Abstract
To assess the safety and efficacy of rilpivirine in combination with emtricitabine and tenofovir (RPV/FTC/TDF) as a once-daily single-tablet regimen (STR) in HIV-1-infected children and adolescents we performed a multicenter case series study of HIV-1-infected patients. Inclusion criteria were initiation of therapy with RPV/FTC/TDF before the age of 18. Patients were divided into undetectable viral load (uVL) group, HIV-1 RNA < 20 copies/mL on stable combined antiretroviral therapy (cART), and detectable viral load (dVL) group, HIV-1 RNA ≥ 20 copies/mL at RPV/FTC/TDF initiation. Patients were monitored from the date of RPV/FTC/TDF initiation until June 30, 2015, RPV/FTC/TDF discontinuation or failure to follow-up. Seventeen patients (8 in uVL and 9 in dVL group) with age between 11.6 and 17.6 were included. Reasons for switching were toxicity (n = 4) and simplification (n = 4) in uVL; viral failure (n = 8) and cART initiation (n = 1) in the dVL group. After a median follow-up of 90 (uVL) and 40 weeks (dVL), 7/8 (86%) patients maintained and 8/9 (89%) achieved and maintained HIV-1 suppression. Median CD4 count increased from 542 to 780/μL (uVL, P = 0.069) and 480 to 830/μL (dVL, P = 0.051). Five patients (2 in uVL and 3 in dVL) improved their immunological status from moderate to no immunosuppression. Serum lipid profiles improved in both groups; cholesterol dropped significantly in the dVL group (P = 0.008). Grade 1 laboratory adverse events (AEs) were observed in 3 patients. No clinical AEs occurred. Adherence was complete in 9 patients (5 in uVL and 4 in dVL); 1 adolescent interrupted treatment. Once-daily STR with RPV/FTC/TDF may be a safe and effective choice in selected HIV-1-infected adolescents and children.
Keywords: adolescents, antiretroviral therapy, children, HIV-1, rilpivirine
1. Introduction
The management of HIV-infected pediatric patients is a challenge due to the complexity of their social environment, the desire to be accepted by their peers, and poor knowledge and a negative attitude in relation to their HIV status. Furthermore, lack of adherence (particularly in adolescents), high pill burden, inadequate support, substance abuse, few age-appropriate formulations to ensure appropriate drug exposure, and differences in pharmacokinetics between children and adults impact efficacy and safety.[1–6]
In adults, single-tablet regimens (STR) are associated with high adherence rates and improvement of quality of life.[7] STR also avoid selective nonadherence—not taking all components of a regimen.[8] Rilpivirine (TMC278), a new non-nucleoside reverse transcriptase inhibitor (NNRTI), is available as a single-agent tablet or in combination with FTC plus TDF in an STR. Several studies have indicated RPV/FTC/TDF STR being a favorable choice in naïve and selected treatment-experienced HIV-1-infected adults.[9–12] This STR has been approved by the FDA in naïve HIV-1-infected adults with HIV-1 RNA ≤100,000 copies/mL or virologically suppressed (HIV-1 RNA < 50 copies/mL) adults with no mutations associated with resistance to NNRTIs, FTC, or TDF.[13]
RPV has been approved in the United States for children age ≥12 years, but not in Europe, and data on its efficacy in this age group is very limited to date. PAINT (Pediatric study in Adolescents Investigating a new NNRTI TMC278) is a multicenter ongoing trial evaluating the pharmacokinetics, safety, and efficacy of RPV in treatment-experienced and naïve HIV-1-infected adolescents age 12 to 18 years.[14,15] Here, we present long-term data from children and adolescents with perinatally acquired HIV-1 infection treated with off-label RPV/FTC/TDF.
1.1. Study patients and methods
In this multicenter observational study, 17 vertically HIV-1-infected children and adolescents (<18 years) were enrolled from March 2013 from 6 pediatric reference hospitals (Cohort of the Spanish Pediatric HIV Network, CoRISpe,[16] see Annex). STR with RPV/FTC/TDF (25/200/245 mg) was prescribed under the Spanish off-label medication use program.[17] Individuals were monitored from baseline (date of RPV/FTC/TDF initiation) until June 30, 2015 (administrative censoring date), RPV/FTC/TDF discontinuation or failure to follow up. Demographic, clinical, and laboratory parameters were extracted from the CoRISpe database. Adherence was evaluated by pill count, assessing the dose taken, and interviewing parents/guardians, and was summarized as a single percentage for each combined antiretroviral therapy (cART) regimen for each patient and categorized as poor (<70%), intermediate (70–90%), good (91–99%), or perfect (100%). Immunological category and clinical stage were based on the CDC classification.[18] Major mutations from the IAS-USA mutation list 2013 were used to define resistance to RPV, TDF, and/or FTC.[19] Plasma VL (HIV-1 RNA) was measured by Amplicor Monitor assay (Roche Diagnostic Systems, Pleasanton, CA, USA) and real-time NASBA (Easy Mag y Nuclisens Easy Q, BioMerieux, Boston, MA, USA) with a detection limit of 20 HIV-1 RNA copies/mL. Virological failure was defined as the inability to achieve or maintain suppression of viral replication below 20 copies/mL. CD4 were quantified by flow cytometry (Beckman Coulter FC-500 Cytometer, Beckman Coulter, Inc., Brea, CA, USA and Becton-Dickinson-FACScalibur, Franklin Lakes, NJ, USA). Toxicity was monitored every 6 months during follow-up. Parameters monitored included renal toxicity, hepatic toxicity, and laboratory parameters such as lipid profile cholesterol and glucose. Rash, dizziness/light-headedness, insomnia or abnormal dreams, headaches, nausea, fatigue, and depression were also evaluated. Adverse events (AEs) severity was graded using the Division of AIDS AEs grading table.[20] Ethical approval was obtained from the ethical committees of all hospitals.
Quantitative variables were summarized using medians and interquartile ranges (IQRs), whereas absolute values and relative frequencies were used for qualitative variables. Demographic, clinical, and laboratory variables were analyzed according to baseline VL: patients with undetectable VL (uVL) and patients with detectable viral load (dVL). Continuous variables were compared between groups using the Mann–Whitney U test. The nonparametric Wilcoxon signed-rank test was applied to determine differences for measurements at different points in time. The differences were considered statistically significant for P values <0.05. The statistical analyses were performed using SPSS software (v. 19.0, Chicago, IL).
2. Results
Seventeen subjects were included in the study. Demographic, clinical, and laboratory baseline characteristics are summarized in Table 1. Two were children age 11.6 and 11.7 years and 15 were adolescents age 16.7 years (IQR: 15.8–17.3). Ten were girls (59%) and 13 (76%) Caucasian. At time of enrolment 7 (41%) subjects presented moderate immunosuppression and 2 (12%) had a clinical stage C. At baseline all patients showed HIV-1 RNA <10,000 copies/mL. At the start of the RPV-based regimen, 1 patient was cART-naïve and the rest had been exposed to cART for a median of 10.0 (IQR: 7.6–12.2) years. Four were on an NNRTI and 12 on a protease inhibitor-based regimen. Five adolescents had accumulated reverse transcriptase resistance-associated mutations (RAMs): in the uVL group, 1 patient had the M184V mutation, 1 individual the M184V and G190A mutations, and 1 subject the T215Y and M41L mutations. In the dVL group, 1 patient had the T215Y mutation and 1 the T215Y and Y181C mutations; the latter reduces susceptibility to RPV 3-fold (Table 1).
Table 1.
Characteristics of the study population at baseline.
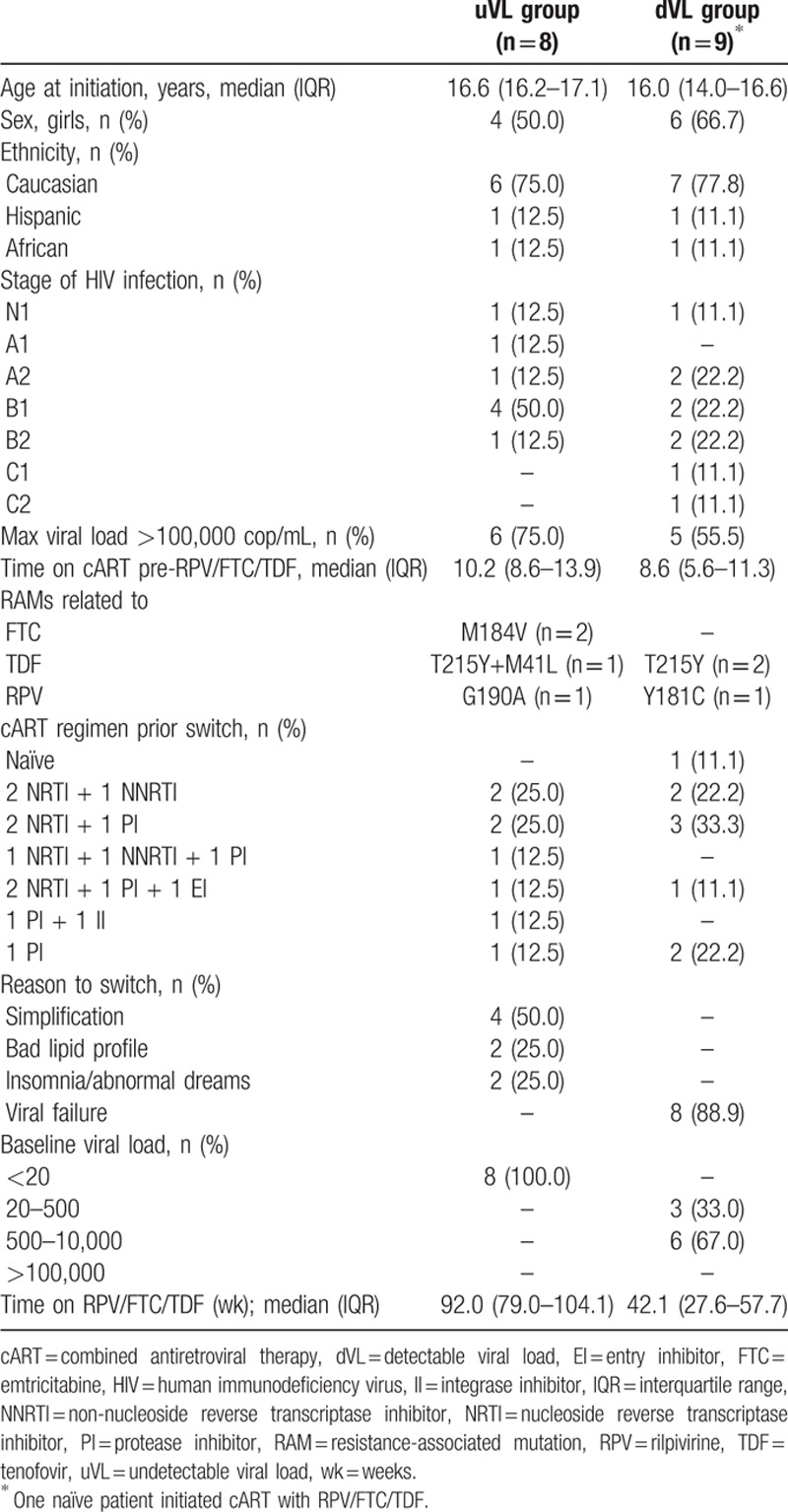
Reasons for RPV/FTC/TDF initiation were simplification (n = 4; 24%); toxicities (neurological, associated with EFV, n = 2, 12%; dyslipidemia, n = 2, 12%); viral failure (n = 8; 47%) and CD4 count below 350/μL in the naïve patient. Overall median time on RPV-based treatment was 61.9 weeks (IQR: 41.1–90.5). According to baseline VL, 8 patients were included in the uVL group and 9 subjects were included in the dVL group. Median time on RPV-based treatment was 89.1 weeks (IQR: 66.5–100.9) and 39.6 weeks (IQR: 23.6–55.4) in the uVL and dVL groups, respectively (P = 0.01). Seven out of 8 adolescents with uVL at baseline (including the 3 patients with RAMs) maintained undetectable viral load (uVL) for a median time of 93.6 weeks (IQR: 87.4–104.3). A 17-year-old boy developed viral failure due to poor adherence (<10%) caused by mental disorders (antisocial personality disorder). Eight out of 9 patients in the dVL group achieved and maintained uVL for a total median time of 41.1 weeks (IQR: 26.8–57.5), although 1 of these experienced a blip at the end of the follow-up because of intermediate adherence (50–90%). On the other hand, 1 adolescent remained persistently detectable during the study period because of poor adherence (<70%) and low-level TDF resistance (T215Y), while the patient who had the T215Y and Y181C mutations reached a viral load below 100 copies/mL (77 copies/mL) after 30.9 weeks.
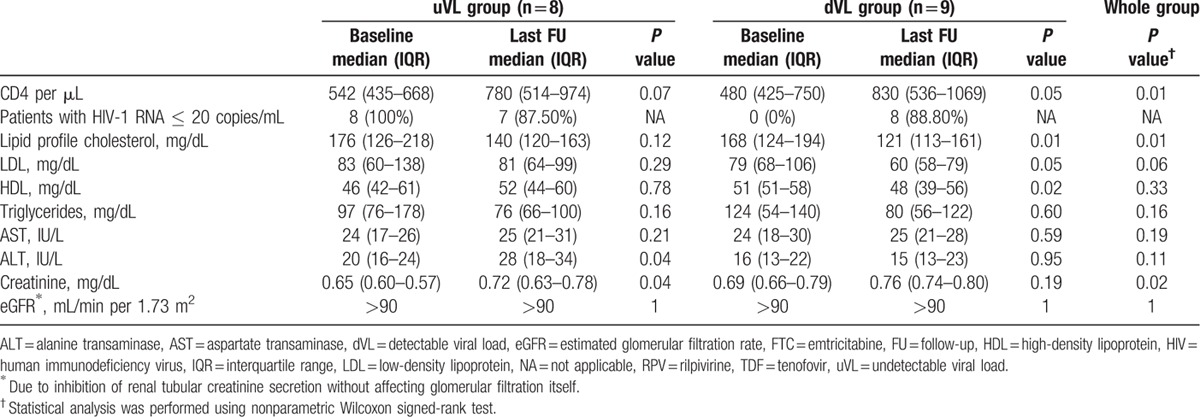
Laboratory parameters are summarized in Table 2. Median CD4 counts as well as CD4/CD8 ratio improved in both groups and a significant difference was observed when analyzing the whole group (P = 0.01 and P = 0.01, respectively). Five patients changed their immunological category from moderate immunosuppression to no suppression (29%); however, 1 patient dropped from category 1 to 2. No patient discontinued the study because of AEs. Grade 1 AE were reported in 3 patients (18%). Increases in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) occurred in 2 adolescents (12%), although in 1 of them it was not considered related to RPV-based treatment, and triglycerides elevation was reported in 1 patient (6%). While lipid profile in the uVL group improved, it did not reach statistical significance. On the other hand, in the dVL group, cholesterol (P = 0.01) improved significantly. Clinical AE related to RPV/FTC/TDF, such as skin rash or central nervous system-related symptoms were not observed during follow-up. Moreover, the 2 adolescents who had suffered insomnia or abnormal dreams with their previous EFV-based regimen did not report any complaints with the new cART. One adolescent with poor adherence (<70%) discontinued the RPV-based regimen and dropped out of care. No deaths were observed.
Table 2.
Laboratory parameters at baseline and at last follow-up in pediatric patients treated with RPV/FTC/TDF.
3. Discussion
In this case series of HIV-1-infected children and adolescents, treatment with RPV/FTC/TDF was associated with good control of HIV infection in both groups (uVL and dVL) after a median follow-up time of 89.1 and 39.6 weeks, respectively, in all but 2 patients with a poor adherence (<70%). While no clinical or laboratory significant adverse effects were observed, CD4 counts and CD4/CD8 ratio improved independently of the study groups. These observations are in line with previous publications in adults comparing RPV with EFV therapy, which describe a significant increase in CD4 counts after the 96-week treatment period in the RPV group.[9,10] As previously described, it is also worth noting that we observed an improvement of fasting serum lipid profile.[12]
Only low-grade AEs were reported during clinical follow-up and the toxicities that lead to cART switch to RPV/FTC/TDF (insomnia/abnormal dreams and bad lipid profile) disappeared. AST, ALT, and creatinine were the commonest observed Grade 3 to 4 laboratory abnormalities described in adults.[11] In this study, Grade 1 AST/ALT and triglycerides elevation were the only laboratory abnormality observed.
In summary, in this first pediatric study, RPV/FTC/TDF was associated with control of viral replication, an improvement of CD4 counts and CD4/CD8 ratio as well as serum lipid profiles, while low-grade AEs were observed. An important limitation of this observational study is the small sample size as well as the different time for follow-up between the 2 groups. However, to date there is limited data on adolescent patients and no data regarding children receiving RPV/FTC/TDF therapy. Our observation is therefore new and probably reassuring, although the presented results need to be confirmed with larger prospective clinical studies in order to conclude whether RPV/FTC/TDF is a good therapeutic option in selected HIV-1-infected children and adolescents where treatment simplification strategies to enhance adherence are crucial.
4. Annex
Hospitals in which the patients were enrolled: Hospital Virgen del Rocío, Seville (6 patients); Hospital Universitario Doce de Octubre, Madrid (2 patients); Hospital Universitari Vall d’Hebron, Barcelona (4 patients); Hospital Sant Joan de Déu, Barcelona (3 patients); Hospital General Universitario Gregorio Marañón, Madrid (1 patient); and Hospital Virgen de las Nieves, Granada (1 patient).
Acknowledgments
The authors would like to thank all the patients for their participation and the collaborating centers for the clinical samples provided. This study was registered under reference number RIS-EPICLIN-13/2015.
Footnotes
Abbreviations: AE = adverse events, ALT = alanine aminotransferase, AST = aspartate aminotransferase, cART = combined antiretroviral therapy, dVL = detectable viral load, EFV = efavirenz, FTC = emtricitabine, NNRTI = nonnucleoside reverse transcriptase inhibitor, PI = protease inhibitor, RAM = resistance-associated mutation, RPV = rilpivirine, STR = single-tablet regimen, TDF = tenofovir, uVL = undetectable viral load.
The first two authors (LF-N and CP) contributed equally to the manuscript.
The last two authors (VB and ON) contributed equally to the manuscript.
LF-N, CP, VB, ON, and MLNG conceived the study and participated in its design. PS-P, MIG-T, LF-L-C, ON, CF, AN-J, and JLS provided the patients’ data. SJD and MAF selected the data. CP, LF-N, PO, VB, and ON designed the database and analyzed the data. LF-N, CP, ON, and VB drafted the manuscript. LF-N, ON, AN-J, CP, and VB discussed the final manuscript. All the authors read and approved the final manuscript.
Financial support was provided by the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en Sida (ISCIII-RETIC RD06/006; RD12/0017/0035, and RD12/0017/0037) and FIPSE (grant number: 36-0910-10). This work has been also supported by grants from Instituto de Salud Carlos III (Ref. MPY 1039/14 to VB). CP is supported by the Portuguese Fundação para a Ciência e Tecnologia (FCT) (grant number SFRH/BPD/77448/2011, part of the EDCTP2 program supported by the European Union). VB is supported by the Miguel Servet program run by the Fondo de Investigación Sanitaria (ISCIII) (grant number CP13/00098).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicts of interest to disclose.
References
- [1].Murphy DA, Sarr M, Durako SJ, et al. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med 2003;157:249–55. [DOI] [PubMed] [Google Scholar]
- [2].Reisner SL, Mimiaga MJ, Skeer M, et al. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med 2009;17:14–25. [PMC free article] [PubMed] [Google Scholar]
- [3].Phelps BR, Rakhmanina N. Antiretroviral drugs in pediatric HIV-infected patients: pharmacokinetic and practical challenges. Paediatr Drugs 2011;13:175–92. [DOI] [PubMed] [Google Scholar]
- [4].Menson EN, Walker AS, Sharland M, et al. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997–2005: cohort study. BMJ 2006;332:1183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fernandez E, Perez R, Hernandez A, et al. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 2011;3:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ma Q, Lu AY. Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol Rev 2011;63:437–59. [DOI] [PubMed] [Google Scholar]
- [7].Airoldi M, Zaccarelli M, Bisi L, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence 2010;13:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Antinori A, Angeletti C, Ammassari A, et al. Adherence in HIV-positive patients treated with single-tablet regimens and multi-pill regimens: findings from the COMPACT study. J Int AIDS Soc 2012;15suppl 4:18098. [Google Scholar]
- [9].Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 2011;378:238–46. [DOI] [PubMed] [Google Scholar]
- [10].Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet 2011;378:229–37. [DOI] [PubMed] [Google Scholar]
- [11].Cohen C, Wohl D, Arribas JR, et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naïve HIV-1-infected adults. AIDS 2014;28:989–97. [DOI] [PubMed] [Google Scholar]
- [12].Palella FJ, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS 2014;28:335–44. [DOI] [PubMed] [Google Scholar]
- [13].European Medicines Agency. Eviplera™ 200 mg/25 mg/245 mg film coated tablets: Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002312//WC500118802.pdf Accessed October 5, 2015. [Google Scholar]
- [14].Crauwels H, Hoogstoel A, Vanveggel S, et al. Rilpivirine pharmacokinetics in HIV-1-infected adolescents: a substudy of PAINT (Phase II Trial) [Abstract 900]. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI 2014); March 3–6, 2014. Boston, MA. [Google Scholar]
- [15].Lombaard J, Bunupuradah T. Week 48 safety and efficacy of a rilpivirine (TMC278)-based regimen in HIV-infected treatment-naïve adolescents: PAINT phase II trial [Abstract O10]. In: 7th International Workshop on HIV Pediatrics; July 17–18, 2015. Vancouver, Canada. [Google Scholar]
- [16].de Jose MI, Jiménez de Ory S, Espiau M, et al. A new tool for the paediatric HIV research: general data from the Cohort of the Spanish Paediatric HIV Network (CoRISpe). BMC Infect Dis 2013;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boletín Oficial del Estado. Real Decreto 1015/2009 de 19 de junio, por el que se regula la disponibilidad de medicamentos en situaciones especiales. Ministerio de Sanidad y Política Social. BOE núm. 174 de 20/7/2009. https://www.boe.es/diario_boe/txt.php?id=BOE-A-2009–12002 Accessed February 15, 2013. [Google Scholar]
- [18].Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed March 18, 2016. [Google Scholar]
- [19].Johnson VA, Calvez V, Gunthard HF, et al. Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med 2013;21:6–14. [PMC free article] [PubMed] [Google Scholar]
- [20].US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0 [November 2014]. http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GRADING_TABLE_v2_NOV2014.pdf Accessed June 23, 2015. [Google Scholar]


