Abstract
The number of dissected lymph nodes (LNs), surgical outcomes, and postoperative recurrence-free survival (RFS) were compared between thoracic duct (TD)-preserved and TD-resected groups. The distribution of metastasis in LNs around TD (TDLN) was reviewed. Transthoracic esophagectomy (TTE) with TD resection for esophageal cancer patients has been one of the standard procedures. Because the adipose tissue surrounding the TD contains LNs, TD resection might be necessary for radical LN dissection. However, few studies have investigated the oncological outcome of TTE with TD resection. Two hundred fifty-six consecutive patients who underwent TTE between 2004 and 2015 were retrospectively reviewed and classified into TD-preserved or TD-resected groups. The number of dissected LNs for each LN station and surgical outcomes were compared. RFS was analyzed in 155 patients who underwent TTE before December 2012. Since 2013, the TDLN number was prospectively examined, independent of the regional LNs (n = 72). Of these, the TDLN number for each location (TDLN-Ut/Mt/Lt) was investigated and the correlation between TDLN metastasis and clinicopathological factors was analyzed. The TD was preserved in 89 patients and resected in 167 patients. Patients with TD resection showed significant advanced stage. There was no significant difference in the incidence of postoperative complications, including pneumonia, anastomotic leakage, and chylothorax. The number of dissected mediastinal LNs was significantly increased in the TD-resected group. The 5-year RFS rate of cStage I patients was 67.3% in the TD-preserved group against 90.3% in the TD-resected group, showing a tendency towards RFS extension that did not quite reach statistical significance (P = 0.055). The mean TDLN-Ut/Mt/Lt numbers were 0.89/0.56/0.44, respectively. Eight of 72 (11%) patients displayed TDLN metastasis. Metastatic TDLNs were observed on the same or cranial level of the primary lesion in 7 of 8 patients. Transthoracic esophagectomy with TD resection could increase the number of dissected mediastinal LNs without increase of postoperative complication. TDLN metastasis was observed in patients with advanced disease. A prospective trial, investigating the survival between TD-preserved and TD-resected groups, should be conducted to clarify if TD should be resected in TTE.
Keywords: esophageal cancer, lymph node dissection, thoracic duct resection
1. Introduction
Transthoracic esophagectomy (TTE) with lymph node (LN) dissection has been recognized as one of the standard treatments for esophageal cancer, especially in esophageal squamous cell carcinoma (ESCC), where LN metastasis spreads from the cervical to abdominal nodes. Therefore, 3-field LN dissection is recognized as a standard treatment in Japan.[1,2] On the other hand, the number of dissected LNs was previously shown to be a prognostic factor.[3,4] Therefore, radical LN dissection, which could increase the number of dissected LNs, was recommended.
The thoracic duct (TD) is the main lymphatic root, which originates from the cistern of chyle and ascends along the thoracic descending aorta, flowing at a left venous angle. Previously, Udagawa et al showed that the mediastinal LNs along TD (TDLN) are the nodes in the adipose tissue surrounding TD and running between the thoracic esophagus and the descending aorta. They indicated that TD resection with dissection of TDLN should be performed routinely.[5] Furthermore, TD ligation was shown to reduce postoperative chylothorax.[6,7] In contrast, TD ligation was reported to induce retroperitoneal fluid retention and lead to intravenous volume loss after surgery.[8,9] In terms of hepatic damage, Guler et al demonstrated that TD ligation had a negative effect on the liver in a canine model of peritonitis, which was induced by the amount of endotoxin to which the liver was exposed.[10] As shown above, although there are controversies in TD resection in TTE, to date, there has been no study to investigate the oncological outcomes of TTE with TD resection.
In this study, we hypothesized that TD resection may increase the number of dissected mediastinal LNs in TTE to clarify the oncological benefit of TD resection in ESCC patients. ESCC patients who underwent TTE with TD resection were reviewed. The number of dissected LNs, surgical outcomes, postoperative complications, and recurrence-free survival (RFS) were compared between TD-preserved and TD-resected groups. Furthermore, recently, TDLNs were examined independent of the regional LNs in our institution. Therefore, we investigated the detection rate of TDLN and reviewed the distribution of TDLN metastasis in this study.
2. Methods
2.1. Patients: TTE with or without TD resection
Two hundred fifty nine patients who were histologically diagnosed with ESCC and underwent TTE with extended LN dissection, which included LNs with bilateral recurrent nerve LNs at our institution between January 2004 and July 2015, were retrospectively reviewed. In our institution, TD-preserved TTE was conducted until 2008. Since 2009, the standard surgical procedure was changed to TTE with TD resection to achieve further radical LN dissection. However, because TD resection might influence postoperative fluid retention and liver function, TD was preserved in patients with liver cirrhosis, kidney disease, or heart disease. In this study, based on TD resection status, all patients were classified into TD-preserved or TD-resected groups. Patient characteristics, clinicopathological factors, and surgical procedure were recorded. To determine clinical stages, all patients were assessed using esophagoduodenoscopy, esophagography, and computed tomography before treatment. This study was conducted with the approval of the ethics committee of Keio University School of Medicine. The extent of tumor spread was evaluated using the seventh edition of the TNM classification, which was established by the Union for International Cancer Control.
2.2. Surgical procedure, TD resection, and LN station numbers
As a curative surgery, TTE with right thoracotomy and gastric tube reconstruction in the posterior mediastinal or presternal route was performed as a standard surgical procedure at our institution. When gastric tube could not be used because of synchronous double cancer of the stomach or previous history of gastrectomy, reconstruction using the terminal ileum and right colon was performed. Regarding LN dissection, mediastinal LNs with bilateral recurrent nerve LNs and abdominal LNs, including pericardial LNs, and LNs along the lesser curvature and the left gastric artery were routinely dissected. If the primary tumor was located between the upper and mid-thoracic esophagus, supraclavicular LNs were also dissected. In the TD-resected group, when LN around the left recurrent nerve was dissected from the aortic arch to the cervical level, TD and surrounding adipose tissue containing TDLNs was dissected en bloc together. TD was clipped and divided at the level of the thoracic inlet. In the middle and lower mediastinal approach, after dividing the esophagus and para-esophageal LNs, TD and the surrounding adipose tissue were divided from the descending aorta with posterior mediastinal LNs. TD was then clipped behind the lower thoracic esophagus and resected above the diaphragm. According to the Japanese Classification of Esophageal Cancer, 10th Edition of the Japan Esophageal Society,[11] the LN station numbers were described as follows: 105, upper thoracic para-esophageal LN; 106recL, left recurrent laryngeal nerve LN; 106recR, right recurrent laryngeal nerve LN; 107, subcarinal LN; 108, middle thoracic para-esophageal LN; 109, main bronchus LN; 110, lower thoracic para-esophageal LN; 111, supradiaphragmatic LN; and 112, posterior mediastinal LN. In this study, based on the location in the mediastinum, TDLNs were classified into the following 3 parts: TDLN-Ut, TDLN in the upper mediastinum, which is dissected with 106recL; TDLN-Mt, TDLN in the middle mediastinum, which is dissected with 112; TDLN-Lt, and TDLN in the lower mediastinum, which is dissected with 112. In our study, TDLN-Ut was added to 106recL and TDLN-Mt and TDLN-Lt were added to 112 (Fig. 1).
Figure 1.
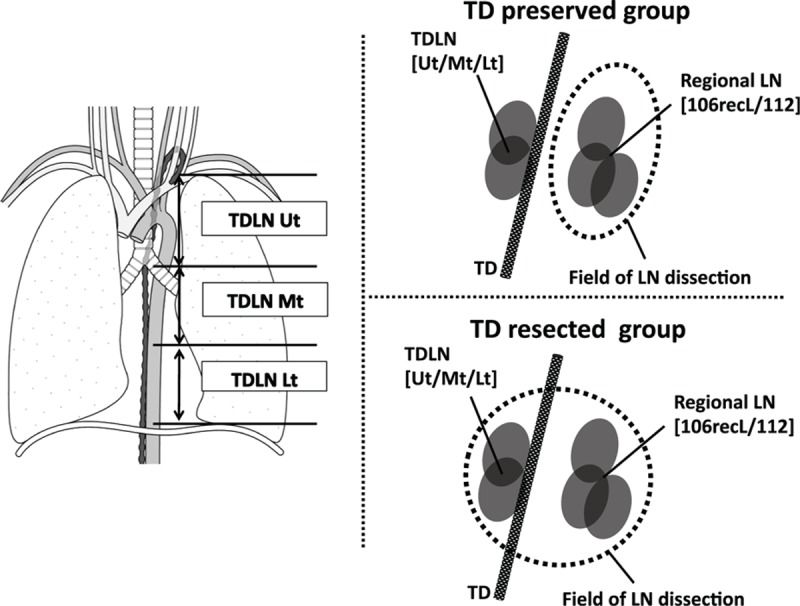
Definition of lymph nodes around the thoracic duct. 106recL = left recurrent laryngeal nerve LN, 112 = posterior mediastinal LN, LN = lymph node, TD = thoracic duct, TDLN-Ut = LN around TD in the upper mediastinum, which is dissected with 106recL, TDLN-Mt and LN around TD in the middle mediastinum, which is dissected with 112; TDLN-Lt and LN around TD in the lower mediastinum, which is dissected with 112.
2.3. Perioperative treatment, postoperative complications
On the basis of JCOG 9907 study, neoadjuvant chemotherapy (NAC), a combination of cisplatin and 5-fluorouracil (FP) twice every 3 weeks,[12] has been standard treatment in our institution since 2007. A regimen consisting of 3 drugs—cisplatin, 5-fluorouracil, and docetaxel (DCF)—administered 3 times every 3 weeks has become a treatment option.[13] As a neoadjuvant chemoradiotherapy, concurrent radiation of 46 Gy was added to the FP therapy. Postoperative complications were evaluated using Claviend–Dindo (C–D) classification. In this study, pneumonia (C–D >grade II), anastomotic leakage (C–D >grade IIIa), chylothorax (C–D >grade III), and postoperative mortality 30 days after esophagectomy were investigated.
2.4. Comparative analysis between TD-preserved and TD-resected groups: surgical outcomes, number of dissected LNs, and RFS
Surgical outcome, operation time, and blood loss were compared between TD-preserved and TD-resected groups. Postoperative complications were evaluated using C–D classification. Incidence of pneumonia (C–D ≥grade II), anastomotic leakage (C–D ≥grade IIIa), chylothorax (C–D ≥grade IIIa), and postoperative mortality were investigated. Number of dissected LNs for each LN station was compared between groups. Furthermore, number of dissected LNs was analyzed under the stratification based on clinical and pathological LN metastasis. RFS was analyzed in 155 patients who underwent TTE before December 31, 2012. The RFS was calculated from the date of surgery to the date of recurrence. Patients were followed up until death or December 31, 2015.
2.5. Existence rate of TDLN in TD-resected group and distribution of metastasis in TDLNs
Since June 2013, the TDLN number was examined independently of 106recL or 112 in our institution (n = 72). TDLN number at each location (TDLN-Ut/Mt/Lt) and the existence rate were investigated. Furthermore, patients with TDLN metastasis were reviewed and we investigated the correlation between TDLN metastasis and clinicopathological factors. Additionally, to clarify the distribution of TDLN and non-TDLN metastasis, mapping of metastatic LNs and the primary tumor were shown in all patients with TDLN metastasis.
2.6. Statistical analysis
Means and standard deviations (SDs) were calculated, and differences were identified using the Student t test. Differences between categories were identified using the chi-square test or Fisher exact test. We produced survival curves using the Kaplan–Meier survival method. Two groups were compared using a 2-sided log-rank test. All statistical analyses were performed using IBM SPSS Statistics version 22 for Windows (Chicago, IL), and differences were considered significant when P < 0.05.
3. Results
3.1. Patient selection and clinicopathological factors between TD-preserved and TD-resected groups
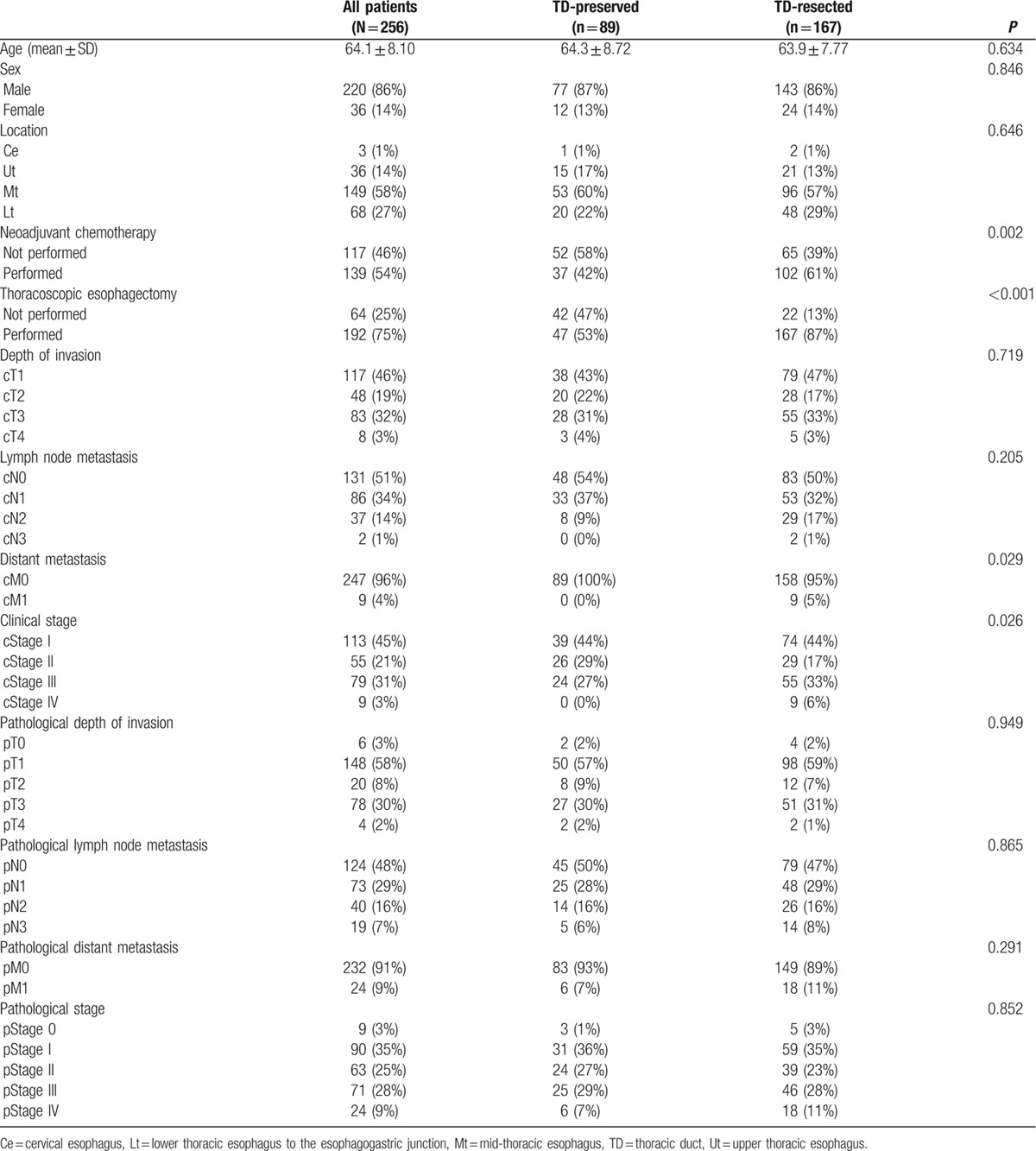
Of 256 patients, TD-preserved TTE was conducted in 89 patients and TD-resected TTE was conducted in 167 patients. The patient characteristics are shown in Table 1. Eighty-six per cent were male, and the primary tumor was located in the midthoracic esophagus of 58% of patients. Nine patients (4%) were evaluated as having cM1 disease due to supraclavicular LN metastasis. The distribution of pretreatment clinical stage of 1/2/3/4 was 113 (45%)/55 (21%)/79 (31%)/9 (3%), and postoperative pathological stage of 0/1/2/3/4 was 9 (3%)/90 (35%)/63 (25%)/71 (28%)/24 (9%). When the patient characteristics and clinicopathological factors were compared between the TD-preserved and TD-resected groups, significantly more patients received NAC and underwent thoracoscopic esophagectomy in the TD-resected group. In terms of pretreatment staging, distant metastasis and advanced pretreatment stage were more likely to be observed in the TD-resected group. There was no difference in pStage between groups.
Table 1.
Patient characteristics, clinicopathologocal factors, and TD resection.
3.2. Surgical outcome, postoperative complications, and RFS
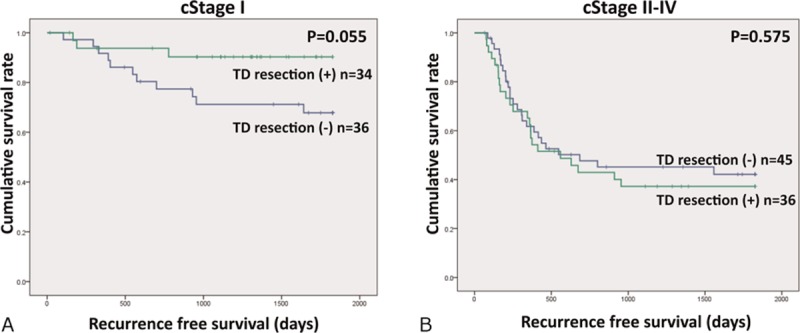
Operation time was significantly longer in the TD-resected group. In contrast, there was no significant difference in blood loss. Regarding postoperative complications, the incidence of postoperative pneumonia, anastomotic leakage, and chylothorax was 20%, 15%, and 1%, respectively, and no differences were observed between the TD-preserved and TD-resected groups. No postoperative mortality 30 days after surgery was observed in either group. Postoperative recurrence is shown in Fig. 2. The 5-year RFS rate of cStage I patients was 67.3% in the TD-preserved group against 90.3% in the TD-resected group, thereby showing a tendency toward RFS extension in the TD-resected group that did not quite reach statistical significance (P = 0.055; Fig. 2A). The median follow-up period was 1475 days in the TD-preserved group and 1252 days in the TD-resected group.
Figure 2.
Thoracic duct (TD) resection and recurrence-free survival (RFS). The 5-year RFS rate of cStage I patients was 67.3% in TD-preserved group against 90.3% in TD-resected group (P = 0.055; A). In cStage II to IV, there was no significant difference in RFS between TD-preserved and TD-resected groups (B).
3.3. Number of dissected LNs for each LN station between TD-preserved and TD-resected groups
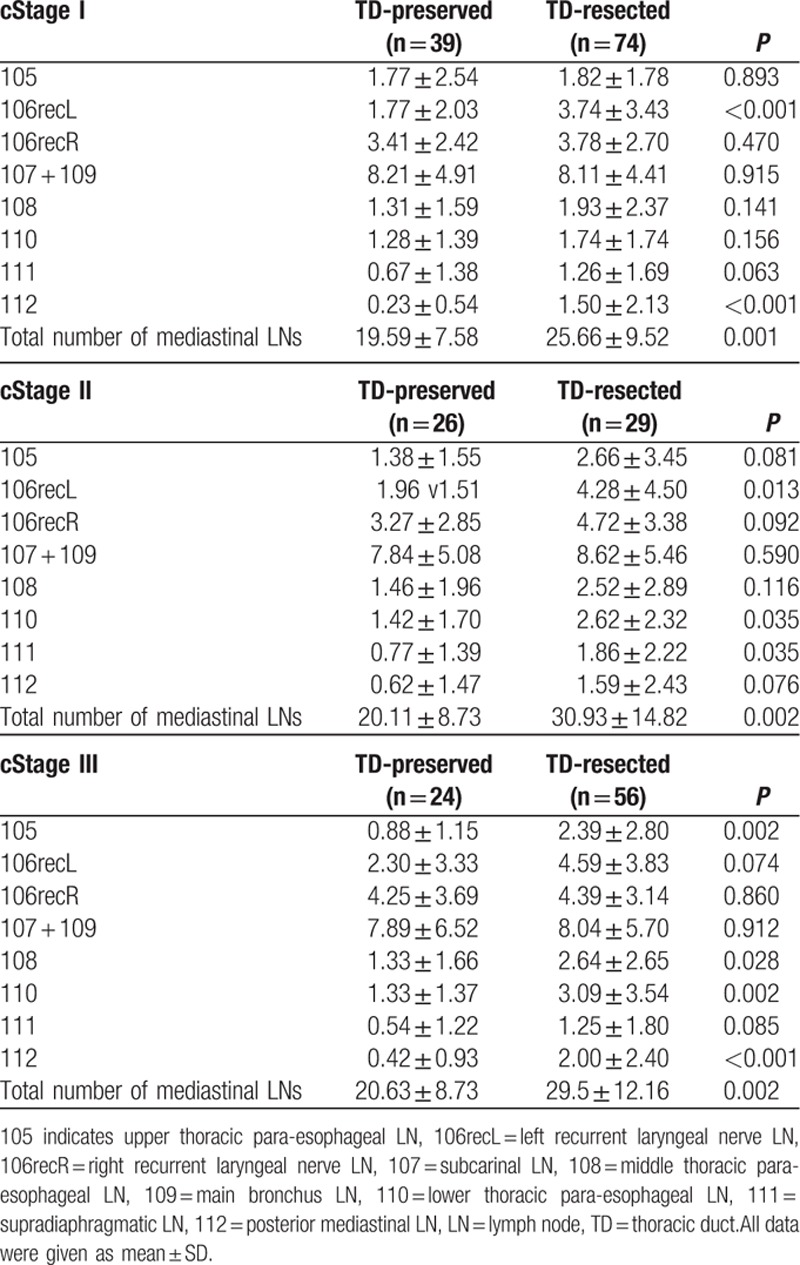
Number of dissected LNs was significantly increased in 105, 106recL, 108, 110, 111, and 112 LNs, resulting in an increased number of the total mediastinal LNs (Table 2). There was no significant difference in the number of dissected 106recR, and 107 ± 109 LNs. When we analyzed by stratification for cStage I, II, and III, the total number of dissected mediastinal LNs was significantly increased in all subgroups (Table 3). When we focused on the number of LNs around TD (106recL, 112), the number of 106recL was significantly increased in the TD-resected group in cStage I and II, and 112 increased in cStage I and III.
Table 2.
Number of dissected LNs and TD resection: all cases.
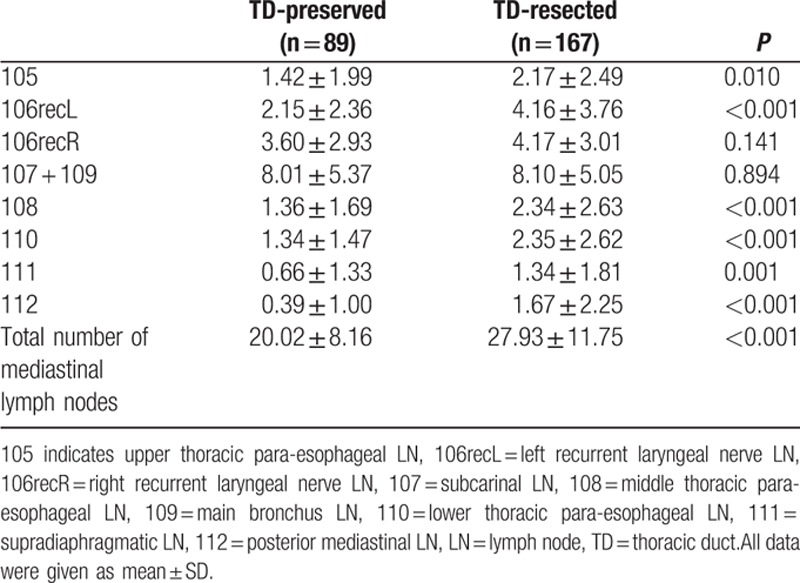
Table 3.
Number of dissected LNs and TD resection: stratification using clinical stage.
3.4. Comparison of the number of regional 106recL/112 except for TDLNs in patients with or without TD resection
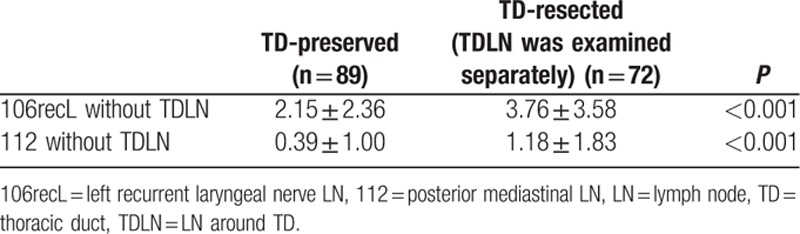
In Table 4, the number of regional 106recL and 112 except for TDLNs in the TD-resected group was compared with those in the TD-preserved group. The number of 106recL and 112 except for TDLNs was shown to be significantly increased in the TD-resected group.
Table 4.
Comparison of the number of regional 106recL/112 LNs without TDLN in patients with or without TD resection.
3.5. Existence rate of TDLNs and metastasis in TDLNs: comparison of clinicophathological factors and mapping
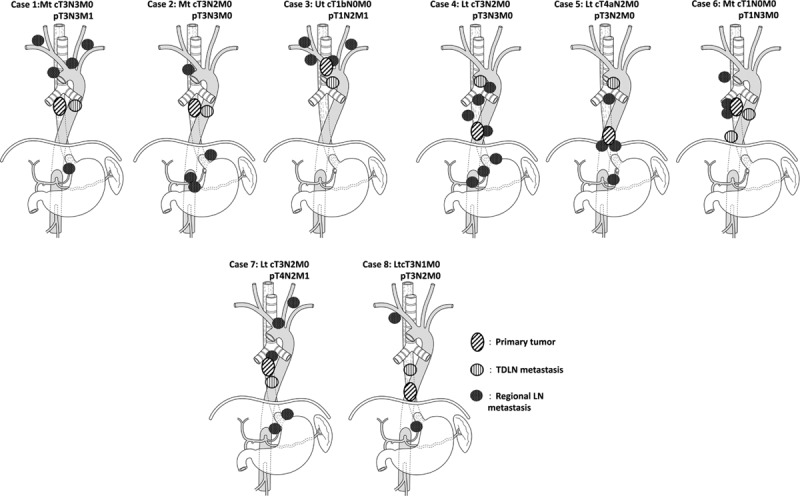
The TDLN-Ut, Mt, and Lt numbers (mean ± SD) were 092 ± 1.74, 0.64 ± 1.42, and 0.47 ± 1.11, respectively (Table 5). The TDLN existence rate was 40% in TDLN-Ut, 31% in TDLN-Mt, and 25% in TDLN-Lt. Consequently, TDLN was detected in 48 out of 72 (67%). LN metastasis in TDLNs was observed in 64 out of 72 patients (11%; Table 6). In patients with pT1 or pT2, incidence of TDLN metastasis was 4% and 26% in patients with pT3 or pT4. When correlations between LN metastasis in TDLN and clinicopathologocal factors was investigated, patients with TDLN metastasis showed significantly higher incidence of clinical LN metastasis and progressive disease. In Fig. 3, location of the primary tumor, TDLN metastasis, and regional metastatic LNs were shown for each patient who was diagnosed with TDLN metastasis. In 7 of 8 cases, metastatic TDLN was observed at the same or cranial level to the primary tumor. Metastatic regional LNs, except for TDLNs, existed on the cranial and caudal side of primary tumor in 6 out of 8 cases, and were observed on the same or at the caudal level to metastatic TDLNs for most of the cases (other than case 6).
Table 5.
Number of TDLNs.
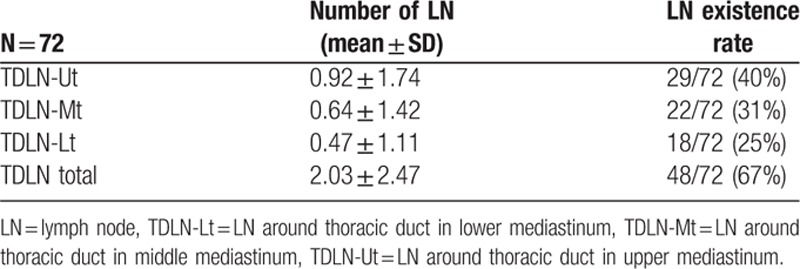
Table 6.
Association tests between LN metastasis in TDLN and clinicopathologocal factors.
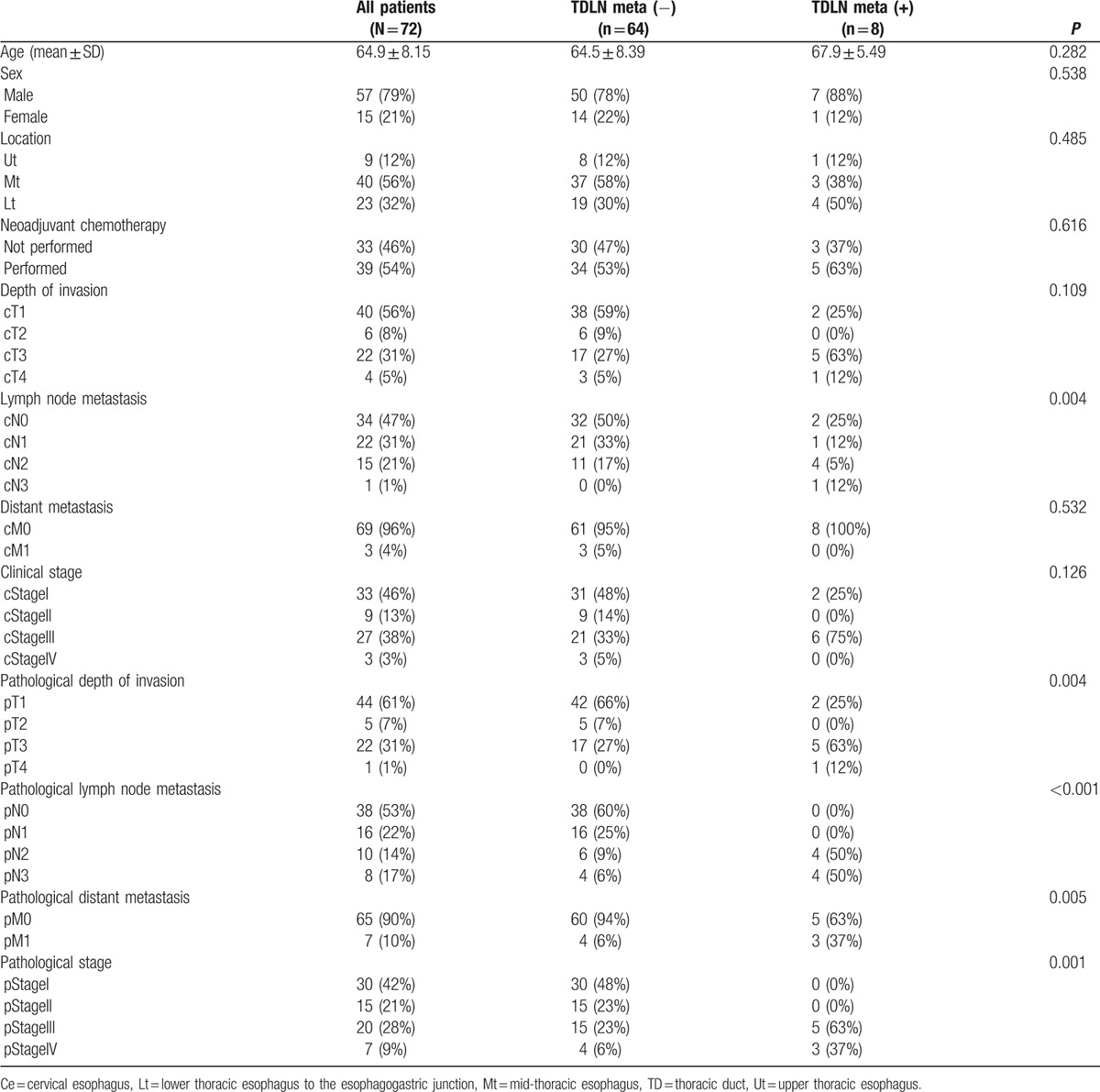
Figure 3.
Mapping of primary tumor, TDLN, and non-TDLN. TDLN = lymph nodes around thoracic duct.
4. Discussion
In the current analysis, the number of dissected mediastinal LNs increased significantly in the TD-resected group. As Udagawa et al[5] described previously, there were LNs in the adipose tissue surrounding TD which could be dissected with TD resection by transthoracic esophagectomy. However, there have been few comparative studies which analyzed oncological outcomes which include number of dissected LNs between TD-preserved and TD-resected group. On the basis of the current result, TD resection might be beneficial for radical esophagectomy.
The TD ascends along the thoracic aorta and transfers from right to left in the upper mediastinum. Therefore, LNs along TD could be classified as 112 in the middle (TDLN-Mt) and lower mediastinum (TDLN-Lt),[11] and 106recL in the upper mediastinum (TDLN-Ut). In the current analysis, number of dissected 106recL and 112 LNs was significantly increased in the TD-resected group. Furthermore, because pretreatment clinical stage was advanced in the TD-resected group, we performed subgroup analysis of a number of dissected LNs for each patient in cStage I, II, and III. Consequently, in most of the subgroups, the number of dissected 106recL and 112 increased significantly, indicating that TD resection directly influenced extended LN dissection. In contrast, other than LNs along TD, para-esophageal LNs were found to be increased, indicating that factors other than TD resection might increase the number of LNs. Since 2009, we developed a hybrid of the prone and left lateral decubitus positions for thoracoscopic TTE.[14] Compared with the hybrid thoracoscopic TTE with classical left decubitus position, mobilization and lymphadenectomy around the middle and lower esophagus are easier in the hybrid thoracoscopic TTE, leading to more radical LN dissection.
When we compared the number of regional 106recL and 112 except for TDLNs between the TD-preserved and TD-resected groups, the number of those specific LNs were not decreased but increased significantly, showing that TDLNs were not intentionally extracted from the regional LNs (106recL, 112) during resection. Furthermore, TD resection might encourage radical LN dissection, leading to an increased number of dissected LNs even in regional LNs, except for TDLN.
In terms of distribution of TDLNs, as shown in Table 6, TDLNs were observed independent of regional LNs (106recL, 112) for each location (TDLN-Ut/Mt/Lt). Although the existence rate of TDLN varied (25%–40%) for each location; 48 out of 72 patients (67%) had TDLN, indicating that TD resection could increase the total number of dissected LNs.
In the current analysis, TDLN metastasis was observed in 8 of 72 patients (11%). Previously, Udagawa et al reported the incidence of TDLN metastasis as 2.2% in pT1/2 and 10% in pT3/4. In this study, 4% of patients with pT1/2 were diagnosed as having TDLN metastasis, and 26% with pT3/4, which was consistent with previous studies. Based on the correlation between TDLN metastasis and clinicopathological factors, patients with TDLN metastasis showed a significantly advanced disease. Furthermore, on the basis of mapping of the primary lesion and metastatic LNs, TDLN metastasis was observed on the same level or the cranial side to the primary lesion despite the fact that metastatic regional LNs existed on the cranial and caudal side of primary tumor in 6 out of 8 cases. Furthermore, in 7 out of 8 cases, metastatic TDLN was observed on the same level or cranial side to metastatic regional LNs. Therefore, we speculated that lymphatic flow in the tissue around TD ascends from the abdomen to the cervix, and also flow in TD.
Prognosis needs to be investigated when considering the oncological benefits of TD resection. Because an increased number of LN dissections were shown to be a favorable prognostic factor in esophageal cancer patients, a survival benefit might exist. The RFS in cStage I patients tended to be extended in the current analysis. With regard to the correlation with TDLN metastases, 2 patients who were diagnosed as cStage I had TDLN metastases. Therefore, TTE with TD resection may lead to a survival benefit in patients with cStage I. However, the number of patients for each cStage was insufficient for firm conclusions to the survival analysis. Furthermore, prospective comparative trial needs to be planned to clarify the survival benefit of TD resection.
There are several drawbacks to TD resection. Simultaneous TD resection might extend the operation time and increase blood loss. However, we found that there was no significant difference in blood loss between the TD-preserved and TD-resected groups. Furthermore, although more patients received NAC in TD-resected group, the incidence of postoperative complications, including chylothorax, did not increase in the TD-resected group. Consequently, TD resection might be safely performed.
The present study was limited to retrospective assessments. However, consecutive patients who underwent TTE were reviewed, and all ESCC patients who underwent TTE between January 2004 and July 2015 were included in this analysis, leading to reduced selection bias. Furthermore, although the TD-preserved group tended to undergo surgery before 2009 and perioperative management may be different between the TD-preserved and TD-resected groups, those factors might not have a strong influence on the number of dissected LNs, which was the primary outcome measure of this study. Regarding survival analysis, the period of TTE was different between groups. However, RFS was chosen as a prognostic indicator, and was evaluated only in patients who underwent TTE before December 31, 2012.
In conclusion, TTE with TD resection could be safely performed and increased the number of dissected mediastinal LNs. Furthermore, LNs were included in adipose tissue around TD and metastasis occurred in TDLN, especially in patients with advanced esophageal cancer. To clarify whether TD should be resected in TTE, a prospective comparative trial, which investigates survival between TD-preserved and TD-resected groups, needs to be coordinated.
Footnotes
Abbreviations: C–D = Claviend–Dindo, DCF = cisplatin, 5-fluorouracil, and docetaxel, ESCC = esophageal squamous cell carcinoma, FP = cisplatin and 5-fluorouracil, LNs = lymph nodes, NAC = neoadjuvant chemotherapy, RFS = recurrence-free survival, TD = thoracic duct, TDLN = LNs around TD, TTE = transthoracic esophagectomy.
The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
References
- [1].Udagawa H, Akiyama H. Surgical treatment of esophageal cancer: Tokyo experience of the three-field technique. Dis Esophagus 2001;14:110–4. [DOI] [PubMed] [Google Scholar]
- [2].Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411–20. [DOI] [PubMed] [Google Scholar]
- [3].Hanna JM, Erhunmwunsee L, Berry M, et al. The prognostic importance of the number of dissected lymph nodes after induction chemoradiotherapy for esophageal cancer. Ann Thorac Surg 2015;99:265–9. [DOI] [PubMed] [Google Scholar]
- [4].Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857–63. [DOI] [PubMed] [Google Scholar]
- [5].Udagawa H, Ueno M, Shinohara H, et al. Shoud lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 2014;11:204–10. [Google Scholar]
- [6].Lai FC, Chen L, Tu YR, et al. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg 2011;91:1770–4. [DOI] [PubMed] [Google Scholar]
- [7].Cagol M, Ruol A, Castoro C, et al. Prophylactic thoracic duct mass ligation prevents chylothorax after transthoracic esophagectomy for cancer. World J Surg 2009;33:1684–6. [DOI] [PubMed] [Google Scholar]
- [8].Imamura M, Shimada Y, Kanda T, et al. Hemodynamic changes after resection of thoracic duct for en bloc resection of esophageal cancer. Surg Today 1992;22:226–32. [DOI] [PubMed] [Google Scholar]
- [9].Aiko S, Yoshizumi Y, Matsuyama T, et al. Influences of thoracic duct blockage on early enteral nutrition for patients who underwent esophageal cancer surgery. Jpn J Thorac Cardiovas Surg 2003;51:263–71. [DOI] [PubMed] [Google Scholar]
- [10].Guler O, Ugras S, Aydin M, et al. The effect of lymphatic blockage on the amount of endotoxin in portal circulation, nitric oxide synthesis, and the liver in dogs with peritonitis. Surg Today 1999;29:735–40. [DOI] [PubMed] [Google Scholar]
- [11].Japan Esophageal Society. Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. 10th ed.Tokyo, Japan: Kanehara Co; 2007. [Google Scholar]
- [12].Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- [13].Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 2013;104:1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaburagi T, Takeuchi H, Kawakubo H, et al. Clinical utility of a novel hybrid position combining the left lateral decubitus and prone positions during thoracoscopic esophagectomy. World J Surg 2014;38:410–8. [DOI] [PubMed] [Google Scholar]


