be 99.4% (negative likelihood ratio: 0.30). On the other hand, the specificity in predicting mortality would be 89.9% if 17% used as the cutoff of RDW (positive likelihood ratio: 3.16). Patients with liver disease, malignancy, or immunocompromised status were more likely to have a wider RDW distribution (all P values for trend <0.05). Patients with a wider RDW tended to have lower hemoglobin, platelet, and albumin and higher BUN, creatinine, total bilirubin, and ammonia levels (all P values for trend <0.05). Patients with wider RDW tended to have higher mortality as well as higher ICU admission rates (all P values for trend <0.05). Interestingly, we also observed more severely septic patients in the wider RDW groups (all P values for trend <0.05).
Figure 2.
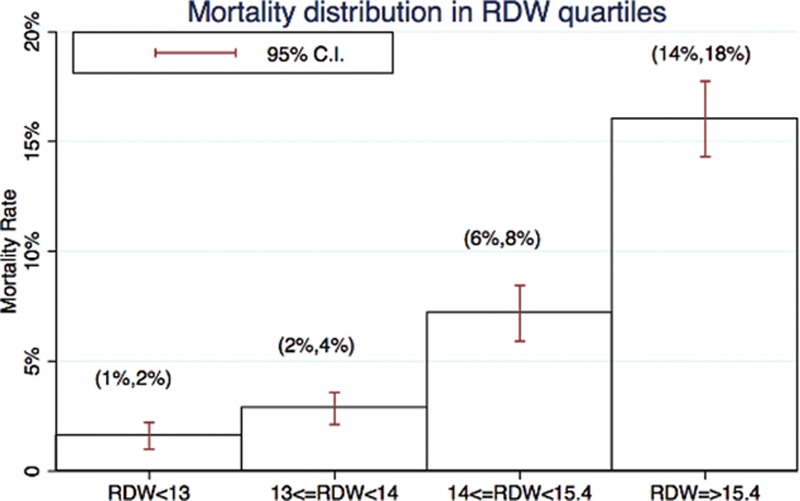
Mortality distribution in RDW quartiles. CI = confidence interval, RDW = red blood cell distribution width.
Fig. 3 illustrates the ROC curves obtained from RDW, different clinical prediction rules, and biomarkers in predicting in-hospital mortality for septic patients. The AUC of RDW to predict mortality was 0.75 (95% confidence interval, 0.72–0.77), which is significantly higher than the AUCs of clinical prediction rules such as SIRS, MEDS, and CURB-65 and common biomarkers utilized clinically such as CRP, PCT, and lactate (Fig. 3A, all P <0.001). After adjusting for possible confounders including age, diabetes, cerebral vascular accident, chronic kidney disease, status of hemodialysis and chemotherapy, hemoglobin level, mean corpuscular volume, white blood cell count, liver disease, immunocompromise, and ICU admission the AUC of RDW was still superior to other scores such as SIRS, MEDS, and CURB-65 (0.74 [95% confidence interval, 0.72–0.76] vs 0.45 [0.43–0.48], 0.60 [0.57–0.63], and 0.55 [0.53–0.59], respectively; all P <0.001; Table 2), and the adjusted Diagnostic Odds Ratio of mortality between the highest and lowest RDW quartiles was 4.94 (95% CI: 3.24–7.54). When we integrated the RDW quartile into these prediction scores as a parameter, all prediction scores performed better, as shown in Fig. 3C (0.73, 0.72, and 0.77 for SIRS, MEDS, and CURB-65, respectively).
Figure 3.
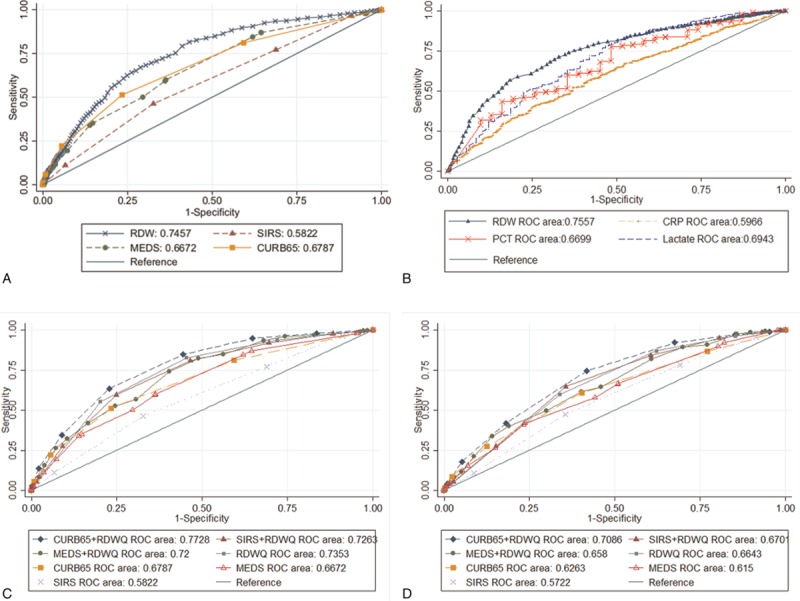
Receiver operating characteristic (ROC) curves. Comparison of the performance of RDW as a continuous variable in predicting mortality with clinical prediction rules (A) and common biomarkers utilized clinically (B). ROC curves of RDW as a quartile indicator with and without clinical prediction rules (C) and in the severely septic group (D). CRP = C-reactive protein, CURB65 = Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older score, MEDS = Mortality of Emergency Department Sepsis score, PCT = procalcitonin, RDW = red blood cell distribution width, RDWQ = RDW in quartile form, SIRS = Systemic Inflammatory Response Syndrome.
Table 2.
Performance comparison between RDW alone, clinical prediction rules alone, and RDW plus clinical prediction rules.
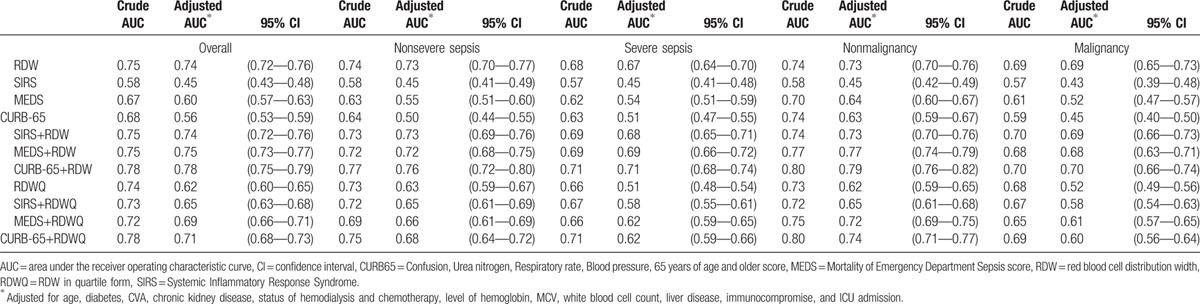
We further divided our patients into 2 subgroups of severe sepsis and nonsevere sepsis. Among the severely septic patients (n = 2110), 13.8% died (n = 290), which is significantly higher compared with the nonsevere septic group (3.8%, n = 187, P < 0.001). We simplified the utilization of RDW as a binary biomarker using 14% as a cutoff value. For patients in the severe and nonsevere septic groups, the mortality rates of the higher RDW group (18.5% and 7.6%, respectively) were significantly higher than the rates for patients in the lower RDW groups (5.6% and 1.5%, respectively; P < 0.001). All clinical prediction scores performed better in mortality prediction among nonsevere septic patients (supplementary Table 1). Furthermore, CURB65 plus RDW also performed better than the others in the nonsevere septic patients (0.69, 0.70, and 0.71, respectively; Table 2). Interestingly, RDW and all the clinical prediction rules performed better among patients without malignancy (Table 2).
4. Discussion
In this retrospective cohort study, we illustrated the significant differences in RDW levels between mortality and survival groups of septic patients. The performance of RDW to predict mortality for patients with sepsis was found to be better than other currently utilized prediction rules such as SIRS, MEDS, and CURB-65. Furthermore, the RDW quartile was positively associated with in-hospital mortality of patients with sepsis. Patients who had the lowest quartile of RDW (<13.1%) had only a 1.6% chance to develop in-hospital mortality. Utilizing this simple and readily available biomarker, RDW, we could predict mortality more accurately than we could with other complicated or more expensive indicators.
RDW has been utilized in diverse diseases other than traditionally for interpretation of anemia. In chronic diseases, elevated RDW was associated with increased mortality among healthy middle-aged and older adults from the general population and patients with cardiovascular disease, stroke, heart failure, and chronic dialysis.[11–18] In acute conditions, RDW can also be used as a mortality predictor among patients with acute pancreatitis, acute dyspnea during an ED visit, out-of-hospital cardiac arrest, and critical illnesses in ICU setting.[5,19–21] For septic patients, RDW was also found to be an independent indicator of mortality in patients with gram-negative bacteremia, community-acquired pneumonia, severe sepsis, and septic shock.[6,7,22]
Although the mechanism of the association between RDW and mortality in septic patients is not well understood, some studies suggested a possible causal pathway. When patients are infected, the microbes and the released lipopolysaccharides could subsequently lead to a catastrophic inflammatory cascade. Inflammatory cytokines such as tumor necrosis factor α, interleukin-1, interleukin-6, interleukin-10, and interferon-γ could subsequently induce direct red blood cell damage by erythrophagocytosis or apoptosis, interfere with iron homeostasis by cytokines or by hepcidin (an acute-phase protein), inhibit erythropoiesis by myelosuppression, and down-regulate erythropoietin-receptor expression.[23] Emans et al,[20] Fornal et al,[23] and Ferruci et al[22] confirmed these hypotheses in studies that found an association between inflammatory markers and erythropoietin activity It has also been shown that elevated inflammatory cytokines are associated with all-cause mortality and early hemodynamic deterioration among severely septic patients.[24,25] This could possibly explain why anisocytosis and elevated RDW in a proinflammatory status was associated with higher mortality.[5]
From our study, higher white blood cell counts, bandemia, increased prothrombin time and activated partial thromboplastin time, and higher BUN, creatinine, aspartate aminotransferase, ammonia, and total bilirubin levels and lower hemoglobin levels and platelet counts were associated with mortality. Not surprisingly, indicators of organ dysfunction that are commonly seen in severe sepsis and septic shock were associated with mortality. These variables could also be associated with chronic illnesses that could contribute to the higher mortality.
From the subgroup analysis, we found that RDW is a good biomarker not only in mortality prediction but also in detailed risk stratification. The performance of RDW in predicting mortality was better for patients who had not yet developed severe sepsis. We hypothesized that RDW could be an indicator of hematologic organ failure in the early stage of sepsis progression, during which the bone marrow is still responsive to the coagulation cascade and producing red blood cells and subsequently resulting in increased RDW. We believe that early sepsis could be a good niche for the clinical utility of RDW; however, clinicians need to be aware of the possible limitation of the utility for severely septic patients. Furthermore, RDW performed better for patients who did not have history of malignancy. Patients with either hematopoietic malignancy or chemotherapy that could impair the process of hematopoiesis may not express wider RDW when encountering sepsis.
In this study, we also demonstrated that integration of this simple biomarker, RDW, into other existing clinical prediction rules, such as SIRS, MEDS, or CURB-65, could increase the performance of these rules easily. For healthcare providers who have limited resources, simply using mental status, respiratory rate, BUN level, age, and RDW could yield potential benefits to their daily care of septic patients. Using RDW along or with other existing clinical prediction rules, clinicians could stratify patients with lowest risk to develop mortality and plan an ordinary ward or even outpatient treatment accordingly.
There are several limitations in our study. First, this is a retrospective cohort study. All of the laboratory data were collected retrospectively. Hence, there are some missing data and potential bias. This could interfere with our result, especially in the subgroup analysis in which we compare the performance of RDW and clinical prediction rules between severe and nonsevere septic patients. However, utilizing partial paired ROC analysis, this bias could be minimized. Second, we could not know if the patients received a blood transfusion before the index ED visits, but we did exclude patients who were transferred from other hospitals to reduce this potential bias. Third, we did not have baseline hemoglobin levels from all patients visiting our ED, since for some patients, the ED visit may have been their first visit at this institution. Nevertheless, we aimed to focus on the acute manifestation of the size of red blood cells, the RDW value, and their associations with mortality. Therefore, chronic anemia may not have biased our observations. Forth, all of the patients and data were collected in a single center. The severity of disease, the patient characteristics, and the value of RDW could be different at other institutions. Fifth, the operational definition of sepsis we utilized in this study, that is, 2 sets of blood cultures ordered by physicians and with a clinical diagnosis of sepsis, could be different in other institutions. Further studies are merited to validate our results in other target populations. Lastly, this single-center study excluded patients with repeated ED visits or transferred from other hospitals; therefore, we caution the reader to apply the results to patients who are still in the early stage of sepsis. Further validation from another population is merited.
In conclusion, we found patients over 65 years of age, with comorbidities such as liver disease, malignancy, immunosuppressive status, and undergoing regular dialysis or chemotherapy, tended to have a higher risk of mortality after experiencing sepsis. Furthermore, RDW is an independent predictor of mortality among septic patients. It is an easy and inexpensive test to risk stratify septic patients in the ED. Simply using RDW quartiles, clinicians could stratify patients according to risk of mortality and treat them accordingly. For more accurate mortality prediction, RDW could be a potential parameter used along with the prediction rules, such as CURB-65 plus RDW in our study.
Acknowledgments
The authors thank Mr Bin-Zhe Hong for the assistance of statistics.
Supplementary Material
Footnotes
Abbreviations: AUC = area under the ROC curve, CRP = C-reactive protein, CURB-65 = Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older, ED = emergency department, ICU = intensive care units, MEDS = Mortality in Emergency Department Sepsis, PCT = Procalcitonin, RDW = red blood cell distribution width, ROC = receiver operating characteristic, SIRS = Systemic Inflammatory Response Syndrome.
This study was supported by the National Science Council and Chang Gung Memorial Hospital in Taiwan (100–2314-B-182A-038-MY3, CMRPG2C0201, CMRPG2B0121, CMRPG2B0271, and CMRPG2B0371). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
References
- [1].Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629–38. [DOI] [PubMed] [Google Scholar]
- [2].Shapiro NI, Wolfe RE, Moore RB, et al. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med 2003;31:670–5. [DOI] [PubMed] [Google Scholar]
- [3].Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marwick CA, Guthrie B, Pringle JE, et al. Identifying which septic patients have increased mortality risk using severity scores: a cohort study. BMC Anesthesiol 2014;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bazick HS, Chang D, Mahadevappa K, et al. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med 2011;39:1913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ku NS, Kim HW, Oh HJ, et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock 2012;38:123–7. [DOI] [PubMed] [Google Scholar]
- [7].Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013;31:72–9. [DOI] [PubMed] [Google Scholar]
- [8].Jo YH, Kim K, Lee JH, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med 2013;31:545–8. [DOI] [PubMed] [Google Scholar]
- [9].Pepe MS. Three approaches to regression analysis of receiver operating characteristic curves for continuous test results. Biometrics 1998;54:124–35. [PubMed] [Google Scholar]
- [10].Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- [11].Sicaja M, Pehar M, Derek L, et al. Red blood cell distribution width as a prognostic marker of mortality in patients on chronic dialysis: a single center, prospective longitudinal study. Croat Med J 2013;54:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Uyarel H, Isik T, Ayhan E, Ergelen M. Red cell distrubition width (RDW): a novel risk factor for cardiovascular disease. Int J Cardiol 2012;154:351–2. [DOI] [PubMed] [Google Scholar]
- [13].Martínez-Velilla N, Ibáñez B, Cambra K, Alonso-Renedo J. Red blood cell distribution width, multimorbidity, and the risk of death in hospitalized older patients. Age 2011;34:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Perlstein TS. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Internal Med 2009;169:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci 2009;65A:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Patel KV. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med 2009;169:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci 2009;277:103–8. [DOI] [PubMed] [Google Scholar]
- [18].Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure. J Am Coll Cardiol 2007;50:40–7. [DOI] [PubMed] [Google Scholar]
- [19].Senol K, Saylam B, Kocaay F, Tez M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med 2013;31:687–9. [DOI] [PubMed] [Google Scholar]
- [20].Kim J, Kim K, Lee JH, et al. Red blood cell distribution width as an independent predictor of all-cause mortality in out of hospital cardiac arrest. Resuscitation 2012;83:1248–52. [DOI] [PubMed] [Google Scholar]
- [21].Hong N, Oh J, Kang SM, et al. Red blood cell distribution width predicts early mortality in patients with acute dyspnea. Clin Chim Acta 2012;413:992–7. [DOI] [PubMed] [Google Scholar]
- [22].Jo YH, Kim K, Lee JH, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med 2013;31:545–8. [DOI] [PubMed] [Google Scholar]
- [23].Guenter Weiss MD, Lawrence T, Goodnough MD. Anemia of chronic disease. N Engl J Med 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- [24].Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181:176–80. [DOI] [PubMed] [Google Scholar]
- [25].Oberholzer A, Souza SM, Tschoeke SK, et al. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock 2005;23:488–93. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


